
Safety and efficacy of delayed completion lobectomy following wedge resection vs. segmentectomy—a retrospective cohort study
Highlight box
Key findings
• Delayed completion lobectomy (CL) occurring over 3 months after sublobar resection is a safe and effective procedure.
What is known and what is new?
• It has been reported that CL is relatively safe at an operative interval of no longer than 5 weeks.
• We reported a longer operative interval, and an initial wedge resection might lead to better results of CL compared with segmentectomy.
What is the implication, and what should change now?
• When considering the potential risk of subsequent CL, segmentectomy should not be overly pursued, and wedge resection might be a better choice for early-stage non-small cell lung cancer. However, surgical decisions should be made in cautious.
Introduction
With lung cancer screening becoming more prevalent, a growing number of patients with early-stage lung cancer are now being detected. Many of these patients can expect an extended post-operative survival but are at risk of developing secondary primary tumors (1) that warrant additional resections. In addition, for patients with multiple nodules that are suspected to be multiple primary lung cancers, surgical resection may only remove the primary, dominant lesion. Any remaining secondary lesions have the potential to progress over follow-up. Subsequent operations, particularly on the ipsilateral side of the lung, can be challenging due to scarring and dense adhesions or fibrosis of hilar structures (2).
Lobectomy has been established as the standard of care for the surgical management of lung cancer (3). However, more recent studies (4,5) have demonstrated that sublobar resections may achieve a comparable prognosis for small, early-stage, less invasive peripheral lung cancers (6). In current clinical practice, a large number of early-stage lung cancer patients now routinely undergo sublobar resections. These patients may require a subsequent completion lobectomy (CL) if they develop secondary primary lesions, metastatic, or recurrent lesions within the same lobe over the course of follow-up. Only a small number of studies with limited sample sizes have been reported to address the safety and efficacy of CL in this growing population of patients (7-10).
A recent multicentric prospective study revealed that wedge resection and segmentectomy were equally effective in a selected group of patients with ground-glass nodule (GGN)-predominant lung tumors (11). However, some experts have warned against pursuing an anatomical segmentectomy (12), citing concerns about the difficulties of a subsequent CL as compared to after an initial wedge resection. We have found no convincing evidence to support these concerns.
Therefore, the aim of our study was to evaluate the safety and efficacy of CL, and to compare the technical difficulty and short-term outcomes of CL after an initial surgery of anatomical segmentectomy vs. wedge resection.
This study used a retrospective cohort design, in which the data of patients were obtained retrospectively, and the patients that had undergone sublobar resection (wedge resection or segmentectomy) were followed up according to the standard process after subsequent CL, and short-term outcomes of the CL of different groups [wedge resection group (WR group) and segmentectomy group (SG group)] were obtained and compared. Wedge resection can be regarded as exposure and segmentectomy as control. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1780/rc).
Methods
Patient selection
Consecutive patients who underwent CL between January 2013 and December 2019 at the Shanghai Pulmonary Hospital for a second ipsilateral lung cancer within the same lobe and at least 3 months after their initial sublobar resection were retrospectively included in this study. “Delayed” CL was thus defined as CL that occurred no less than 3 months after the initial sublobar resection in this study. Exclusion criteria included (I) time interval between resections of less than 3 months (to exclude patients who underwent salvage lobectomy due to insufficient resection margin at initial surgery); (II) secondary resections consisting of sublobar resections (Figure 1). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of the Shanghai Pulmonary Hospital (K23-222), and individual consent for this retrospective analysis was waived.
Treatment
Clinical (marked with “c”) and pathological (marked with “p”) staging was done according to the eighth edition of the International Association for the Study of Lung Cancer (IASLC) on tumor, node, and metastasis (TNM) staging system for non-small cell lung cancer (NSCLC) (13). Sublobar resection was considered as an alternative to lobectomy for patients with early-stage NSCLC (cTNM stage: IA, n=24, IB, n=1) in an attempt to preserve lung parenchyma. In this study, whether to choose anatomical segmentectomy or wedge resection was determined by the size and location of the tumor.
Subsequent CL was performed in the following situations: (I) tumor recurrence occurred in the same lobe after primary sublobar resection; (II) a new secondary primary tumor identified in the same lobe; and (III) residual lesions in the same lobe enlarged during follow-up. During the standardized follow-up process after initial sublobar resection, if tumor recurrence was suspected or if the residual nodules were increased (for patients presented initially with multiple primary nodules), a positron emission tomography (PET)-computed tomography (CT) examination was performed. If the lesions were suspected to be malignant and no distant metastasis was found, a CL was recommended in cases of recurrence. If the patient presented with multiple primary nodules that progressed over follow-up, the appropriate subsequent resection (i.e., wedge resection, segmentectomy, or lobectomy) was determined based on the size and location of the lesions, with a parenchymal-preserving resection favored if possible.
All resections were performed under general anesthesia, either by video-assisted thoracoscopy (VATS) (single port or two ports) or thoracotomy. At our institute, surgical procedure decisions between thoracotomy and VATS were made after a comprehensive evaluation by operating surgeons. In general, a camera port was made to determine whether single-port resection was appropriate. In some cases, conversion from VATS to thoracotomy was inevitable. For our single center, total lung adhesions (usually due to previous pleural inflammation) remain the most important reason for conversion to thoracotomy, followed by large vessel injury, and if the adhesions are separated during VATS for more than 4 hours, conversion to thoracotomy should be conducted to avoid prolonged surgery.
Follow-up
All patients were recommended to return 3 to 4 weeks after their surgeries. The follow-up was scheduled every 3 to 4 months for the first 2 years, and then every 6 to 12 months after that. Every 6 to 12 months, a chest CT was recommended. Phone calls and emails were also used to acquire information about the patient’s status and postoperative complications. The deadline for follow-up after the second surgery was January 23rd, 2024.
Outcomes of CL
Outcome variables such as “use of angioplasty/bronchoplasty”, “blood loss”, “operative duration”, “length of hospital stay”, and “postoperative complications” were acquired according to the surgery records or hospital records retrospectively, long-term survival variables (such as “recurrences”) were obtained during the standard follow-up process.
Statistical analysis
Continuous variables were shown as mean ± standard deviation, range, or interquartile range (IQR), and categorical variables as number and percentage. Continuous variables were compared using the t-test or Wilcoxon rank-sum test, depending on whether they were normally distributed, and categorical variables by Fisher’s exact test to examine relationships between groups. All P values were two-sided, except for the following post-operative variables: “postoperative complication”, “length of hospital stay”, “use of angioplasty/bronchoplasty”, “blood loss”, and “operative duration”, which were one-sided to provide a non-inferiority analysis. In this study, recurrence in the stump was specifically characterized as local recurrence. Regional recurrence was defined as metastasis in the ipsilateral hilar or mediastinal lymph nodes. Recurrence at all other places was regarded as distant recurrence. Recurrence-free survival (RFS) was defined as the duration between surgery and either recurrence or death from any cause. Overall survival (OS) was defined as the period between surgery and death from any cause. RFS and OS were estimated based on Kaplan-Meier methods, and compared using log-rank test. Hazard ratios (HRs) were estimated using univariate Cox proportional hazard (Coxph) regression modeling. P<0.05 was considered to be significant. IBM SPSS Statistics (version 25; RRID: SCR_016479) and R Project for Statistical Computing (version 4.1.2; RRID: SCR_001905) were used for statistical analysis.
Results
Patient characteristics
A total of 236 patients underwent a repeat lung resection for ipsilateral second NSCLC during the study period. Of these, 25 patients underwent CL at least 3 months after their initial sublobar resection, including nine segmentectomies (SG group) and 16 wedge resections (WR group).
Baseline characteristics of the patient cohort are listed in Table 1. There were no missing values in all relevant variables, except for lymph node dissection, which was marked as “unknown” when missing. No significant differences existed between the SG and WR groups in regard to gender, age, comorbidities, history of contralateral lung surgery, smoking history, operative interval, and site of lesion.
Table 1
| Characteristics | Patient cohort (n=25) | Segmentectomy (n=9) | Wedge resection (n=16) | P |
|---|---|---|---|---|
| Gender, n (%) | 0.68 | |||
| Male | 14 (56.0) | 6 (66.7) | 8 (50.0) | |
| Female | 11 (44.0) | 3 (33.3) | 8 (50.0) | |
| Age (years) | 0.73 | |||
| Mean ± SD | 58.4±8.05 | 57.7±4.12 | 58.9±9.70 | |
| Median (Q1, Q3) | 58.0 (54.0, 63.0) | 57.0 (55.0, 60.0) | 60.0 (53.5, 63.8) | |
| Comorbidities, n (%) | >0.99 | |||
| Without | 16 (64.0) | 6 (66.7) | 10 (62.5) | |
| With | 9 (36.0) | 3 (33.3) | 6 (37.5) | |
| History of contralateral lung surgery, n (%) | >0.99 | |||
| Without | 22 (88.0) | 8 (88.9) | 14 (87.5) | |
| With | 3 (12.0) | 1 (11.1) | 2 (12.5) | |
| Smoking history, n (%) | 0.60 | |||
| No | 21 (84.0) | 7 (77.8) | 14 (87.5) | |
| Yes | 4 (16.0) | 2 (22.2) | 2 (12.5) | |
| Operative interval (months) | 0.45 | |||
| Mean ± SD | 32.7±22.1 | 37.3±24.7 | 30.1±20.9 | |
| Median (Q1, Q3) | 26.0 (14.0, 46.9) | 35.8 (14.0, 56.5) | 25.7 (14.7, 36.0) | |
| Site of lesion (lobe), n (%) | 0.96 | |||
| LUL | 9 (36.0) | 4 (44.4) | 5 (31.3) | |
| LLL | 2 (8.0) | 1 (11.1) | 1 (6.3) | |
| RUL | 6 (24.0) | 2 (22.2) | 4 (25.0) | |
| RLL | 7 (28.0) | 2 (22.2) | 5 (31.3) | |
| RML | 1 (4.0) | 0 (0.0) | 1 (6.3) | |
SD, standard deviation; Q1, the first quartile; Q3, the third quartile; LUL, left upper lobe; LLL, left lower lobe; RUL, right upper lobe; RLL, right lower lobe; RML, right middle lobe.
Characteristics of the initial sublobar resection are listed in Table 2. Lymph node dissection data were missing in five patients who had undergone resection at another hospital. There were no significant differences between the two groups in lesion diameter, pathological grade, cTNM or pTNM staging, surgical procedure, or postoperative adjuvant therapy undertaken. Additionally, the two groups had similar CT radiographic characteristics and CT uptake values. All of the surgical or pathological margins were negative. Of note, about 64.0% (16/25) of the patients underwent “compromised” sublobar resections, which was done due to poor pulmonary function or underlying diseases of the patients. Others underwent intentional ones (36.0%, 9/25). The distribution of pathological subtypes (i.e., adenocarcinoma, squamous carcinoma, and others) was significantly different between WR and SG groups (P=0.01), and adenocarcinoma seems to be predominant in WR group (WR: 16/16, 100.0% vs. SG: 5/9, 55.6%). However, when comparing the proportions of different subtypes of adenocarcinoma, no significant differences were found (P=0.17). No significant differences were found in lymph node dissection (i.e., yes or no, P=0.36) or type of lymph node dissection (i.e., sampling or systemic lymph node dissection, P=0.17), although there were fewer patients with lymph node dissection in the WR group (WR: 4/16, 25.0% vs. SG: 5/9, 55.6%).
Table 2
| Characteristics | Patient cohort (n=25) | Segmentectomy (n=9) | Wedge resection (n=16) | P |
|---|---|---|---|---|
| Diameter of lesion (cm) | 0.15 | |||
| Mean ± SD | 1.47±0.830 | 1.79±0.991 | 1.29±0.695 | |
| Median (Q1, Q3) | 1.20 (0.800, 2.00) | 1.50 (1.20, 2.00) | 1.10 (0.700, 1.70) | |
| Pathological grade of lesion, n (%) | >0.99 | |||
| I | 14 (56.0) | 5 (55.6) | 9 (56.3) | |
| II | 8 (32.0) | 3 (33.3) | 5 (31.3) | |
| III | 3 (12.0) | 1 (11.1) | 2 (12.5) | |
| cTNM stage, n (%) | 0.36 | |||
| IA | 24 (96.0) | 8 (88.9) | 16 (100.0) | |
| IB | 1 (4.0) | 1 (11.1) | 0 (0.0) | |
| pTNM stage, n (%) | >0.99 | |||
| IA | 19 (76.0) | 7 (77.8) | 12 (75.0) | |
| IB | 5 (20.0) | 2 (22.2) | 3 (18.8) | |
| IIB | 1 (4.0) | 0 (0.0) | 1 (6.3) | |
| Surgical procedure, n (%) | 0.52 | |||
| VATS | 23 (92.0) | 9 (100.0) | 14 (87.5) | |
| Thoracotomy | 2 (8.0) | 0 (0.0) | 2 (12.5) | |
| Positive surgical margins, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Positive pathological margins, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Type of sublobar resection, n (%) | 0.09 | |||
| Compromised | 16 (64.0) | 8 (88.9) | 8 (50.0) | |
| Intentional | 9 (36.0) | 1 (11.1) | 8 (50.0) | |
| Postoperative pathological subtype, n (%) | 0.01 | |||
| Adenocarcinoma | 21 (84.0) | 5 (55.6) | 16 (100.0) | 0.17 |
| AAH/AIS | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| MIA | 6 (28.6) | 1 (20.0) | 5 (31.2) | |
| Invasive, LPA | 4 (19.0) | 3 (60.0) | 1 (6.3) | |
| Invasive, ACA | 8 (38.1) | 1 (20.0) | 7 (43.8) | |
| Invasive, PAP | 2 (9.5) | 0 (0.0) | 2 (12.5) | |
| Invasive, MIP | 1 (4.8) | 0 (0.0) | 1 (6.3) | |
| Invasive, SOL | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Squamous carcinoma | 2 (8.0) | 2 (22.2) | 0 (0.0) | |
| Others | 2 (8.0) | 2 (22.2) | 0 (0.0) | |
| Radiographic characteristics of lesion, n (%) | 0.14 | |||
| Pure GGN or part solid nodule | 11 (44.0) | 2 (22.2) | 9 (56.3) | |
| Solid nodule | 13 (52.0) | 6 (66.7) | 7 (43.8) | |
| Mass | 1 (4.0) | 1 (11.1) | 0 (0.0) | |
| CT uptake value | 0.20 | |||
| Mean ± SD | −178±217 | −103±183 | −221±227 | |
| Median (Q1, Q3) | −78.0 (−400, −24.9) | −30.0 (−122, 18.0) | −101 (−407, −50.2) | |
| Lymph node dissection, n (%) | 0.36 | |||
| No | 11 (44.0) | 3 (33.3) | 8 (50.0) | |
| Yes | 9 (36.0) | 5 (55.6) | 4 (25.0) | 0.17 |
| Sampling | 3 (33.3) | 3 (60.0) | 0 (0.0) | |
| Systemic dissection | 6 (66.7) | 2 (40.0) | 4 (100.0) | |
| Unknown | 5 (20.0) | 1 (11.1) | 4 (25.0) | |
| Postoperative adjuvant therapy, n (%) | 0.41 | |||
| Without | 12 (48.0) | 3 (33.3) | 9 (56.3) | |
| With | 13 (52.0) | 6 (66.7) | 7 (43.8) | |
| Chemotherapy | 13 (100.0) | 6 (100.0) | 7 (100.0) | |
| Targeted therapy | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Radiotherapy | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
SD, standard deviation; Q1, the first quartile; Q3, the third quartile; cTNM, clinical tumor, node, and metastasis; pTNM, pathological tumor, node, and metastasis; VATS, video-assisted thoracoscopy; AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; LPA, lepidic predominant; ACA, acinar predominant; PAP, papillary predominant; MIP, micropapillary predominant; SOL, solid predominant; GGN, ground glass nodule; CT, computed tomography.
For the subsequent CL, there were no significant differences between the two groups regarding several preoperative variables of the tumor (Table 3), including the reason for resection, lesion diameter, pathological grade, cTNM or pTNM staging, surgical procedure, CT uptake values, postoperative adjuvant therapy, and follow-up time after surgery. There was, however, a significant difference in the radiographic characteristics of the lesion between the SG and WR groups (P=0.04) at the time of CL. Similarly, all surgical or pathological margins were negative. No significant difference was found in pathological type between groups.
Table 3
| Characteristics | Patient cohort (n=25) | Segmentectomy (n=9) | Wedge resection (n=16) | P |
|---|---|---|---|---|
| Reason for CL, n (%) | 0.58 | |||
| Tumor recurrence | 12 (48.0) | 4 (44.4) | 8 (50.0) | |
| Newly identified secondary primary tumor | 11 (44.0) | 5 (55.6) | 6 (37.5) | |
| Enlarging residual lesions over follow up | 2 (8.0) | 0 (0.0) | 2 (12.5) | |
| Diameter of lesion (cm) | 0.60 | |||
| Mean ± SD | 1.88±0.678 | 1.98±0.653 | 1.82±0.706 | |
| Median (Q1, Q3) | 1.70 (1.50, 2.50) | 1.80 (1.50, 2.50) | 1.60 (1.35, 2.50) | |
| Pathological grade of lesion, n (%) | 0.29 | |||
| I | 8 (32.0) | 2 (22.2) | 6 (37.5) | |
| II | 14 (56.0) | 7 (77.8) | 7 (43.8) | |
| III | 3 (12.0) | 0 (0.0) | 3 (18.8) | |
| cTNM stage, n (%) | >0.99 | |||
| IA | 20 (80.0) | 7 (77.8) | 13 (81.3) | |
| IB | 2 (8.0) | 1 (11.1) | 1 (6.3) | |
| IIA | 3 (12.0) | 1 (11.1) | 2 (12.5) | |
| pTNM stage, n (%) | >0.99 | |||
| 0 | 1 (4.0) | 0 (0.0) | 1 (6.3) | |
| IA | 21 (84.0) | 8 (88.9) | 13 (81.3) | |
| IB | 2 (8.0) | 1 (11.1) | 1 (6.3) | |
| IIIA | 1 (4.0) | 0 (0.0) | 1 (6.3) | |
| Surgical procedure, n (%) | 0.69 | |||
| VATS | 10 (40.0) | 3 (33.3) | 7 (43.8) | |
| Thoracotomy | 15 (60.0) | 6 (66.7) | 9 (56.3) | |
| Positive surgical margins, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Positive pathological margins, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Postoperative pathological subtype, n (%) | 0.23 | |||
| Adenocarcinoma | 21 (84.0) | 7 (77.8) | 14 (87.5) | 0.93 |
| AIS | 1 (4.8) | 0 (0.0) | 1 (7.1) | |
| MIA | 2 (9.5) | 0 (0.0) | 2 (14.3) | |
| Invasive, LPA | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Invasive, ACA | 14 (66.7) | 6 (85.7) | 8 (57.1) | |
| Invasive, PAP | 1 (4.8) | 0 (0.0) | 1 (7.1) | |
| Invasive, MIP | 2 (9.5) | 1 (14.3) | 1 (7.1) | |
| Invasive, SOL | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Invasive, mucinous | 1 (4.8) | 0 (0.0) | 1 (7.1) | |
| Squamous carcinoma | 2 (8.0) | 2 (22.2) | 0 (0.0) | |
| Others | 2 (8.0) | 0 (0.0) | 2 (12.5) | |
| Radiographic characteristics of lesion, n (%) | 0.04 | |||
| Pure GGN or part solid nodule | 4 (16.0) | 0 (0.0) | 4 (25.0) | |
| Solid nodule | 16 (64.0) | 5 (55.6) | 11 (68.8) | |
| Mass | 5 (20.0) | 4 (44.4) | 1 (6.3) | |
| CT uptake value | 0.65 | |||
| Mean ± SD | −52.3±208 | 21.9±24.3 | −94.0±252 | |
| Median (Q1, Q3) | 22.2 (−23.6, 36.9) | 23.6 (20.7, 36.5) | 17.9 (−85.8, 50.0) | |
| Postoperative adjuvant therapy, n (%) | 0.40 | |||
| Without | 9 (36.0) | 2 (22.2) | 7 (43.8) | |
| With | 16 (64.0) | 7 (77.8) | 9 (56.3) | |
| Chemotherapy | 15 (93.8) | 7 (100.0) | 8 (88.9) | |
| Targeted therapy | 1 (6.3) | 0 (0.0) | 1 (11.1) | |
| Radiotherapy | 0 (0.0) | 0 (0.0) | 0 (0.0) |
CL, completion lobectomy; SD, standard deviation; Q1, the first quartile; Q3, the third quartile; cTNM, clinical tumor, node, and metastasis; pTNM, pathological tumor, node and metastasis; VATS, video-assisted thoracoscopy; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; LPA, lepidic predominant; ACA, acinar predominant; PAP, papillary predominant; MIP, micropapillary predominant; SOL, solid predominant; GGN, ground glass nodule; CT, computed tomography.
Perioperative outcomes of CL
Postoperative variables after CL are also compared and depicted in Table 4. Postoperative complications were defined as blood transfusion (n=5), prolonged air leak (n=2), or pneumonia (n=1) in this study. Patients in the WR group had fewer postoperative complications than those in the SG group (WR: 2/16, 12.5% vs. SG: 5/9, 55.6%, P=0.03). The major complication after CL was blood transfusion, whose risk was significantly higher in the SG group (WR: 1/16, 6.3% vs. SG: 4/9, 44.4%, P=0.04), while the minor complications, including prolonged air leak over 7 days (n=2) and pneumonia (n=1), were comparable (WR: 1/16, 6.3% vs. SG: 1/9, 11.1%, P=0.60). A higher proportion of patients in the SG group also underwent bronchoplasty or angioplasty during the time of CL, as compared to patients in the WR group (SG: 3/9, 33.3% vs. WR: 0/16, 0.0%, P=0.04).
Table 4
| Characteristics | Patient cohort (n=25) | Segmentectomy (n=9) | Wedge resection (n=16) | P |
|---|---|---|---|---|
| With postoperative complications, n (%) | 7 (28.0) | 5 (55.6) | 2 (12.5) | 0.03 |
| Major complication(s) | 5 (20.0) | 4 (44.4) | 1 (6.3) | 0.04 |
| Blood transfusion | 5 (20.0) | 4 (44.4) | 1 (6.3) | 0.04 |
| Minor complications | 2 (8.0) | 1 (11.1) | 1 (6.3) | 0.60 |
| Prolonged air leak >7 days | 1 (4.0) | 1 (11.1) | 0 (0.0) | 0.36 |
| Pneumonia | 1 (4.0) | 0 (0.0) | 1 (6.3) | 0.64 |
| Length of hospital stay (days) | 0.68 | |||
| Mean ± SD | 5.76±3.17 | 6.22±3.53 | 5.50±3.03 | |
| Median (min, max) | 5.00 (2.00, 14.0) | 5.00 (3.00, 13.0) | 4.50 (2.00, 14.0) | |
| Median (Q1, Q3) | 5.00 (4.00, 7.00) | 5.00 (4.00,7.00) | 4.50 (3.75, 6.25) | |
| Median (IQR) | 5.00 (3.00) | 5.00 (3.00) | 4.50 (2.50) | |
| Use of angioplasty/bronchoplasty, n (%) | 0.04 | |||
| No | 22 (88.0) | 6 (66.7) | 16 (100.0) | |
| Yes | 3 (12.0) | 3 (33.3) | 0 (0.0) | |
| Blood loss (mL) | 0.30 | |||
| Mean ± SD | 218±440 | 406±700 | 113±123 | |
| Median (min, max) | 100 (10.0, 2,200) | 100 (50.0, 2,200) | 100 (10.0, 500) | |
| Median (Q1, Q3) | 100 (50.0, 100) | 100 (50.0, 400) | 100 (45.0, 100) | |
| Median (IQR) | 100 (50.0) | 100 (350.0) | 100 (55.0) | |
| Operative duration (hours) | 0.19 | |||
| Mean ± SD | 2.82±1.59 | 3.17±1.39 | 2.63±1.71 | |
| Median (min, max) | 2.00 (1.00, 7.50) | 3.00 (1.50, 5.50) | 2.00 (1.00, 7.50) | |
| Median (Q1, Q3) | 2.00 (2.00, 3.00) | 3.00 (2.00, 4.00) | 2.00 (2.00, 2.63) | |
| Median (IQR) | 2.00 (1.00) | 3.00 (2.00) | 2.00 (0.625) | |
| 30-days mortality, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
CL, completion lobectomy; SD, standard deviation; Q1, the first quartile; Q3, the third quartile; IQR, interquartile range.
Blood transfusion was also compared between the two groups and stratified according to the site of lesion (Table 5) or lymph node dissection (Table 6). When the first surgery occurred in the left upper lobe (LUL), a significantly higher proportion of patients requiring blood transfusion during CL were found in the SG group compared to the WR group (SG: 3/4, 75.0% vs. WR: 0/5, 0.0%, P=0.04). In contrast, no significant difference was found in the right upper lobe (RUL). Whether lymph node dissection was performed (P>0.99) or not (P=0.27), the proportion of patients requiring blood transfusion between the SG and WR groups was similar.
Table 5
| Site of lesion (lobe) | Patient cohort | Segmentectomy | Wedge resection | P |
|---|---|---|---|---|
| LUL | 3/9 (33.3) | 3/4 (75.0) | 0/5 (0.0) | 0.04 |
| LLL | 0/2 (0.0) | 0/1 (0.0) | 0/1 (0.0) | – |
| RUL | 2/6 (33.3) | 1/2 (50.0) | 1/4 (25.0) | >0.99 |
| RLL | 0/7 (0.0) | 0/2 (0.0) | 0/5 (0.0) | – |
| RML | 0/1 (0.0) | 0/0 (0.0) | 0/1 (0.0) | – |
Data are presented as n/N (%). CL, completion lobectomy; LUL, left upper lobe; LLL, left lower lobe; RUL, right upper lobe; RLL, right lower lobe; RML, right middle lobe.
Table 6
| Lymph node dissection | Patient cohort | Segmentectomy | Wedge resection | P |
|---|---|---|---|---|
| No | 1/11 (9.1) | 0/3 (0.0) | 1/8 (12.5) | 0.27 |
| Yes | 3/9 (33.3) | 2/5 (40.0) | 1/4 (25.0) | >0.99 |
| Sampling | 1/3 (33.3) | 1/3 (33.3) | 0/0 (0.0) | |
| Systemic dissection | 2/6 (33.3) | 1/2 (50.0) | 1/4 (25.0) | |
| Unknown | 1/5 (20.0) | 1/1 (100.0) | 0/4 (0.0) | 0.20 |
Data are presented as n/N (%). CL, completion lobectomy.
There were no significant differences between the WR and SG groups in regards to length of hospital stay (WR: 5.50±3.03 vs. SG: 6.22±3.53 days, P=0.68), blood loss (WR: 113±123 vs. SG: 406±700 mL, P=0.30), or operative duration (WR: 2.63±1.71 vs. SG: 3.17±1.39 hours, P=0.19).
Oncologic outcomes of CL
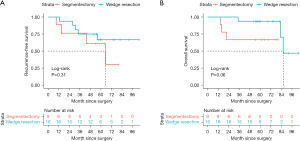
Median follow-up for all patients was 61.1 months, and nine patients had recurrence (Table 7). The 5-year RFS rate was 64.4%, and the 5-year OS rate was 84.0%. No significant difference was found in RFS (Figure 2A) between WR and SG groups (WR: 66.7% vs. SG: 61.0%, P=0.31). Likewise, no significant difference was seen in OS (Figure 2B) between WR and SG groups (WR: 93.8% vs. SG: 66.7%, P=0.06). However, the difference in OS was almost significant, and there was a tendency for better survival in the WR group [univariate HR =0.15, 95% confidence interval (CI): 0.02–1.48, P=0.10], especially in the early follow-up period.
Table 7
| Characteristics | Patient cohort (n=25) | Segmentectomy (n=9) | Wedge resection (n=16) | P |
|---|---|---|---|---|
| Recurrence, n (%) | 9 | 4 | 5 | 0.37 |
| Local | 2 (22.2) | 0 (0.0) | 2 (40.0) | |
| Regional | 2 (22.2) | 2 (50.0) | 0 (0.0) | |
| Distant | 5 (55.6) | 2 (50.0) | 3 (60.0) |
CL, completion lobectomy.
Discussion
The optimal surgical approach for a secondary NSCLC after initial surgical resection remains contested (14-17). Recently, a study conducted by Hattori et al. (2) indicated that a non-completion pneumonectomy (non-CP) procedure may be a promising alternative to CP in subsequent ipsilateral resections, which raises the possibility of considering other strategies such as CL. However, studies regarding CL are limited. Our analysis demonstrates that (I) CL occurring over 3 months following a sublobar resection is safe and feasible; (II) compared to wedge resection, an initial operative strategy of segmentectomy may be associated with a higher level of technical difficulty and higher risk of postoperative complications at the time of subsequent CL; and (III) after CL, RFS and OS were comparable between WR and SG group.
The operative interval may contribute greatly to the technical difficulty of the operation. A second surgery on the ipsilateral side is challenging because pleural adhesions must be divided, and performing a CL after segmentectomy is even more challenging as it necessitates the mobilization of the hilum structure, which is impeded by the strong adhesion to hilum tissue that was previously divided and manipulated throughout the initial segmentectomy procedure. Therefore, this difficulty comes largely from the adhesion formation, which gets more severe over time. One study has indicated that CL may become more challenging approximately 5 weeks after segmentectomy (8). This may be due to the presence of extensive adhesions, which can lead to increased bleeding, longer operative duration, and a higher risk of pulmonary artery injury. Particularly, securing the main pulmonary artery at 5 weeks following segmentectomy proves challenging due to the potential for arterial damage in such circumstances. It has been reported that taping or clamping may be required to prevent catastrophic hemorrhage when hilum adhesion makes it challenging to expose and divide the pulmonary artery (18), and in our institution, sometimes conversion from VATS to thoracotomy is also necessary to prevent pulmonary artery injury and avoid prolonged surgery due to severe adhesion.
CL completed at shorter intervals following an initial resection can be performed with relative ease compared to CL following longer operative intervals (7,9). Most of the former cases are for remedial purposes, such as a positive surgical margin from the initial surgery. Recent evidence suggests that VATS CL after a diagnostic wedge resection is safe, as compared to an initial VATS lobectomy when it is performed at a relatively short interval (33 days; IQR, 27–41 days) following the initial resection (9). Another study reported no perioperative deaths among patients undergoing CL “a few days” (exact duration not specifically documented) after segmentectomy as a salvage operation for micrometastasis found in the sentinel node (7). These studies suggest that it is relatively safe to perform CL after sublobar resections, especially within an operative interval of fewer than 5 weeks.
Few studies, primarily case series, exist examining the outcomes of CL occurring at longer operative intervals (over 5 weeks) after initial resection. One study analyzed ten patients who underwent CL at least 1 month (ranging from 2 to 108 months) after segmentectomy and reported no operative deaths (18). Similarly, a recent study examined eight cases of CL after segmentectomy, with an operative interval of 24 months (IQR, 1.9–20.3 months), and two patients with complications (air leakage and arrhythmia) were reported (10). These studies suggest that CL after segmentectomy can be performed relatively safely following longer operative intervals.
In our study, the operative interval was at least 3 months with a mean of 32.7±22.1 months (median, 26 months; IQR, 14.0–46.9 months), which is a relatively longer duration than that of the previously cited studies, to the extent of our knowledge. Similarly, we also reported no operative deaths and a low rate of postoperative complications, further supporting CL after sublobar resection as safe and feasible.
We also examined the effects of the type of initial sublobar resection on the technical difficulty of subsequent CL, as it has previously been observed to play a role (10). Hilar adhesions are an important factor affecting the difficulty of CL after an initial resection. Recent work has shown that they may be more severe following an anatomical resection than a wedge resection (P=0.004) (19), which may increase bleeding risk and prolong operative duration. In our study, there was a trend towards increased blood loss during CL in the SG group (406±700 mL) vs. the WR group (113±123 mL), and longer operative duration (SG: 3.17±1.39 vs. WR: 2.63±1.71 hours). This is likely a reflection of more extensive, denser adhesions following segmentectomy vs. a wedge resection.
We also found differences in rates of CL complications following initial segmentectomy vs. wedge resection (P=0.03, one-sided). A higher risk of major complications, or blood transfusion in the SG group may indicate the injury of the pulmonary artery during surgery, which indirectly reflects the difficulty of the operation. We also investigated if the specific lobe of surgery might influence the result. Interestingly, when stratified by lobe of operation for initial surgery, the WR group showed a lower rate of blood transfusion during CL in the LUL, which was not the case in other lobes. It has already been reported that the LUL has the highest blood transfusion rate (2.72%) during lobectomy compared to other lobes (20), which demonstrates that CL in the LUL may be sufficiently complicated, and severe adhesion formation in the hilar region after segmentectomy can exacerbate the situation. In this case, wedge resection could be a choice in reducing injury in the possible second surgery. Theoretically, mediastinal lymph node dissection may also have implications for CL, as it requires an incision of the mediastinal pleura at the hilum, which may further aggravate adhesions. However, when stratified by lymph node dissection, blood transfusion rates were similar between the WR and SG groups, no matter whether mediastinal lymph node dissection was performed or not. Considering the small sample size of each stratum for lymph node dissection, this issue should be further investigated.
In particular, the incidence of the need for angioplasty/bronchoplasty (P=0.04, one-sided) was higher in the SG group compared to the WR group. Angioplasty and bronchoplasty may be a consequence of pulmonary artery damage or bronchial injuries, which may be associated with a more difficult operation. All three patients who had angioplasty or bronchoplasty were from the SG group, suggesting that CL after segmentectomy may be more difficult.
Regarding oncology outcomes, we found no significant differences between the WR and SG groups in RFS or OS, although a seemingly better survival was observed in the WR group. Considering the difficulty of subsequent CL, wedge resection seems to be a better option.
However, it is worth noting that decisions on surgical procedures need to be made cautiously. Firstly, controversy over the differences between wedge resection and segmentectomy still exists. Wedge resection is a simpler surgical operation compared to anatomical segmentectomy, both in terms of the surgical techniques used and the amount of tissue removed (21). Therefore, it may be more appropriate for high-risk (patients who are elderly, have impaired pulmonary function, or have underlying medical conditions that render them unsuitable candidates for lobectomy) operable populations. As for the oncology results, wedge resection has been discovered as a negative prognostic factor for the chance of locoregional recurrence-free outcome for clinical stage IA NSCLC in a study (22). In contrast, another study reported comparable oncologic outcomes between wedge resection and segmentectomy for NSCLC of the same clinical stage (23). At the time of drafting this article, The Japan Clinical Oncology Group (JCOG) is now conducting a clinical trial (JCOG 1909) to assess the surgical outcomes of wedge resection and segmentectomy in high-risk operable patients diagnosed with clinical stage IA NSCLC (24). Given that 64.0% (16/25) of patients in this study underwent compromised sublobar resection, the outcomes of JCOG 1909 might give valuable insight into this topic.
Secondly, the necessity of a second surgery should also be considered. For example, the use of intraoperative frozen sections can differentiate between benign and malignant lesions intraoperatively, thus effective in guiding resection strategy for peripheral small-sized lung adenocarcinoma (LUAD) (25). Specifically, for patients who are having a limited resection procedure for invasive LUAD that was first misdiagnosed as atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), or minimally invasive adenocarcinoma (MIA) based on frozen section analysis, further subsequent treatments are not necessary (26), which is important in preventing injury to the patient due to a second surgery.
This study has several limitations. First, it was a retrospective analysis from a single institution. Therefore, a multi-center, prospective study is needed to further verify our conclusions. Second, although our case volume was larger than that of any other similar studies, the number was still limited, owing to the rarity of CL following initial resection. The smaller sample size may lead to inconclusive conclusions, as it may be influenced by large random factors and inherent postoperative differences between the SG and WR groups. Third, the two groups were not completely comparable. Among patients undergoing CL, the pathological subtype and the radiographic characteristics of the lesion differed statistically between groups (Tables 2,3), which could be potential confounding factors. This mismatch was inevitable given the limited number of cases, and thus the conclusions should be interpreted cautiously.
Conclusions
In spite of its limitations, this study adds to our understanding of CL, demonstrating that CL after sublobar resection even after 3 months is a safe and effective procedure, with no deaths occurring within 30 days postoperatively. Compared to segmentectomy, an initial strategy of wedge resection may be associated with a lower blood transfusion rate (especially in the LUL) and a lower risk of needing bronchoplasty or angioplasty during subsequent CL. After CL, RFS and OS were comparable between WR and SG groups. Of note, decisions on surgical procedures should be made in cautious, and prospective, multi-center clinical trials comparing different sublobar resections are warranted.
Acknowledgments
We would like to thank Dr. Xiaoying Lou (Division of Cardiothoracic Surgery, Emory University School of Medicine, Atlanta, Georgia) for English language editing.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1780/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1780/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1780/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1780/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Shanghai Pulmonary Hospital (K23-222), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995;109:120-9. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Watanabe Y, et al. Repeated anatomical pulmonary resection for metachronous ipsilateral second non-small cell lung cancer. J Thorac Cardiovasc Surg 2021;162:1389-1398.e2. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Villamizar N, Swanson SJ. Lobectomy vs. segmentectomy for NSCLC (T<2 cm). Ann Cardiothorac Surg 2014;3:160-6. [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- National Comprehensive Cancer Network. The NCCN clinical practice guidelines in oncology for non-small cell lung cancer (version 3. 2023). Accessed May 06, 2023. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450
- Nomori H, Mori T, Izumi Y, et al. Is completion lobectomy merited for unanticipated nodal metastases after radical segmentectomy for cT1 N0 M0/pN1-2 non-small cell lung cancer? J Thorac Cardiovasc Surg 2012;143:820-4. [Crossref] [PubMed]
- Omasa M, Date H, Takamochi K, et al. Completion lobectomy after radical segmentectomy for pulmonary malignancies. Asian Cardiovasc Thorac Ann 2016;24:450-4. [Crossref] [PubMed]
- Holbek BL, Petersen RH, Hansen HJ. Is it safe to perform completion lobectomy after diagnostic wedge resection using video-assisted thoracoscopic surgery? Gen Thorac Cardiovasc Surg 2016;64:203-8. [Crossref] [PubMed]
- Takamori S, Oizumi H, Suzuki J, et al. Completion lobectomy after anatomical segmentectomy. Interact Cardiovasc Thorac Surg 2022;34:1038-44. [Crossref] [PubMed]
- Suzuki K, Watanabe SI, Wakabayashi M, et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg 2022;163:289-301.e2. [Crossref] [PubMed]
- Li Q, Jin K, Dai J, et al. Limited-anatomic resection for ground-glass like lung cancer-simplicity does not mean inefficacy. Transl Lung Cancer Res 2022;11:1722-4. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Hamaji M, Ali SO, Burt BM. A meta-analysis of resected metachronous second non-small cell lung cancer. Ann Thorac Surg 2015;99:1470-8. [Crossref] [PubMed]
- Yang X, Zhan C, Li M, et al. Lobectomy Versus Sublobectomy in Metachronous Second Primary Lung Cancer: A Propensity Score Study. Ann Thorac Surg 2018;106:880-7. [Crossref] [PubMed]
- Lee DS, LaChapelle C, Taioli E, et al. Second Primary Lung Cancers Demonstrate Similar Survival With Wedge Resection and Lobectomy. Ann Thorac Surg 2019;108:1724-8. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Surgical Management of Multifocal Ground-Glass Opacities of the Lung: Correlation of Clinicopathologic and Radiologic Findings. Thorac Cardiovasc Surg 2017;65:142-9. [PubMed]
- Takahashi Y, Miyajima M, Tada M, et al. Outcomes of completion lobectomy long after segmentectomy. J Cardiothorac Surg 2019;14:116. [Crossref] [PubMed]
- Kamigaichi A, Tsutani Y, Handa Y, et al. Feasibility of repeated ipsilateral anatomical pulmonary resection. Surg Today 2023;53:379-85. [Crossref] [PubMed]
- Linden PA, Block MI, Perry Y, et al. Risk of Each of the Five Lung Lobectomies: A Society of Thoracic Surgeons Database Analysis. Ann Thorac Surg 2022;114:1871-7. [Crossref] [PubMed]
- Tsutani Y, Kagimoto A, Handa Y, et al. Wedge resection versus segmentectomy in patients with stage I non-small-cell lung cancer unfit for lobectomy. Jpn J Clin Oncol 2019;49:1134-42. [Crossref] [PubMed]
- Koike T, Koike T, Yoshiya K, et al. Risk factor analysis of locoregional recurrence after sublobar resection in patients with clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2013;146:372-8. [Crossref] [PubMed]
- Altorki NK, Kamel MK, Narula N, et al. Anatomical Segmentectomy and Wedge Resections Are Associated with Comparable Outcomes for Patients with Small cT1N0 Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:1984-92. [Crossref] [PubMed]
- Shimoyama R, Tsutani Y, Wakabayashi M, et al. A multi-institutional randomized phase III trial comparing anatomical segmentectomy and wedge resection for clinical stage IA non-small cell lung cancer in high-risk operable patients: Japan Clinical Oncology Group Study JCOG1909 (ANSWER study). Jpn J Clin Oncol 2020;50:1209-13. [Crossref] [PubMed]
- Liu S, Wang R, Zhang Y, et al. Precise Diagnosis of Intraoperative Frozen Section Is an Effective Method to Guide Resection Strategy for Peripheral Small-Sized Lung Adenocarcinoma. J Clin Oncol 2016;34:307-13. [Crossref] [PubMed]
- Zhang Y, Deng C, Fu F, et al. Excellent Prognosis of Patients With Invasive Lung Adenocarcinomas During Surgery Misdiagnosed as Atypical Adenomatous Hyperplasia, Adenocarcinoma In Situ, or Minimally Invasive Adenocarcinoma by Frozen Section. Chest 2021;159:1265-72. [Crossref] [PubMed]



