Validation of EORTC and CALGB prognostic models in surgical patients submitted to diagnostic, palliative or curative surgery for malignant pleural mesothelioma
Introduction
Malignant pleural mesothelioma (MPM) is an aggressive and lethal malignant tumour of the pleura, with limited treatment options and with a curative-intent treatment feasible in only a selected group of patients.
Prognosis of patients affected by MPM is multifactorial and includes anatomical (stage and classification), tumor-related (histology, genetics), patient-related (age, performance score, comorbidities) and environment-related (geography, health-care system) prognostic factors. Identification of prognostic groups is important to provide an estimation of the clinical outcome, to design trials and to test treatments. Also, combination of prognostic factors and integration in a prognostic model is useful to identify patient subgroups that may benefit from multimodality treatments, including surgery. The European Organisation for Research and Treatment of Cancer (EORTC) proposed a prognostic scoring system that has been used in the assessment of survival and patients’ stratification into randomised clinical studies of mesothelioma patients (1-3).
Aim of this study is to examine the trend of our surgical patients affected by MPM and submitted to diagnostic, palliative or curative surgical procedures performed and to validate, for the same cohort of patients, the EORTC prognostic factors.
Methods
This is a cohort study of patients submitted to surgery for MPM from January 2007 to December 2013. Cases were retrospectively collected from the surgical registry of the Department of Thoracic Surgery, San Giovanni Battista Hospital (Torino, Italy). All patients submitted to diagnostic, palliative or curative procedures were included in the study. Patients’ data were acquired from hospital records.
For the purpose of this study, the nomenclature of the surgical procedures matches the International Association for the Study Lung Cancer (IASLC)/International Mesothelioma Interest Group (iMIG) (4).
All patients were submitted to a routine preoperative assessment inclusive of radiological investigations (chest X-ray, CT scan), blood samples [white blood cells (WBC) count, platelets count, haemoglobin], electrocardiogram and anaesthesiologist review. PET scans were available for patients undergoing a process of disease staging/diagnosis and rarely for patients undergoing palliative procedures.
Diagnosis of MPM was mostly obtained by means of a video-assisted thoracic surgery (VATS) biopsy of the pleura or by a mini-thoracotomy; only in few cases diagnosis was achieved by a CT-guided biopsy or cytology from pleural effusion. Since several years, fit patients affected by MPM and with a surgically approachable pleural disease were offered a pleurectomy/decortication (P/D) through a postero-lateral thoracotomy, followed by chemotherapy regimens and eventually radiotherapy.
A palliative thoracoscopic procedure (drainage of pleural effusion and talc pleurodesis), eventually followed by chemotherapy, was the treatment of choice for unfit and/or elderly patients and/or in advanced disease. Talc pleurodesis was performed only in patients with an expandable, non-trapped lung. Board qualified thoracic surgeons performed the surgical procedures.
A clinical stage assessment for each patient was performed through a retrospective review of preoperative CT scans, PET scans and surgical notes (e.g., thoracoscopy) according to the IASLC-American Joint Committee on Cancer (AJCC)-International Union Against Cancer (UICC) Staging Manual in Thoracic Oncology (5).
Follow-up (FU) data were obtained by routine outpatient clinic appointment or telephone contact and was completed in June 2014.
Scores and indexes
The EORTC parameters include the Eastern Cooperative Oncology Group (ECOG) performance status (PS), histological subtype, sex, certainty of diagnosis and WBC count, as shown in Table 1.

Full table
Patients’ comorbidities were assessed by the Charlson’s Comorbidity Index (CCI) and by the Colinet Comorbidity Score (6).
Data variables and outcomes
Primary outcome was overall survival (OS), computed from the date of diagnosis to the date of death by any cause; patients alive were censored on the date of last FU.
We chose in the final dataset the following variables of interest, which include the IASLC CORE and supplementary prognostic variables (7): gender (female vs. male); smoking habit (no vs. yes); asbestos exposure (no vs. yes); chest pain (no vs. yes); dyspnoea (no vs. yes); ECOG PS (no vs. yes); Hb <14.5 g/dL (no vs. yes); WBC count >15,500/mm3 (no vs. yes); platelets count >400×103/mm3; CCI (as continuous); CCS >9 (no vs. yes); epithelioid histology (yes vs. no); clinical tumor stage (iMIG, I–II vs. III–IV); kind of procedure (diagnostic-palliative vs. P/D vs. EPP); chemotherapy treatment (no vs. yes) and EORTC (low risk vs. high risk) prognostic score.
Statistical analysis
Continuous data are presented as median interquartile range (IQR), categorical ones as number (percentage, %). Survival curves were assessed using the Kaplan-Meier method and significant differences in survival between groups were estimated with log rank test. Univariate and multivariate adjusted comparisons by prognostic score (i.e., EORTC) for OS were accomplished using Cox regression method. Adjusted models included the following clinical variables: kind of procedure, smoking habit, asbestos exposure, CCI, clinical tumor stage, adjuvant chemotherapy, dyspnoea, chest pain and haematological variables according to the score features.
All statistical analyses were obtained using STATA (version 12.1).
Results
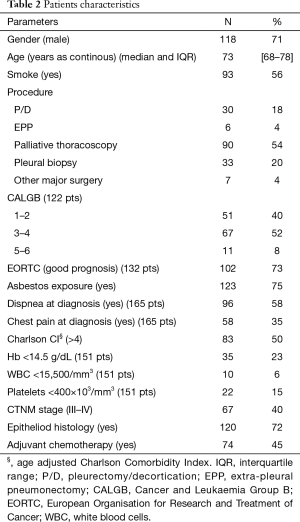
One hundred sixty-six consecutive cases were collected during the study period. Population’s demographics and clinical, surgical and pathologic characteristics are showed in Table 2. The majority of patients were male (118, 71%) and the median age at surgery was 73 (IQR 68–78). Ninety-three patients (56%) were smokers and 123 (75%) had a history of exposure to asbestos. In 96 cases (58%) dyspnoea was present at the time of diagnosis and 58 patients presented with chest pain.
Full table
Most of the patients were offered a palliative thoracoscopic procedure with talc pleurodesis (90, 54%); a P/D was performed in 30 patients and in 6 an EPP. Thirty-three patients (20%) underwent a diagnostic pleural biopsy. In seven patients, another major surgery was performed (e.g., lobectomy), usually driven by an undefined pre-operative diagnosis: these patients were excluded from the final analysis.
Clinical TNM stages were as follows: 99 (60%) stage I–II, 34 (20%) stage III and 33 (20%) stage IV. Eighty-nine patients (54%) received a complementary treatment: 74 (45%) had adjuvant chemotherapy, 12 (7%) an adjuvant chemo- and radiotherapy treatment and 3 (2%) an induction chemotherapy.
Generally, most of the patients were classified in the good prognosis group according to the EORTC score (102, 73%).
Survival analysis
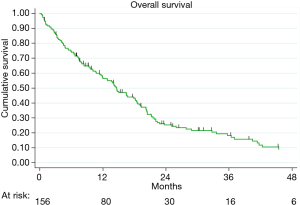
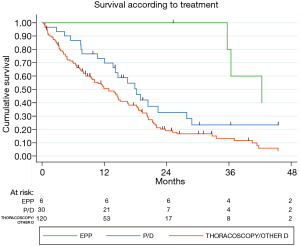
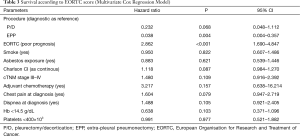
The mean FU was 19 months (IQR 9–31) and the FU-completeness was 98%. At the end of the study period, 130 patients died (78%). The overall 1- and 3-year survival was 60% and 36%, respectively (Figure 1). Patients submitted to EPP and P/D showed a better survival than those submitted to diagnostic or palliative procedure (P=0.013). Moreover, multivariate-adjusted models indicated an independent effect of EPP and P/D on survival (Table 3).
Full table
EORTC
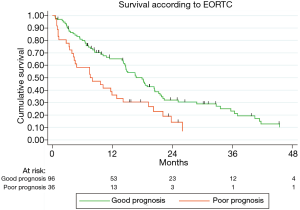
Figure 2 illustrates the survival curves according to the EORTC score: patients in the good prognosis group had a slightly better survival than those in the poor prognosis group (P=0.0013). Multivariable model showed an independent prognostic value of EORTC score (HR 2.87, P<0.001; Table 3).
Discussion
In recent years, a considerable interest has been directed to the association of systemic markers and prognosis in cancer. The role of prognostic factors is necessary in cancer treatment, since specific factors capable of predicting survival or progression of disease help in selecting a better cure according to the patient profile/characteristics. This is particularly felt for mesothelioma patients due to the long incubation and aggressiveness of this disease, along with the limited treatment options (8-10).
Through this work we aimed to validate the EORTC prognostic score in a surgical population of patients submitted to diagnostic, curative and palliative surgical procedures for MPM since the EORTC is a very well recognized prognostic scoring system and is a helpful tool in the assessment of survival and patient stratification for statistical analysis. Furthermore, for the purpose of this study, we selected the variables of interest according to the IASLC CORE and the supplementary prognostic variables, which proved to be predictors of survival as described in the paper published on behalf of the IASLC staging committee in 2014 (7).
Our results showed an independent prognostic value of the EORTC score at the multivariable model (HR 2.86; P<0.0001) and are in line with the work of Edwards et al. (2) who validated the EORTC and the Cancer and Leukaemia Group B (CALGB) prognostic scores in their surgical series of patients.
Although substantially in line with the literature (7), the 1- and 3-year survival of our study, confirms the poor prognosis and high aggressiveness of MPM. However, both multivariate-adjusted models point out an independent effect of curative and cytoreductive surgery (EPP and P/D) on survival. In fact, patients of our cohort submitted to EPP and P/D, showed a better survival than those submitted to a diagnostic or palliative procedure (P=0.013; Figure 3) probably referable to patient selection (fit and young, with a good PS) and disease stage (favourable histology, surgically approachable). This has been evidenced by a meta-analysis with a consistent number of patients treated with P/D and EPP, concluding that P/D should be preferred to EPP whenever feasible (11). The debate on whether P/D is superior to EPP or vice-versa is still on going but with a growing recognition that P/D offers a good compromise between long-term survival and postoperative quality of life if compared to EPP (11-18).
The analysis of the clinical staging identified a high percentage of clinical stage I–II (60%), and nearly the same percentage of stage III and IV (20%, both) underlying that patients referred to our center are primarily in an early clinical stage rather than in an advanced one. However, most of these patients were not suitable candidates for major surgical procedures since nearly 54% of them were submitted to a palliative treatment (VATS talc pleurodesis) and only 36 patients were submitted to either P/D or EPP.
This study is one of the few studies available in literature with a detailed preoperative staging and a strong FU. Its importance is clear since it is the only method to evaluate surgical diagnostic procedures that do not contemplate a pathological staging (e.g., diagnostic and palliative surgery). Among the limitation of the present study, there were the mono-institutional setting and the retrospective nature of the data.
In conclusion, outcomes of patients affected by MPM are evidently poor and prognosis is multifactorial. The role of surgery and its extent remains still matter of debate; in fact, in selected patients, surgical aggressiveness, although not radical (P/D), may still be beneficial within a multimodal approach, and could be a good compromise in terms of survival and quality of life.
Also, we were able to validate the EORTC prognostic index in our cohort of patients, which proved to be an independent prognostic factor and therefore a reliable and valid instrument that may be implemented in the daily practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the local Institution Review Board and written informed consent was obtained from all patients.
References
- Curran D, Sahmoud T, Therasse P, et al. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. J Clin Oncol 1998;16:145-52. [PubMed]
- Edwards JG, Abrams KR, Leverment JN, et al. Prognostic factors for malignant mesothelioma in 142 patients: validation of CALGB and EORTC prognostic scoring systems. Thorax 2000;55:731-5. [Crossref] [PubMed]
- Fennell DA, Parmar A, Shamash J, et al. Statistical validation of the EORTC prognostic model for malignant pleural mesothelioma based on three consecutive phase II trials. J Clin Oncol 2005;23:184-9. [Crossref] [PubMed]
- Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the international association for the study of lung cancer international staging committee and the international mesothelioma interest group. J Thorac Oncol 2011;6:1304-12. [Crossref] [PubMed]
- Goldstraw P. IASLC staging handbook in thoracic oncology. 1st edition. Orange Park: Editorial Rx Press, 2009.
- Ball D, Thursfield V, Irving L, et al. Evaluation of the Simplified Comorbidity Score (Colinet) as a prognostic indicator for patients with lung cancer: a cancer registry study. Lung Cancer 2013;82:358-61. [Crossref] [PubMed]
- Pass HI, Giroux D, Kennedy C, et al. Supplementary prognostic variables for pleural mesothelioma: a report from the IASLC staging committee. J Thorac Oncol 2014;9:856-64. [Crossref] [PubMed]
- Steele JP. Prognostic factors for mesothelioma. Hematol Oncol Clin North Am 2005;19:1041-52. vi. [Crossref] [PubMed]
- Steele JP, Klabatsa A, Fennell DA, et al. Prognostic factors in mesothelioma. Lung Cancer 2005;49 Suppl 1:S49-52. [Crossref] [PubMed]
- Meniawy TM, Creaney J, Lake RA, et al. Existing models, but not neutrophil-to-lymphocyte ratio, are prognostic in malignant mesothelioma. Br J Cancer 2013;109:1813-20. [Crossref] [PubMed]
- Taioli E, Wolf AS, Flores RM. Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann Thorac Surg 2015;99:472-80. [Crossref] [PubMed]
- Rena O, Casadio C. Extrapleural pneumonectomy for early stage malignant pleural mesothelioma: a harmful procedure. Lung Cancer 2012;77:151-5. [Crossref] [PubMed]
- Lang-Lazdunski L, Bille A, Lal R, et al. Pleurectomy/decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J Thorac Oncol 2012;7:737-43. [Crossref] [PubMed]
- Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. [Crossref] [PubMed]
- Weder W, Stahel RA, Baas P, et al. The MARS feasibility trial: conclusions not supported by data. Lancet Oncol 2011;12:1093-4; author reply 1094-5. [Crossref] [PubMed]
- Sugarbaker DJ, Richards WG, Bueno R. Extrapleural pneumonectomy in the treatment of epithelioid malignant pleural mesothelioma: novel prognostic implications of combined N1 and N2 nodal involvement based on experience in 529 patients. Ann Surg 2014;260:577-80; discussion 580-2. [Crossref] [PubMed]
- Mollberg NM, Vigneswaran Y, Kindler HL, et al. Quality of life after radical pleurectomy decortication for malignant pleural mesothelioma. Ann Thorac Surg 2012;94:1086-92. [Crossref] [PubMed]
- Jenkins P, Milliner R, Salmon C. Re-evaluating the role of palliative radiotherapy in malignant pleural mesothelioma. Eur J Cancer 2011;47:2143-9. [Crossref] [PubMed]