
Clinically important pulmonary vascular variations: a narrative review
Introduction
Accurate knowledge of vascular structures allows surgeons to ligate them during anatomical resections. Pulmonary veins also allow the determination of intersegmental and intersubsegmental planes (1). Pulmonary vessels and their anatomical variations are important factors in determining successful thoracic operations, and segmentectomy is an emerging approach in treating early non-small-cell lung cancer. Therefore, there is a great need for a more thorough approach to describe the vascular anatomy of lungs because pulmonary vessels are crucial in defining landmarks of segments and avoiding misidentification (2-6).
The nonpathological nature of such variants may pose risks of overlooking them and causing intra- and postoperative complications, especially with the rising popularity of video-assisted thoracic surgery (VATS) (7). In comparison to open thoracotomy, VATS is a less invasive technique but provides a smaller range of visibility for surgeons, and certain vessels can be easily mistaken, either resulting in increased difficulty of the procedure or requiring conversion to thoracotomy (8-10). Pulmonary fissures are highly variable, and more than half of them are incomplete. When combined with an atypical pattern of pulmonary veins, these factors can result in an increased risk of complications (11,12). Detailed radiological examination using computed tomography (CT) and recently three-dimensional (3D)-CT is key in successful procedures, but surgeons should be aware of possible variations to better deal with them (3,13-16).
Although there is already extensive literature about pulmonary vessels, it seems that there has been no systemic review of both pulmonary veins and arteries of each lobe. Therefore, we examined data from case reports and reviews with more scope to provide a more comprehensive view of pulmonary vessels’ variations in a manner that could be useful in both lobar and segmental resections. We have also summarized recent findings on incidence rates. Our goal is to draw attention to the wide range of possible variations and their clinical implications in a systematized way and to provide a practical guide. We present this article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1715/rc).
Methods
The PubMed and Embase databases were searched from January 2023 to June 2023 with the following terms to identify papers of interest: “pulmonary vessels variations”, “pulmonary vascular variations”, “pulmonary veins variations”, and “pulmonary arteries variations”. In addition, relevant bibliographies were was reviewed. Meta-analyses, case reports, case series, retrospective studies, and review articles were examined for this review. To limit the study to data sourced from cutting-edge imaging, we excluded studies published before the year 2000. Articles were also excluded if they were written in a non-English language and described pediatric patients or patients with diseases that may affect their pulmonary vascular trees, such as pulmonary embolism and chronic thromboembolic disease (Table 1). We used the nomenclature of Nagashima, Yamashita, Boyden, Maciejewski, and Shimizu (17-21).
Table 1
| Items | Specification |
|---|---|
| Date of search | January 3rd, 2023–June 3rd, 2023 |
| Databases and other sources searched | PubMed, Embase |
| Search terms used | “pulmonary vessels variations”, “pulmonary vascular variations”, “pulmonary veins variations”, “pulmonary arteries variations” |
| Timeframe | 2000–June 2023 |
| Inclusion and exclusion criteria | Exclusion: articles written in non-English language, describing pediatric or patients with diseases that may affect their pulmonary vascular tree such as pulmonary embolism, and chronic thromboembolic disease |
| Inclusion: cited articles from reviewed papers | |
| Selection process | Search was conducted independently by M.T.P., K.B., Z.G. |
Right upper lobe (RUL) variations
There is no consensus on the variability of the RUL. Some authors maintain that the variations in this lobe rarely cause any complications, while others report it to be the most variable lobe of the right lung or at least more variable than the right middle lobe (RML) (2,11,22). However, there is no doubt that there has been extensive literature in recent years on analyses of its vascular patterns.
Normally, the RUL consists of three bronchopulmonary segments: the apical (S1R), posterior (S2R), and anterior (S3R) segments. Each of them has a correspondingly named segmental artery and vein. Before analyzing patterns of segmental arteries, one should note the branching of the superior trunk and right pulmonary artery (RPA). Typically, the first branch of the RPA is the superior trunk, while the second is the ascending A2R. However, one analysis of 214 cases found that 17.1% of them had an additional inferior trunk (Figure 1A) (17). That study also redefined the ascending A2R as a vessel branching from the interlobar portion distal to A4R. The inferior trunk is the second branch of the RPA and not of the superior trunk. It arises from the mediastinal portion distal to the superior trunk and proximal to A4R. It is difficult to establish a boundary between the interlobar and mediastinal portion, and the ascending A2R and inferior trunk can be easily confused, so these new terms should greatly help surgeons perform right superior segmentectomies (17).

For A1R, in the majority of cases, both its branches arise from superior trunk, although we found reports of separate branching of A3R and A1R with recurrent A2R directly from the RPA (Figure 1B) (23,24). The anatomy of S2R is much more complex. Notably, this segment has an arterial supply from both the interlobar part of the RPA (ascending A2R) and the superior trunk (recurrent A2R). This typical pattern is present in only 46.4% of cases, and in almost one-third, recurrent A2R is lacking (Figure 1C). In approximately 15% of cases, an ascending A2R is not present (Figure 1D) (17).
Zhang and colleagues described the segmentectomy of S2R with multiple variations, including a lack of ascending A2R, which made exposing subsegmental arteries difficult and forced them to release the segmental hilum first (25). Other potentially problematic variants are a common root of A2R with A6 (with incidence between 1.53% to 12%) (Figure 1E), A3R, the inferior trunk, or three separate branches to S2R (17,23,24,26). According to one retrospective analysis, the doubling of A2R is the most common arterial variation of the right lung (Figure 1F) (27). The branching pattern of A3R is mostly consistent except for a split supply of S3R from the ascending A2R and the inferior trunk, as well as a recently identified variant of A3R arising from the middle lobe artery (Figure 2A) (17).

When considering venous patterns, it is necessary to take into account the importance of the topography of pulmonary veins and their use in establishing intersegmental planes. The sole location and outflow of a segmental vein can influence not only lung-related surgeries, but also esophagostomy or ablation procedures (28,29). V1R and V2R are two of the most notorious pulmonary veins in the literature. One of the most common possibly problematic variations is the right upper variant of the retrobronchial course of V2R, which has an incidence rate of 8% (Figure 2B). This also occurs with V1R, but much less frequently (24,30).
Other problematic variations involve both of these veins having a trajectory that is proximal to the mediastinum and RPA, as well as V1R running proximally to the superior vena cava and doubling of these pulmonary veins (30). One study noted anomalous drainage of the V2R with a rate of 7.4% (Figure 2C) (2). The most common anomalous outflow site is the right inferior pulmonary vein (RIPV) with a frequency of 3.7–6.67% (Figure 2B), while anomalous outflow into V6R has a rate of 1.6%, and the rate into the left atrium is 1.1% (2,30).
The right top pulmonary vein is difficult to categorize as it is both anomalous and supernumerary. However, the literature points either to RUL as a whole or to S2R as a place of origin, so we decided to provide a description (28). It runs from the RUL behind the right main or intermediate bronchus and penetrates subcarinal lymph nodes. Some minor subsegmental veins from the RLL flow into it, and it empties into the superior or inferior pulmonary vein, the left atrium, or V6R (Figure 2D). If it enters the left atrium, it usually does so in an area that is superomedial to the right superior pulmonary vein (RSPV), which is the origin of the name of this vein (28,31). Studies report different incidence rates varying between 0.28% and 8.01% and different locations as the most common inflow area (31). Hiroshi et al. indicates that it is the RPSV, while Miyamoto et al. indicate the RIPV, and both of their studies were from 2021 (32,33). However, there is consensus that this particular variation is important for both surgeons and interventional cardiologists to keep in mind.
Right upper venous variations can be problematic during segmentectomies as well. Normally, S1R consists of intrasegmental V1a and intersegmental V2a and V1b. S2R consists of intrasegmental V2b, surface V2t, intersegmental V2a, and V2c. S3R consists of intrasegmental V3a, surface V3b and V3c, and intersegmental V2b and V2c (Figure 2E) (18). The anterior vein originates from V1b, runs anteriorly to the right main bronchus, and empties into the mediastinal site of RSPV. The central vein begins as V2a, runs between B2R and B3R, and drains into an interlobar aspect of RSPV. This pattern is present in 81–83.2% of cases, so it is assumed to be a normal one. However, it can be further divided into different types. In type Iab, V1a and V1b drain into the anterior vein, which occurs in the majority of cases (Figure 2F). In type Ib, V1a and V1b drain into the central vein and anterior vein, respectively, which occurs in approximately 26% of cases. In the anterior type, V1-3R drains exclusively into the anterior vein, which has an incidence of 8.8% to 12%, and in the central type, V1-3 drains into the central vein, which has an incidence of 7% (17,18). The aforementioned case reported by Zhang also included a lack of second intrasegmental vein (V2b), which could potentially result in misidentification of other vessels as the intersegmental vein (25).
RML variations
Despite being the smallest lung lobe, the blood supply and drainage of the RML can cause significant challenges because of the variation rate. Cory and Valentine found that the left upper lobe (LUL) is the most variable, while both Subotich et al. (52% of all variations) and Shiina et al. reported that the majority of variations were in the RML (2,11,30,34). The most common arterial pattern is a two-stemmed artery (69.6%), which arises from the anteromedial aspect of the interlobar artery anteriorly to the intermediate bronchus (22,35). However, between studies, the possibility of an alternate pattern (more frequently single or three-stemmed) can vary between 30% and 50% (35). Normally, there is one corresponding artery (A4R and A5R) and vein (V4R and V5R) for each bronchopulmonary segment (30). However, both the branching point and number of vessels can differ (Figure 3A,3B).

The topography of the middle lobe arteries is usually invariant, with the exception of A4R running posteriorly to the middle lobe bronchus in 2.85% of cases, as noted by Sivrikoz and Tulay (36). For the branching point, one can distinguish A4R arising from the common basal trunk or from A7R, which has not been reported extensively (11,23,35). Both the RML artery and particular segmental arteries can be doubled and potentially deceive surgeons during procedures involving other lobes (Figure 3C) (11,23).
The venous pattern is much more variant than the arterial pattern. Typically, V4R and V5R merge into the middle lobe vein (MLV), which then drains into the RSPV in 68% of cases (18). However, the incidence rate of the MLV draining directly into the left atrium can vary between 7.9% and 20.74% (Figure 3D), especially in patients with atrial fibrillation (2,29). Other variants include drainage into the RIPV, split drainage of segmental veins into the RSPV and RIPV, or emptying into the left atrium (Figure 3E,3F) (29,30). In regard to abnormal numbers of veins, one study reported that accessory MLVs are the most common variation in the right lung (37). Other possible variants are doubled V4R or V5R with or without direct outflow into the left atrium (30).
Right lower lobe (RLL) variations
The RLL consists of five bronchopulmonary segments, and each of them typically has a corresponding arterial and venous vessel. S6R or the superior segment of RLL is connected to an artery, which branches off posteriorly from the interlobar artery. In 70% of cases, the second arterial branch is the medial basal artery (A7R), after which the common basal trunk divides into anterior basal (A8R), lateral basal (A9R), and posterior basal (A10R) parts (22). One report notes that in approximately 75% of cases, A6R has a single stem, but it also mentions an incidence rate of a subsuperior segmental artery of 20.4% (35). Duplicated superior segmental arteries are one of the most common variations regarding the RLL (23,30). Many reports note this variation, and Sivrikoz and Tulay estimated its incidence rate as 12.5% (36). A6R can also originate proximally to the middle lobe artery, it can have a common root with ascending A2R or A4+5R, or it can be bifurcated or trifurcated (23,27,36).
In 60% of cases, A7R and A8R have a common root. A7R branches independently in 34% of cases and is lacking in 6% of cases (38). One case report described A7R arising directly from the RPA, which passed a thin branch toward the posterior region of the RIPV, which could have been easily injured (Figure 4A). However, there is severely limited literature mentioning the mediastinal basal pulmonary artery (14). Another retrospective analysis reported a common trunk of A7R and the middle lobe artery (Figure 4B) (27). One study observed type A7a or single-stemmed arteries in 74.8% of cases, while 14.8% showed two-stemmed A7ab. A7b (or an artery running over and posteriorly to the RIPV according to original categorization) is more likely to run between V6R and the common basal root, while in most cases, an indirect A7R branches off other segmental arteries rather than the common basal root and is supplied by A10R (Figure 4C-4E) (35).

The most common branching patterns of other basal segmental arteries are the A8R and A9+A10R type (which is found in 68.1–90.8% of cases), the A8+A9R and A10R type (11.1%), and the recently discovered split-bifurcated A8R and A8+A9+A10R or A8+A9R and A9+A10R types (16.7%). The trifurcation type is found in only 4.1% of cases (35). Such classifications provide more systematic knowledge that can be invaluable, especially during segmentectomies, where the operation field is severely limited. Every segmental artery has a corresponding vein, which all fuse and form the RIPV. Similarly to arterial patterns, most venous variations involve S6R. According to one study, V6R drains directly into the left atrium in 7.9% of cases and into the RSPV in 1.6% (Figure 5A). Some studies note that the accessory V6R, which runs posteriorly to the intermediate bronchus, poses a potential risk of injury during surgeries in the posterior mediastinum and empties into the RSPV (Figure 5B) (7,27,30). The frequency of this variation is difficult to estimate as one study mentions a rate of >1%, while others report up to 22% (30).

There is limited data about V7R and its branches. However, V8R, V9R, and V10R present quite diverse patterns (Figure 5C) (35). One can distinguish simple and split bifurcation types. A slightly less frequent simple type consists of two venous roots, one of which is shared by either V8R and V9R or V9R and V10R. The split type can be divided into the V8+V9+V10R and V10R type and the V8+V9R and V9+V10R type. The frequency of each is difficult to estimate due to the limited amount of studies, but in general, it varies between 20% and 31%.
There is also a trifurcation type, but it occurs rarely (35). During VATS lobectomies, Amore and colleagues observed V7R draining into the MLV and crossing interlobar planes, which caused additional challenges during surgery (39). An absence of V8R with drainage substituted by V5R and V9R has also been reported, which is potentially troublesome during segmentectomy (Figure 5D) (30).
LUL variations
Anomalous patterns of pulmonary artery branching have been documented, and a higher frequency has been observed on the left side (23). Compared to other lobes, the relationship between the pulmonary artery and bronchus appears to be less rigid in the upper left lobe, especially within the context of subsegmental anatomy (40). The left main pulmonary artery’s general course presents an anatomical feature as it curves behind the bronchus of the upper left lobe before extending into a shared basal trunk, which subsequently branches out and supplies blood to the basal segments (22). In some documented instances, the left pulmonary takes a path through the division point between the apicoposterior and common bronchus, necessitating an alternate surgical approach (8).
The count of pulmonary arterial branches leading to the upper left lobe is known to display a wide range of variability, spanning from two to seven (22,36). According to Fourdrain, the LUL receives blood from three arteries in 22.7% of cases, four arteries in 50%, five arteries in 22.7%, and six arteries in 4.5% (40). In approximately 80% of cases, a singular branch of the pulmonary artery supplies the lingula with blood before branching off to provide arteries for both the superior and inferior segments (22).
To more easily group variations, a division into a proper S1+2L and S3L segment and lingular S4 and S5L segment is proposed. Reports differ on whether the common apicoanterior trunk or independent A1+2L and A3L type is more common (22,41). Moreover, both of these types can be further differentiated. The common trunk of A3L and A1+2L type can be differentiated into three subtypes: subtype E with a common trunk of A3L+A1+2(a+b)L and separate A1+2cL, subtype F with a common artery of A3L+A1+2aL and separate artery A1+2(b+c)L, and subtype G with a common artery of A3L+A1+2aL and two separate arteries to subsegments S1+2b and S1+2cL.
The independent A3L type can be further divided into four subtypes: subtype A with separate A3L, A1+2(a+b)L, and A1+2cL, subtype B with A3L, a common artery to S1+2(b+c)L, and separate A1+2aL, subtype C with A3L and three subsegmental arteries, and subtype D with A3L and a common artery to all three subsegments of S1+2L. Subtypes F, G, A, and D are the most common (Figure 6A-6D). Each of them differs in the particular subsegment supply, with implications for lesion localization and the choice between subsegmental resection and segmentectomy (3).

The left upper pulmonary lingular artery shows diversity with three distinct branching patterns into either or both S1+2L and S3L in approximately 20% of cases (Figure 6E) (3). Sivrikoz and Tulay noted a common origin of A3L with A4+5L and five separate arteries to the proper segment with a reported incidence of 0.93–19% (36). Both of these variations may significantly influence segmentectomies as accurate knowledge of segmental supply is needed. The origin of the lingular artery is commonly situated along the oblique cleft of the lingular bronchus, necessitating adjustments in surgical approaches based on oblique fissure development (14). Variations in the origin of A4+5L from the lingular bronchus have significant ramifications for surgical planning, particularly in cases involving an underdeveloped oblique fissure, which is present in 10–21% of left lungs (11).
Among these anomalies, the most common variation may be in the mediastinal lingular artery (26–27% incidence), which courses between the superior pulmonary vein and the upper bronchus in both lungs (8,23,41). This variation can be divided into a partially mediastinal type with mediastinal A4L and interlobar A5L, a wholly mediastinal type with both originating from the interlobar part of LPA, and an interlobar type (Figure 7A-7C) (41). One case report noted a common trunk of mediastinal A4+5L and the lateral branch of A3L, which can greatly influence anterior segmentectomies and lingulectomies (8). Additionally, A5L can arise from or with A8L, or three lingular branches can be present (23,36).

The left upper division shows distinct venous patterns. A semi-central vein or ascending central type predominate, constituting a substantial 62.87–72.8% of cases, followed by the central vein type, which contributes significantly at 17.4–28.22%. The non-central or descending central vein type represents a smaller yet notable proportion at 8.91–9.7% (Figure 8A-8C) (3,41). Misidentification of these types has significant implications for left upper segmentectomies and subsegmentectomies as each of them requires precise ligation. Although rare, V1L crossing LPA proximally and V2L running retrobronchially need to be kept in mind (30). V3L seems to be mostly invariable (3).

Typically, there is one lingular pulmonary vein, but a two-branched pattern is present in 16.34% of cases. Especially important during segmentectomies is the presence of three lingular branches with one draining into V3L, which are observed in 0.25% of cases (Figure 8D-8F) (41). Shiina et al. documented an instance (0.5%) where lingular pulmonary veins displayed an atypical pattern by draining into the left inferior pulmonary vein. In 1.1% of cases, they exhibited distinct drainage into the left atrium (2). Remarkably, an exceptional occurrence (0.5%) was documented where V5L exhibited a unique dual discharge into both the inferior pulmonary vein and the left atrium (2).
Left lower lobe (LLL) variations
Typically, the pulmonary artery undergoes a transition within its basal part, where a solitary superior segmental artery is commonly found. This division subsequently branches into two terminal arteries, further giving rise to anteromedial (A8L), lateral (A9L), and posterior (A10L) segmental arteries (22). Within the basal segment, the most prevalent vascular pattern consists of the A8 and A9+A10 configuration, accounting for 52.0% of cases, closely followed by the A8+A9 and A10 type, which constitutes 41.7% of cases (1). Regarding the LLL, Fourdrain et al.’s observations revealed that the upper segment is primarily supplied by a single artery in 65.9% of cases, by two separate arteries in 27.3%, and by three separate arteries in 6.8% (27,40). In 50% of cases, the left basal pulmonary artery bifurcates into two trunks: the anteromedial basal and posterolateral basal trunks (40). Other possible variants regarding A6L are also possible, such as A6L branching with A4+5 or A8L branching from A4+5L (Figure 9A) (1,27).
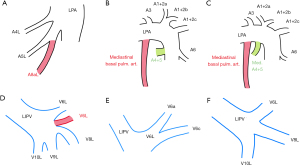
A mediastinal basal pulmonary artery is a rare variant that can be dangerous when present. Different segments and subsegments ranging from A5L to A10L may be supplied by this vessel (42-44). Two categories can be distinguished: single (without a mediastinal lingular artery) and complex (with the artery) (Figure 9B,9C). Both of these vessels can be misidentified and transected with grave consequences (45). Additionally, they can either be displaced or supernumerary (44). Either way, the potential presence of this artery can greatly handicap segmentectomy and lobectomy.
Normally, S7L is not present, and segmental V6L, V8L, V9L, and V10L are present. Basal veins are known to display a multitude of branching variations, which are often characterized by patterns where subsegmental veins originate from other segmental veins (1). S7L and therefore V7L are present in just 8% of cases (1). Furthermore, approximately 1% of cases have the superior segment of the LLL being drained by two segmental veins (Figure 9D). The anatomy of branches of V6L is limited, although in approximately 20% of cases, an intersegmental vein (V6b) between S6L, S8, and S9L could not be identified (Figure 9E). The venous anatomy of basal segments is quite diverse and clinically prominent. A lack of intersegmental V9bL was noted in 15.8% of cases and V9L in 2.4%, which would make it difficult to perform S9L or S10L solo segmentectomy (Figure 9F) (1). Also worth mentioning is an abnormal course of the aorta running from the proximal to thoracic directions of the left inferior pulmonary vein (30).
Common pulmonary veins
Common trunks of left and right pulmonary veins are difficult to classify into any specific lobe. Therefore, a separate subsection is dedicated to this variation. Although the common right pulmonary vein trunk is much less common than its counterpart on the left, one study reported a combined frequency of 4.10% in patients without atrial fibrillation (29). Other studies reported an incidence of 2–13% (46,47). The presented data make it difficult to estimate the frequency more accurately.
Despite it being relatively rare, a potential erroneous injury may cause dramatic results. A common left pulmonary vein is more common and similarly dangerous (30). Cutting it without proper identification in lobectomy may result in lung edema in the unresected lobe and fatal complications (48). Afterwards, open thoracotomy with anastomosis of the pulmonary trunk and the left atrium or pneumonectomy remain the only options (1,13,48). Significantly more data are available regarding this variation, but with a reported frequency of 0.93–17.8% (not including case reports), more studies are needed (1,30,48). Thankfully, these variations are possible to identify preoperatively after careful CT examination (13).
Discussion
Accurate knowledge of pulmonary vessels variations is crucial for not only thoracic surgeons but also any specialist operating within the thorax (28-30). The topography of pulmonary arteries and pulmonary veins can greatly influence both the outcome of lobectomies and seemingly unrelated procedures such as esophagectomies (28). A recent study provided information that segmentectomies have reportedly decreased complications, shorter operative time, and shorter hospital stay, which had a significant impact on older patients (49,50). Therefore, it is important to highlight and summarize the knowledge presented in this review.
Minimally invasive techniques such as VATS combined with less extensive resections using bronchopulmonary segments can greatly improve the outcomes of operations. There are several indications that segmentectomy is non-inferior to lobectomy in treating non-small cell lung carcinoma (51-53). Using 3D-CT, one study reported a preoperative identification rate of abnormal vessels of 98% in comparison with intraoperative identification (17). Therefore, the importance of proper radiological preparation cannot be overstated (4-6,15,16,54,55). However, Decaluwe et al. reported a vascular complication rate of 2.9% in an analysis of VATS surgeries, of which the majority ended in conversions to open thoracotomy (56). The extent of this review should not cloud the clinical implications of the mentioned variations. Therefore, we decided to highlight the importance of some of them.
There is inconsistency among authors in regard to which of the lungs or lobes are variable. According to Marom et al., a classic venous pattern on the right side is observed in only 60–68% of cases, and another author observed a similar frequency on the left side (37,46). However, in general, more authors agree that the right lung is more variable in regard to pulmonary veins (2,27,29,39). This may not be the case in arterial branching patterns (44). Therefore, each lobe and segment should be treated individually.
In comparison to other lobes, the RUL undergoes more lobectomies and segmentectomies (17). Okami et al. reported that nearly a third of lung cancers occur in this lobe (57). Its location near the subcarinal lymph nodes and the complex vascular anatomy of bronchopulmonary segments make it extremely important to deepen knowledge about clinical implications. Right top pulmonary vein draining of this lobe can easily injure it, or it can be misidentified as V1R or V2R directly draining into the RIPV or the left atrium during resection, lobectomy, or esophagectomy (28). What distinguishes this particular vessel is the possible additional supply from the RLL (31). Any abnormal retrobronchial course of the pulmonary vein, whether it involves V2R or V6R, can cause major bleeding during procedures in the posterior mediastinum (7,30).
As there is no haptic feedback during VATS resections, patterns provided by Yamashita and new definitions included in this review may be invaluable (21). Anterior or central vein type in RUL segmentectomy, where every intersegmental vein flows into one of these pulmonary veins, can impact the whole procedure and require a lobectomy (18). Although almost every abnormal vessel can be identified by 3D-CT, this is not always the case. Even though arterial branching is normally less variable, it may be problematic as well (17). The RPA and its branches, even with preoperative identification, may pose a challenge. Many authors report a simultaneous absence of a normal artery and the presence of a supernumerary artery (for example, a lack of ascending A2R with doubled A6R) (36). Even supposedly minor variations such as a low origin of certain arteries can be impactful. Depending on the study, as much as more than half of pulmonary fissures are incomplete (12,58). Absent or incomplete fissures combined with any variations in an interlobar artery may be dangerous (59).
Due to the many possible vascular variations of the RML, this region warrants attention. There have been reports of doubling of middle lobe arteries, split drainage from the RML, or direct outflow of the RMV into the left atrium, which is especially common in patients with atrial fibrillation (11,29,30). Overlooking such variations during RLL lobectomy may necessitate resection of healthy RMLs, which would have grave consequences for the patient (11).
Another finding from this review is that the amount of data about vascular variations in RLL is limited. Although there are many reports about vascular variations of S6R, others have not been studied as much. To our knowledge, only four cases of right mediastinal basal pulmonary artery have been reported, and three of the reports were written in Japanese (14). Despite the fact that it is a rare abnormality, its clinical implications when cut can be grave. Additionally, implications for segmentectomies should be mentioned. As for arteries, multi-stemmed and indirectly branching vessels may be significantly challenging to identify properly, even with 3D-CT (35). Different branching patterns of pulmonary veins, which are quite diverse in basal segments, can render selective resections difficult and require larger ones. The functional benefit of segmentectomies of more than two segments is uncertain (6).
Depending on the arterial variation, either segmentectomy of S1+2L or subsegmentectomy is advised. Additionally, accurate identification of venous patterns is needed to safely resect the desired segment and not damage the venous return from others. Pulmonary veins should serve as a protective guide for safe surgeries. Gao et al. indicate that the mediastinal lingular artery may be the most common variation that does not parallel the bronchus (41). Therefore, the development of an oblique fissure and the point of origin of the lingular artery greatly influence the surgical approach (3). An intrapericardial basal artery or the aforementioned mediastinal basal pulmonary artery can also be present in the left lung, which can be even more troublesome as they are difficult to distinguish from a mediastinal lingular artery and can potentially transect (14). Although more practical data is available on LLL variations, some useful information for performing S7L segmentectomy is still lacking. Similarly to the RLL, correct identification of pulmonary veins is crucial to perform selective and useful resections (1).
Our study has several limitations. There was no analysis of bronchial variations, which is a major factor. Furthermore, available data from studies and case reports are not sufficient to fully comprehend the scope of vascular variations, selection bias is possible, and different classifications and terminology make it difficult to compare findings. Due to the relatively small amount of data for some variations, additional studies should be conducted to verify incidence rates, and more systematized classifications should be implemented. Future research should focus particularly on segmental pulmonary veins as they are a major factor in segmentectomies.
Moreover, the use of 3D-CT can be beneficial and should be utilized more widely. Even though vascular variations are assigned to specific lobes, some of them may influence procedures in other lobes. Accurate knowledge of both the variations and their patterns is clinically important because it gives surgeons a broader perspective. Ultimately, saving lung parenchyma is one of the goals of thoracic surgery (18).
Conclusions
The knowledge about vascular variations can be applied to many different procedures, however, more studies are needed to be conducted to better estimate the frequencies and types of variations.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1715/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1715/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1715/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maki R, Miyajima M, Ogura K, et al. Pulmonary vessels and bronchial anatomy of the left lower lobe. Surg Today 2020;50:1081-90. [Crossref] [PubMed]
- Shiina N, Kaga K, Hida Y, et al. Variations of pulmonary vein drainage critical for lung resection assessed by three-dimensional computed tomography angiography. Thorac Cancer 2018;9:584-8. [Crossref] [PubMed]
- Deng Y, Cai S, Huang C, et al. Anatomical variation analysis of left upper pulmonary blood vessels and bronchi based on three-dimensional reconstruction of chest CT. Front Oncol 2022;12:1028467. [Crossref] [PubMed]
- Kanzaki M, Maeda H, Wachi N, et al. Complete video-assisted thoracoscopic multi-subsegmentectomy based on patients' specific virtual 3-D pulmonary models. Asian J Endosc Surg 2013;6:110-5. [Crossref] [PubMed]
- Wu WB, Xu XF, Wen W, et al. Thoracoscopic Pulmonary Sub-Subsegmentectomy Based on Three-Dimensional Images. Ann Thorac Surg 2016;102:e389-91. [Crossref] [PubMed]
- Brunelli A, Decaluwe H, Gonzalez M, et al. European Society of Thoracic Surgeons expert consensus recommendations on technical standards of segmentectomy for primary lung cancer. Eur J Cardiothorac Surg 2023;63:ezad224. [Crossref] [PubMed]
- Amore D, Muto E, Caterino U, et al. Anomalous segmental pulmonary vein: additional V6 behind the bronchus intermedius draining into the superior pulmonary vein. Monaldi Arch Chest Dis 2022; [Crossref] [PubMed]
- Amore D, Caterino U, Casazza D, et al. Uncommon anomalies of the left pulmonary artery during VATS lobectomy for lung cancer. Clin Case Rep 2020;8:1057-60. [Crossref] [PubMed]
- Igai H, Kamiyoshihara M, Yoshikawa R, et al. Algorithm-based troubleshooting to manage bleeding during thoracoscopic anatomic pulmonary resection. J Thorac Dis 2019;11:4544-50. [Crossref] [PubMed]
- Shah RD, D'Amico TA. Modern impact of video assisted thoracic surgery. J Thorac Dis 2014;6:S631-6. [PubMed]
- Subotich D, Mandarich D, Milisavljevich M, et al. Variations of pulmonary vessels: some practical implications for lung resections. Clin Anat 2009;22:698-705. [Crossref] [PubMed]
- Bostanci K, Ozyurtkan MO, Polat MO, et al. Variations in pulmonary fissural anatomy: a medicolegal autopsy study of 256 cases. ANZ J Surg 2020;90:608-11. [Crossref] [PubMed]
- Nakamura T, Koide M, Nakamura H, et al. The common trunk of the left pulmonary vein injured incidentally during lung cancer surgery. Ann Thorac Surg 2009;87:954-5. [Crossref] [PubMed]
- Atari M, Nakajima Y, Fukuhara M, et al. Abnormal branch of right pulmonary artery (A7): a case report and literature review. Surg Case Rep 2016;2:16. [Crossref] [PubMed]
- Akiba T, Marushima H, Harada J, et al. Importance of preoperative imaging with 64-row three-dimensional multidetector computed tomography for safer video-assisted thoracic surgery in lung cancer. Surg Today 2009;39:844-7. [Crossref] [PubMed]
- Yamada S, Suga A, Inoue Y, et al. Importance of preoperative assessment of pulmonary venous anomaly for safe video-assisted lobectomy. Interact Cardiovasc Thorac Surg 2010;10:851-4. [Crossref] [PubMed]
- Nagashima T, Shimizu K, Ohtaki Y, et al. An analysis of variations in the bronchovascular pattern of the right upper lobe using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2015;63:354-60. [Crossref] [PubMed]
- Shimizu K, Nagashima T, Ohtaki Y, et al. Analysis of the variation pattern in right upper pulmonary veins and establishment of simplified vein models for anatomical segmentectomy. Gen Thorac Cardiovasc Surg 2016;64:604-11. [Crossref] [PubMed]
- Maciejewski R. Branches of the left pulmonary artery supplying the basal segments of the lung. Acta Anat (Basel) 1991;140:284-6. [Crossref] [PubMed]
- Boyden EA. An analysis of variations in the bronchial pattern of the right upper lobe of 50 lungs. Anat Rec 1947;97:381. [PubMed]
- Yamashita H. Variations in the pulmonary segments and the bronchovascular trees. Roentgenologic anatomy of the lung. Tokyo: Igaku-syoin; 1978:46-58.
- Kandathil A, Chamarthy M. Pulmonary vascular anatomy & anatomical variants. Cardiovasc Diagn Ther 2018;8:201-7. [Crossref] [PubMed]
- Amore D, Casazza D, Caterino U, et al. Variations in the branching patterns of pulmonary artery during thoracoscopic pulmonary resection. Surg Radiol Anat 2021;43:1331-6. [Crossref] [PubMed]
- Li B, Li Z, Duan G, et al. Variation of the right upper pulmonary lobe vein. Asian J Surg 2023;46:1400-1. [Crossref] [PubMed]
- Zhang J, Zhu Y, Li H, et al. VATS right posterior segmentectomy with anomalous bronchi and pulmonary vessels: a case report and literature review. J Cardiothorac Surg 2021;16:60. [Crossref] [PubMed]
- Kayawake H, Chen-Yoshikawa TF, Tanaka S, et al. Variations and surgical management of pulmonary vein in living-donor lobectomy. Interact Cardiovasc Thorac Surg 2020;30:24-9. [Crossref] [PubMed]
- Polaczek M, Religioni J, Orłowski T. Thoracic surgery. Anatomic variations of pulmonary vessels relevant with regard to lung tissue resections – literature review and personal experiences. Kardiochirurgia i Torakochirurgia Polska 2013;10:232-8. [Crossref]
- Sato Y, Tanaka Y, Ohno S, et al. Right top pulmonary vein is a venous anomaly of which surgeons should be aware in subcarinal dissection for thoracoscopic esophagectomy: a case report and literature review. World J Surg Oncol 2022;20:160. [Crossref] [PubMed]
- Polaczek M, Szaro P, Baranska I, et al. Morphology and morphometry of pulmonary veins and the left atrium in multi-slice computed tomography. Surg Radiol Anat 2019;41:721-30. [Crossref] [PubMed]
- Polaczek M, Szaro P, Jakubowska L, et al. Pulmonary veins variations with potential impact in thoracic surgery: a computed-tomography-based atlas. J Thorac Dis 2020;12:383-93. [Crossref] [PubMed]
- Arslan G, Dincer E, Kabaalioglu A, et al. Right top pulmonary vein: evaluation with 64 section multidetector computed tomography. Eur J Radiol 2008;67:300-3. [Crossref] [PubMed]
- Hiroshi Y, Ken-Ichiro T, Masashi U. Right top pulmonary veins associated with lung incomplete fissure and displaced bronchus: a retrospective study using multidetector computed tomography. Gen Thorac Cardiovasc Surg 2021;69:290-6. [Crossref] [PubMed]
- Miyamoto N, Yoshida M, Takashima M, et al. Classifying the destination of right top pulmonary vein in 31 clinical cases. Gen Thorac Cardiovasc Surg 2021;69:1192-5. [Crossref] [PubMed]
- CORY RA. VALENTINE EJ. Varying patterns of the lobar branches of the pulmonary artery. A study of 524 lungs and lobes seen at operation of 426 patients. Thorax 1959;14:267-80. [Crossref] [PubMed]
- Nagashima T, Shimizu K, Ohtaki Y, et al. Analysis of variation in bronchovascular pattern of the right middle and lower lobes of the lung using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2017;65:343-9. [Crossref] [PubMed]
- Sivrikoz MC, Tulay CM. Variations of lobar branches of pulmonary arteries in thoracic surgery patients. Surg Radiol Anat 2011;33:509-14. [Crossref] [PubMed]
- Altinkaynak D, Koktener A. Evaluation of pulmonary venous variations in a large cohort: Multidetector computed tomography study with new variations. Wien Klin Wochenschr 2019;131:475-84. [Crossref] [PubMed]
- Nomori H, Okada M. Right lower segmentectomy. In: Asai H, editor. Atlas of segmentectomy. Tokyo: Bunko-do; 2011:81.
- Amore D, Casazza D, Imitazione P, et al. Common and Uncommon Variations of Pulmonary Venous Drainage in Patients Undergoing Thoracoscopic Lobectomy. Thorac Cardiovasc Surg 2020;68:256-60. [Crossref] [PubMed]
- Fourdrain A, De Dominicis F, Blanchard C, et al. Three-dimensional CT angiography of anatomic variations in the pulmonary arterial tree. Surg Radiol Anat 2018;40:45-53. [Crossref] [PubMed]
- Gao C, Xu WZ, Li ZH, et al. Analysis of bronchial and vascular patterns in left upper lobes to explore the genesis of mediastinal lingular artery and its influence on pulmonary anatomical variation. J Cardiothorac Surg 2021;16:306. [Crossref] [PubMed]
- Iijima Y, Kinoshita H, Nakajima Y, et al. Branching anomaly of the pulmonary ventrobasal and laterobasal arteries from the mediastinal lingular pulmonary artery. Gen Thorac Cardiovasc Surg 2020;68:1558-61. [Crossref] [PubMed]
- Uchida S, Watanabe SI, Yoshida Y, et al. Aberrant mediastinal trunk of pulmonary artery. J Surg Case Rep 2019;2019:rjy359. [Crossref] [PubMed]
- Liu Y, Zhang S. Mediastinal basal pulmonary artery identification and classification by three-dimensional reconstruction. Surg Radiol Anat 2022;44:447-53. [Crossref] [PubMed]
- Yamada S, Inoue Y, Suga A, et al. Surgical risk of vessel injury: an unusual anatomical variant of the right medial basal segmental pulmonary artery. Gen Thorac Cardiovasc Surg 2011;59:301-3. [Crossref] [PubMed]
- Marom EM, Herndon JE, Kim YH, et al. Variations in pulmonary venous drainage to the left atrium: implications for radiofrequency ablation. Radiology 2004;230:824-9. [Crossref] [PubMed]
- Akiba T, Marushima H, Odaka M, et al. Pulmonary vein analysis using three-dimensional computed tomography angiography for thoracic surgery. Gen Thorac Cardiovasc Surg 2010;58:331-5. [Crossref] [PubMed]
- Asouhidou I, Karaiskos T, Natsis K. Pulmonary vein anatomical variation during videothoracoscopy-assisted surgical lobectomy. Surg Radiol Anat 2017;39:229-31. [Crossref] [PubMed]
- Linden D, Linden K, Oparka J. In patients with resectable non-small-cell lung cancer, is video-assisted thoracoscopic segmentectomy a suitable alternative to thoracotomy and segmentectomy in terms of morbidity and equivalence of resection? Interact Cardiovasc Thorac Surg 2014;19:107-10. [Crossref] [PubMed]
- Kilic A, Schuchert MJ, Pettiford BL, et al. Anatomic segmentectomy for stage I non-small cell lung cancer in the elderly. Ann Thorac Surg 2009;87:1662-6; discussion 1667-8. [Crossref] [PubMed]
- Kodama K, Doi O, Higashiyama M, et al. Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single-institution study. J Thorac Cardiovasc Surg 1997;114:347-53. [Crossref] [PubMed]
- Martin-Ucar AE, Nakas A, Pilling JE, et al. A case-matched study of anatomical segmentectomy versus lobectomy for stage I lung cancer in high-risk patients. Eur J Cardiothorac Surg 2005;27:675-9. [Crossref] [PubMed]
- Keenan RJ, Landreneau RJ, Maley RH Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228-33; discussion 228-33. [Crossref] [PubMed]
- Shimizu K, Nakano T, Kamiyoshihara M, et al. Segmentectomy guided by three-dimensional computed tomography angiography and bronchography. Interact Cardiovasc Thorac Surg 2012;15:194-6. [Crossref] [PubMed]
- Villa M, Sarkaria IS. Great Vessel Injury in Thoracic Surgery. Thorac Surg Clin 2015;25:261-78. [Crossref] [PubMed]
- Decaluwe H, Petersen RH, Hansen H, et al. Major intraoperative complications during video-assisted thoracoscopic anatomical lung resections: an intention-to-treat analysis. Eur J Cardiothorac Surg 2015;48:588-98; discussion 599. [Crossref] [PubMed]
- Okami J, Shintani Y, Okumura M, et al. Demographics, Safety and Quality, and Prognostic Information in Both the Seventh and Eighth Editions of the TNM Classification in 18,973 Surgical Cases of the Japanese Joint Committee of Lung Cancer Registry Database in 2010. J Thorac Oncol 2019;14:212-22.
- West CT, Slim N, Steele D, et al. Are textbook lungs really normal? A cadaveric study on the anatomical and clinical importance of variations in the major lung fissures, and the incomplete right horizontal fissure. Clin Anat 2021;34:387-96. [Crossref] [PubMed]
- Wąsik J, Tubbs RS, Zielinska N, et al. Lung segments from anatomy to surgery. Folia Morphol (Warsz) 2024;83:20-34. [Crossref] [PubMed]


