
Multimodal prehabilitation in patients with non-small cell lung cancer: a feasibility study
Highlight box
Key findings
• Prehabilitation before non-small cell lung cancer surgery is feasible and may lead to improved functional capacity.
What is known and what is new?
• Prehabilitation in colorectal surgery has been proven to be safe. It enhances functional capacity and reduces postoperative severe complications.
• Our results implicate that prehabilitation in lung surgery within a 3-week timeframe is safe and feasible and is associated with enhancing preoperative functional capacity.
What is the implication and what should change now?
• Prehabilitation in lung surgery is safe and feasible, further research on reducing complications, length of stay and enhancing functional capacity is necessary. Due to the multimodal aspect of this intervention and the short timeframe we strongly advocate for a coordinator in the preoperative lung cancer pathway.
Introduction
Lung cancer is the leading cause of cancer-related death worldwide (1). For non-small cell lung cancer (NSCLC) the preferred curative treatment is surgery. Postoperative complications may be significant (2) and lead to a reduced disease-free survival (3). To improve postoperative outcome, guidelines for enhanced recovery after surgery (ERAS) advise both optimisation of the patient preoperatively, as well as clear instructions on postoperative care (4).
Optimisation of patient status preoperatively is called prehabilitation (5). It consists of a multimodal intervention program and has proven to be successful in care pathways for -among others- abdominal surgery and colorectal cancer surgery (5,6). The goal of prehabilitation is to improve functional capacity, meaning the ability to transport oxygen to the muscles (7). In lung surgery functional capacity is particularly at risk since lung parenchyma is removed. Therefore, it seems logical that prehabilitation should play a role in the care pathway of lung surgery, potentially even making patients who are deemed unfit for surgery operable again (8).
The main challenge in prehabilitation in lung surgery is the short preoperative period, which is limited due to the possible risk on tumour progression or dissemination (8,9). In the Netherlands, the guideline for the treatment of NSCLC states that 80% of patients should have surgery within 3 weeks after confirming the diagnosis in the multidisciplinary team (MDT). This is monitored by the Dutch Lung Cancer Audit-Surgery (DLCA-S) that records and benchmarks the diagnosis and treatment in lung surgery (10). This implies that the window of opportunity for prehabilitation is limited to 3 weeks. Other research groups have already shown positive results in achieving improvement of preoperative functional capacity in lung surgery (11,12). However, due to limited research, a clear recommendation on optimal approach of prehabilitation in lung surgery is not available yet.
The aim of this two-centre study was two-fold: (I) to determine the feasibility of a multimodal prehabilitation programme for patients with NSCLC undergoing anatomical pulmonary resection within a 3-week timeframe; (II) to determine whether this programme resulted in improved functional capacity. We present this article in accordance with the TREND reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1929/rc).
Methods
Study design
This study was designed by an MDT of health care professionals from two large teaching hospitals [Albert Schweitzer Hospital (ASz) Dordrecht and Maxima Medical Centre (MMC) Veldhoven, the Netherlands]. The published study protocol is summarised below (13). This study is registered as NL8080 in the Netherlands Trial Register on October 10th 2019. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Central Committee on Research Involving Human Subjects (CCMO) (number NL70578.015.19, reference number of the Medical Ethical Review Committee of Maxima Medical Centre: W19.045). Patients were included after providing written informed consent.
Subjects
Patient recruitment started in January 2020, yet was temporarily interrupted due to the coronavirus disease 2019 (COVID-19) pandemic, and ended July 2021. Consecutive patients >18 years with pathologically confirmed or suspected NSCLC, and an indication for anatomical lung resection were deemed eligible for participation. Furthermore, a straightforward preoperative work-up was necessary to ensure patients could enter the programme directly after diagnosis for an optimal use of the small window of opportunity between diagnosis and surgery.
Patients were excluded in case of contra-indications for training, such as paraplegia or orthopaedic impairments, inability to consume protein supplementation due to renal insufficiency [estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2] or participation in other trials. Referred patients from other hospitals were excluded as travel distance could affect feasibility of the programme. If patients did not want to participate in the programme, they were asked to participate in the control group, where only assessments were completed. Since feasibility and safety of the program was the main outcome, only adverse events due to the program were scored.
Assessments
Assessments consisting of functional capacity measurements and questionnaires took place at baseline (T0), end of program preoperatively (T1), and 6 weeks postoperatively (T2). Three months postoperatively questionnaires were assessed (T3). Functional capacity was determined during assessments at T0, T1 and T2 by a physiotherapist, and consisted of the 6-minute walk test (6MWT) (14), steep ramp test (SRT) (15), maximal inspiratory pressure (MIP; T0 and T1), and indirect one repetition maximum (1RM) (16).
Nutritional status was assessed at T0 and T1 by a dietician by measuring weight, height, and calculating body mass index (BMI). The Patient-Generated Subjective Global Assessment (PG-SGA) was used to screen for malnutrition (17). To determine body composition, a standardised bioelectrical impedance analysis (BIA) was performed. Hand grip strength (HGS) was determined using a hand-held hydraulic dynamometer.
Intervention
The programme had a maximum duration of 3 or 5 weeks in case of a preceding mediastinoscopy. It consisted of the following six interventions [described in more detail in the published study protocol is summarised below (13)].
Exercise programme
The exercise programme consisted of supervised training by a physiotherapist (3 non-consecutive days a week) and home-based non-supervised training (other weekdays). Supervised training of approximately 60 minutes consisted of high-intensity interval training (HIIT) and strength exercises. Intensity of the exercise programme was individualised, based on the T0 assessment, and evaluated and adjusted during the programme. Home-based training consisted of low-intensity training (LIT) exercises (walking or cycling) for at least 60 minutes daily. LIT was continued until the day of hospital admission.
Nutritional support
Nutritional status was optimised through counselling by a dietitian. Based on the T0 assessment, the dietitian provided tailored dietary advice, including energy and protein requirements.
All patients received a protein powder supplement of 30 grams (Refit®-TMP-90-Shake, Friesland Campina Domo, Amersfoort, the Netherlands) for daily use, and twice a day on supervised training days. Additionally, vitamin D (dosage based on the guideline of the Health Council of the Netherlands) and multivitamin supplements were provided for daily use (18).
Psychological support
Psychological support consisted of a 45-minute consultation with a clinical psychologist. During this consultation, burden of disease and the patient’s coping strategies were assessed, and—when indicated—empowerment or psychoeducation was provided. The clinical psychologist determined whether follow-up sessions were needed during the perioperative phase and/or referral to a psychiatrist was indicated.
Smoking cessation programme
If applicable, patients were offered to follow a smoking cessation programme. This programme was outsourced to Sinefuma (19). At T0 and T3, patients were asked about their smoking status by the case manager or physiotherapist.
Patient empowerment
To maximise patient empowerment, patients were informed and educated by the research team about the programme, the surgical care pathway, and their own contribution. A handbook containing information on interventions of the programme and a logbook was provided to register all study-related activities and to register LIT and daily protein and vitamin supplementation. Breathing and relaxation techniques were provided to patients.
Optimisation of respiratory status
Additional to standard pulmonary functioning assessment by spirometry, the MIP was measured at T0 and T1 using the MicroRPM (Respiratory Pressure Meter, Care Fusion, San Diego, CA, USA). An inspiratory muscle training (IMT) device (Philips-Respironics Threshold IMT, Philips, Eindhoven, the Netherlands) was provided and intensity of IMT was based on the baseline MIP. IMT was performed twice daily for fifteen minutes at home. Additionally, a physiotherapist taught breathing and sputum clearance techniques prior to surgery.
Primary outcomes
The first primary outcome of the study was feasibility, defined as ≥80% of all patients completed a sufficient multimodal prehabilitation programme. Feasibility was scored on a total of eighteen interventions in six pillars which all were graded equally due to its synergistic effect. A successful programme would be if a patient had ≥80% out of 18 interventions achieved. Different interventions and goals per intervention are shown in Table 1.
Table 1
| Interventions in prehabilitation programme | Completion goal | Number of patients who completed interventions (%) |
|---|---|---|
| 1. Exercise programme | ||
| 1× Baseline test moment (SRT, 6MWT, 1RM) | 1 | 24/24 (100.0) |
| 6× HIIT | ≥4 and ≥80% planned sessions completed | 17/24 (70.8) |
| LIT (on non-training days between T0 and surgery) | ≥80% actual followed | 18/24 (75.0) |
| 6× Supervised strength exercises | ≥4 and ≥80% planned sessions completed | 17/24 (70.8) |
| 1× End of program tests (SRT, 6MWT, 1RM) | 1 | 24/24 (100.0) |
| 2. Nutritional support | ||
| 1× Dietician consultation with BIA and HGS | 1 | 24/24 (100.0) |
| 1× Dietary advice | 1 | 24/24 (100.0) |
| 27× Protein supplementation | ≥22 and ≥80% actual followed | 13/24 (54.2) |
| Vitamin supplementation (daily between T0 and surgery) | ≥80% actual followed | 22/24 (91.7) |
| 3. Mental support | ||
| 1× Breathing/relaxation exercises | 1 | 24/24 (100.0) |
| 1× Consultation medical psychologist | 1 | 23/24 (95.8) |
| 4. Smoking cessation | ||
| Smoking cessation (when active smoker) | 1 | 5/6 (83.3) |
| Non-active smoker at T3 (cessation/never smoked) | 1 | 23/24 (95.8) |
| 5. Patient empowerment | ||
| 1× Consultation with research nurse | 1 | 24/24 (100.0) |
| 1× Handbook handout | 1 | 24/24 (100.0) |
| 6. Respiratory optimisation | ||
| 1× Baseline test moment (IMT) | 1 | 24/24 (100.0) |
| IMT (twice a day between T0 and surgery) | ≥80% actual followed | 23/24 (95.8) |
| 1× Instructions on breathing/sputum clearing technique | 1 | 24/24 (100.0) |
Number of patients per intervention with percentage score. T0: baseline; T3: 3 months after surgery. SRT, steep ramp test; 6MWT, 6-minute walk test; 1RM, one repetition maximum; HIIT, high-intensity interval training; LIT, low-intensity training; BIA, bioelectrical impedance analysis; HGS, hand grip strength; IMT, inspiratory muscle training.
The number of supervised training sessions differed depending on the time available between T0 and surgery. Table 1 presents the number of sessions that could be planned for these interventions in case of an “optimal window of opportunity” (i.e., with T0 performed the day after MDT and surgery performed 3 weeks after MDT). If surgery is planned earlier than 3 weeks after MDT, or if T0 is delayed, the number of sessions that can be planned will be smaller. If surgery is delayed due to mediastinoscopy, the number of sessions that can be planned will be larger. To take into account these individual differences in the number of plannable sessions, we defined our goal for these interventions in two-fold. The interventions were feasible if: (I) ≥80% of the planned sessions for that patient were attended and completed; and (II) a minimum amount of sessions could was attended and completed (for details see Table 1 and below).
The following definitions of threshold of a successfully completed intervention were applied:
- Exercise programme: threshold for successful HIIT was defined as the presence at both T0 and T1 assessment, and ≥80% of scheduled training sessions attended and finished, with a minimum of four. The threshold for strength exercises was identical. We set the initial threshold of minimum amount of training sessions (HIIT and strength) at five, as we hypothesized that we could include patients before the MDT meeting. Since this was not the case, we lowered the threshold to a minimum of four. The threshold for LIT was defined as ≥80% of scheduled sessions attended and finished, registered in the self-reported logbook.
- Nutritional support: the threshold was defined as presence at T0 for consultation with the dietitian with BIA and HGS. The threshold for protein supplementation was defined as ≥80% of scheduled consumption with a minimum of 22 portions. The threshold for vitamin supplementation was defined as ≥80% of scheduled consumption. Consumption was self-reported by patients in the study logbook.
- Psychological support: the threshold was defined as presence at consultation with a medical psychologist and receiving instructions for breathing and relaxation exercises.
- Smoking cessation: the threshold was defined as: (i) starting a cessation programme (provided by Sinefuma, or if active smokers quitted smoking with the help of a general practitioner or by themselves); (ii) non-smoking at T3.
- Patient empowerment: the threshold was defined as presence at consultation with the research nurse and receiving the handbook.
- Optimisation of respiratory status: the threshold was defined as presence at T0 IMT assessment, and instruction of breathing and sputum clearance techniques. The threshold for IMT was defined as ≥80% of scheduled sessions performed.
As second primary outcome measure, functional capacity, was assessed on T0, T1, and T2 measured with the SRT (W), 6MWT (m), the 1RM for the leg press (LP), low row (LR), lateral pull down (LPD), chest press (CP), HGS (kg; only on T0 and T1), and MIP (cmH2O).
Secondary outcomes
Secondary outcome was the time frame between MDT diagnosis and treatment. It was assessed by collecting time from MDT-surgery, time from T0 to T1 and time from T1 to surgery in order to evaluate our prehabilitation programme against the DLCA-S standard. Additionally, surgical outcomes such as, length of stay (LoS), complication rate (%) as defined by Clavien-Dindo score (20), comprehensive complication index (CCI) (21), readmission rate within 30 days after surgery and mortality within 30 days after surgery were registered.
Baseline characteristics such as gender, age, BMI, American Society of Anaesthesiologists (ASA) score, respiratory function such as forced vital capacity (FVC), forced expiratory volume in the first second (FEV1) and the percentage of predicted FEV1, chronic obstructive pulmonary disease (COPD), tumour, node, metastasis (TNM) stage, operation type and operation time were collected. Finally, a patient evaluation questionnaire on satisfaction and usefulness, drafted by the research team, was sent by email to patients on T1 and T3.
Statistical analyses
Data were checked for normality using Kolmogorov-Smirnov test and Shapiro-Wilk test. The population was described using mean [standard deviation (SD)] or median [interquartile range (IQR)], as appropriate. Due to minimal response on participating in the control group, only the prehabilitation group was analysed with complete case analysis. To assess feasibility, the timeframe of the programme was described as days between MDT and T0, days between T0 and T1 and days between T1 and surgery. To evaluate the feasibility per intervention type, completion rate (%) was calculated as number of completed interventions of total scheduled interventions. To evaluate the effects of the programme on functional capacity over time (T0, T1, and T2), mixed model analyses were performed for SRT, 6MWT, 1RM (LP, LR, CP, LPD), MIP, and HGS (T0–T1), taking into account the correlation between different measurements, corrected for age, gender and centre. Assessment of the patient satisfaction was described as median with IQR. Analyses were conducted using the statistical package for the social sciences (version 22; IBM, SPSS Inc., Chicago, IL, USA). A two-tailed P<0.05 was considered statistically significant.
Results
All 131 patients (ASz: 68, MMC: 63) with high suspected NSCLC and an indication for anatomical resection were assessed for eligibility, of whom 42 met the inclusion criteria. Of those, 14 were not willing to participate, and 28 signed informed consent. Final inclusion contained 24 patients (ASz: 14, MMC: 10) (Figure 1). Most common reasons that patients did not meet the inclusion criteria were study-related (other trials, other hospitals, no NSCLC, COVID stop, inability to give informed consent). Furthermore, 19 patients could not participate because the pre-operative work-up was not straightforward. Only 15 patients could not participate because of patient characteristics. Baseline characteristics are summarised in Table 2. All included patients had video-assisted thoracoscopic surgery (VATS). No adverse events due to the program were seen.
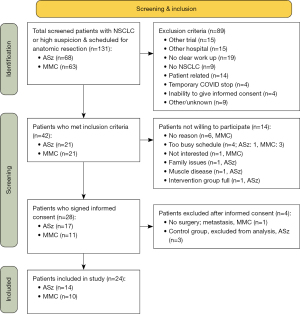
Table 2
| Baseline characteristics | Total group (n=24) | ASz (n=14) | MMC (n=10) |
|---|---|---|---|
| Gender (male) | 11 (45.8) | 5 (35.7) | 6 (60.0) |
| Age (years) | 64.5 [59–71.8] | 64 [59–71.3] | 65.6 [60–74.3] |
| ASA score | |||
| I | 2 (8.3) | – | 2 (20.0) |
| II | 13 (54.2) | 9 (64.3) | 4 (40.0) |
| III | 8 (33.3) | 4 (28.6) | 4 (40.0) |
| IV | 1 (4.2) | 1 (7.1) | – |
| BMI (kg/m2) | 25.3 [23.7–30.3] | 27.1 [24–30.5] | 24 [21.7–29.8] |
| Smoking status | |||
| Active† | 6 (25.0) | 5 (35.7) | 1 (10.0) |
| Never | 3 (12.5) | 1 (7.1) | 2 (20.0) |
| Past | 15 (62.5) | 8 (57.1) | 7 (70.0) |
| Pack years | 23 [14–45] | 22 [8–45.8] | 23 [14–45] |
| Comorbidities‡ | 16 (66.7) | 14 (100.0) | 10 (100.0) |
| Myocardial infarction | 3 (12.5) | 8 (57.1) | 8 (80.0) |
| Peripheral arterial disease | 3 (12.5) | 1 (7.1) | 2 (20.0) |
| Hypertension | 3 (12.5) | – | 3 (30.0) |
| Cerebral vascular accident | 4 (16.7) | 3 (21.4) | 3 (30.0) |
| Chronic pulmonary disease | 10 (41.7) | 5 (35.7) | 1 (10.0) |
| Mild liver disease | 2 (8.3) | – | 5 (50.0) |
| Diabetes Mellitus | 2 (8.3) | 2 (14.3) | 2 (20.0) |
| Renal insufficiency | 1 (4.2) | – | 1 (10.0) |
| Solid tumour in past | 4 (16.7) | 1 (7.1) | 1 (10.0) |
| Leukemia | 1 (4.2) | 1 (7.1) | 3 (30.0) |
| Respiratory function | |||
| FVC (L) | 3.5 (3.3) | 3.5 (1.1) | 3.6 (0.9) |
| FEV1 (L) | 2.6 (0.9) | 2.4 (1.0) | 2.7 (0.7) |
| FEV1 (%predicted) | 89.6 (20.1) | 86.1 (20.8) | 94.4 (19.2) |
| COPD GOLD (%) | 7 (29.2) | 5 (35.7) | 2 (20.0) |
| I | 6 (85.7) | 4 (80.0) | 2 (100.0) |
| II | 1 (14.3) | 1 (20.0) | – |
| AJCC staging (%) | |||
| IAI | 1 (4.2) | 1 (7.1) | – |
| IAII | 6 (25.0) | 4 (28.6) | 2 (20.0) |
| IAIII | 2 (8.3) | – | 2 (20.0) |
| IB | 6 (25.0) | 5 (35.7) | 1 (10.0) |
| IIA | 4 (16.7) | 1 (7.1) | 3 (30.0) |
| IIB | 2 (8.3) | 1 (7.1) | 1 (10.0) |
| IIIA | 2 (8.3) | 1 (7.1) | 1 (10.0) |
| IIIB | 1 (4.2) | 1 (7.1) | – |
| Operation procedure | |||
| Lobectomy | 20 (83.3) | 12 (85.7) | 8 (80.0) |
| Bilobectomy | 3 (12.5) | 1 (7.1) | 2 (20.0) |
| Segment resection | 1 (4.2) | 1 (7.1) | – |
| Operation side | |||
| Left | 10 (41.7) | 8 (57.1) | 2 (20.0) |
| Right | 14 (58.3) | 6 (42.9) | 8 (80.0) |
| Duration of surgery (minutes) | 165.2 (56.9) | 184 (55.2) | 126 (140.3) |
Data are presented as median [IQR], n (%), or mean (SD). †, smoking at T0; ‡, number of patients with comorbidities. T0: baseline. NSCLC, non-small cell lung cancer; ASz, Albert Schweitzer Hospital; MMC, Maxima Medical Centre; ASA, American Society for Anesthesiologists; BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; AJCC, American Joint Committee on Cancer; IQR, interquartile range; SD, standard deviation.
Primary outcomes
Feasibility
All included patients completed the programme, of whom 23 patients (95.8%) completed a sufficient (>80% of goals reached) programme (Table 1).
Completion rate of the exercise programme was 70.8% (n=17) for both supervised HIIT and strength exercises, with a mean amount of supervised exercises of 5.2±2.2. Completion rate of LIT was 75.0% (n=18).
Completion rate of nutritional support was 54.2% (n=13) for protein supplementation and 91.7% (n=22) for vitamin supplementation. Completion rate of psychological support was 95.8% (n=23), whereas one patient refused consultation. No follow-up sessions were deemed necessary.
Smoking cessation resulted in a rate of 95.8% (n=23) at T3, smokers and non-smokers combined. A smoking cessation programme was started in five out of six active smokers (83.3%); the other patients stopped by themself. One patient was enrolled in the Sinefuma cessation programme but did not start it due to no financial reimbursement and failed to stop smoking.
Patient empowerment had a completion rate of 100% (n=24) and psychological support had a completion rate of 100% (n=24).
For optimisation of respiratory status, all patients (100%, n=24) had baseline testing and instructions on how to use the IMT device. Completion rate of IMT was 95.8% (n=23).
Functional capacity
Overall, the second primary outcome, functional capacity, resulted in statistically significant preoperative progression (T1–T0) with regard to SRT, 6MWT, 1RM, and MIP (Figure 2). With regard to 1RM LP, 1RM LR, and 1RM CP the programme also resulted in statistically significant improved postoperative functional capacity compared with baseline (T2–T0). No statistically significant differences were found for HGS.

Secondary outcomes
Time frame of prehabilitation
In total, 83.3% (n=20) of the patients had surgery within the timeframe of the DLCA-S criteria (ASz: 71.4% and MMC: 100%). In ASz and MMC, 20 patients (ASz: 10, MMC: 10) had surgery within 3 or 5 weeks in case of mediastinoscopy. In ASz, six patients had a mediastinoscopy prior to surgery compared to one in MMC. In four patients in ASz, surgery was scheduled 4 weeks after MDT for reasons other than prehabilitation. Timeframe of the logistics of the programme is shown in Table S1. Main differences between hospitals were time MDT to surgery and the time T0–T1.
Surgical outcomes
A total of 15 complications were seen in 12 patients (50%), of which five were major in 5 patients (33.3%). Median CCI score was 4.3 [0–20.9], median LoS was 4 days [3–8] and 4 patients (16.7%) were readmitted within 30 days. Mortality rate was 0% (Table 3).
Table 3
| Surgical outcomes | Total group (n=24) | ASz (n=14) | MMC (n=10) |
|---|---|---|---|
| CCI | 4.3 [0–20.9] | 0 [0–20.9] | 8.7 [6.5–28] |
| Complications† | n=15 | n=5 | n=10 |
| Total patients | 12 (50.0) | 4 (28.6) | 8 (80.0) |
| Minor | 10 (66.7) | 3 (60.0) | 7 (70.0) |
| Major | 5 (33.3) | 2 (40.0) | 3 (30.0) |
| Type of complications‡ | n=15 | n=5 | n=10 |
| Persistent air leakage | 4 (26.7) | 1 (20.0) | 3 (30.0) |
| Atrial fibrillation | 3 (20.0) | – | 3 (30.0) |
| Urinary tract infection | 1 (6.7) | – | 1 (10.0) |
| Pneumonia | 2 (13.3) | 1 (20.0) | 1 (10.0) |
| Meralgia paresthetica after operation | 1 (6.7) | – | 1 (10.0) |
| Infection other | 1 (6.7) | 1 (20.0) | – |
| Other§ | 3 (20.0) | 2 (40.0) | 1 (10.0) |
| Re-intervention | 4 (16.7) | 1 (7.1) | 3 (30.0) |
| Length of hospital stay¶ (days) | 4 [3–8] | 3 [2–8] | 5 [3–9] |
| Readmissionǁ | 4 (16.7) | 1 (7.1) | 3 (30.0) |
| Mortality | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Data are presented as median [IQR] or n (%). †, number of patients with any type of postoperative complications as defined by Clavien-Dindo (minor: Clavien-Dindo <3a, major >3a); ‡, type of complications: 15 complications in 12 patients; §, other complications: exacerbation COPD, COVID-19 infection, electrolyte dysfunction; ¶, length of hospital stay in days; ǁ, readmission within 30 days after surgery. NSCLC, non-small cell lung cancer; ASz, Albert Schweitzer Hospital; MMC, Maxima Medical Centre; CCI, comprehensive complication index (ranging from 0 to 100); IQR, interquartile range; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019.
Patient satisfaction and usefulness
Median patient satisfaction about the program on a 5-point scale was 5 [4–5] and usefulness was graded 5 [5–5] at T1. At T3, patient satisfaction was 4 [4–5] and usefulness was 5 [4–5].
Discussion
In this pilot study, we found that it is feasible to conduct a multimodal prehabilitation programme for patients with NSCLC undergoing elective lung surgery. We identified that the logistics of the programme complied with the timeframe of the DLCA-S which is 3–5 weeks, and patients were satisfied at the end of the programme and 3 months after surgery. Moreover, we observed improved preoperative functional capacity after completion of the prehabilitation programme and sustained improved postoperative muscle strength.
Feasibility
To the best of our knowledge, this is the first study to implement a multimodal prehabilitation programme consisting of six interventions, within a preoperative timeframe of 3 weeks in NSCLC patients. Barriers we encountered in the programme were with planning and logistics of the program, but also with the missing data of self-reporting components in the daily diary such as nutritional consumption and LIT exercise. Nevertheless, overall in 95.8% of the patients it proved feasible to complete at least 80% of the interventions sufficiently. Recent literature showed that with an increase in number of interventions, it is less likely that patients will comply with all of them (22). van Wijk (22) showed a compliance rate of 33.3% for a prehabilitation programme consisting out of three interventions, whereas it was up to 90% in case of only one exercise intervention (23). Our multimodal prehabilitation programme with six interventions and a feasibility rate of 95.8% is higher than other successful prehabilitation programmes with fewer interventions (22,23). Moreover, 83.3% (n=20) of our patients had surgery within the 3 or 5 weeks in case of a mediastinoscopy as obliged by the DCLA-S criteria; in the remaining patients the criteria were exceeded due to reasons other than prehabilitation. Therefore, the extensive prehabilitation programme can be completed within this short time frame. However, completion rates in protein supplementation and training were relatively low compared to the other interventions. Reasons for the low completion rate included: no filled in dairies on protein- and vitamin supplementation but also in problems of planning the training sessions within the timeframe. Due to the multimodal aspect of this intervention program, which is conducted in a short time period, we strongly advise reserving time slots for prehabilitation patients and preferably appointing a prehabilitation coordinator in hospitals implementing prehabilitation.
Functional capacity
Although our pilot study was not powered to confirm that a multimodal prehabilitation programme results in improved functional capacity, our favourable results do point in that direction. All SRT, 6MWT, MIP, and 1RM tests improved significantly preoperatively (T1–T0). The 6MWT, a test commonly used to assess improvement in functional capacity, improved with 20.1 m (P=0.045) which is considered a clinically relevant improvement (24). We also demonstrated significant improvements on postoperative 1RM LP, 1RM CP, and 1RM LR (T2–T0), suggesting that prehabilitation works protective in maintaining muscle strength postoperatively. Nevertheless, our findings should be interpreted with caution, due to a possible learning effect in retesting. The significant postoperative decrease in the aerobic tests SRT and 6MWT when compared to baseline (T2–T0) were rather expected due to the loss in functional lung capacity as a result of parenchyma resection (25), although it should be kept in mind that no training sessions were held postoperatively. A sufficient functional capacity is of utmost importance in the postoperative period and patients with low postoperative functional capacity should be assessed for rehabilitation. In order to definitely assess the beneficial effect of prehabilitation on functional capacity, both preoperative and postoperatively, large cohort studies are necessary.
Surgical outcome
Prehabilitation has proven to decrease postoperative complications in colorectal (6) and lung surgery (9,12), yet patient groups in lung surgery are small. For translating this positive trend into implementation of prehabilitation, more studies with emphasis on complications are necessary. The complication rate of 50% [predominantly minor, median CCI 4.3 (IQR, 0–20.9)] in this study was relatively high but firm conclusions cannot be made due to the relatively small sample size (2) high rate of comorbidities (66.7%) and ASA-III score (33.3%).
Limitations
Our study was a pilot study to assess feasibility of our programme within the short timeframe of 3 weeks, but not powered on demonstrating improvements in functional capacity. Therefore, the favourable results on this outcome should be interpreted with caution. Especially, since patients were not willing to participate in the control group, limiting the option to analyse differences in functional capacity between groups.
Another limitation lies in a potential selection bias. Patients who are willing to exercise are more prone to participate than patients who are not willing to exercise, which could have influenced the results on functional capacity. Nevertheless, 28 patients out of 42 patients (66.7%) who met the inclusion criteria participated in the study (Figure 1).
However, our study had specific requirements for participation, which should be kept in mind when defining the generalizability of the results. The 42 out of 131 screened patients seems low, however the main reasons for not participating were study-related, such as: other hospitals, no NSCLC surgery or participation in other trials. Due to the limited time available in lung cancer surgery we chose to prove feasibility in a specific type of lung cancer patients and offer training in hospital to assess safety. However, after implementation the number of patients participating in prehabilitation will increase, since these factors could possibly be no longer of influence. Possible solutions for offering prehabilitation to more patients could be offering training in physiotherapy practices in the neighbourhood and appointing a coordinator to optimise the preoperative care pathway, including prehabilitation. Future studies should focus on offering prehabilitation to every patient planned for lung cancer surgery to increase participation rate, so the value of prehabilitation in lung surgery can be determined.
Additionally, while conducting the study, we made some alterations from the original protocol (13). Although we designed our study including a control group to test for functional capacity without multimodal prehabilitation, only three patients were willing to join the control group. After the prehabilitation group was filled, we found it unethical to only offer participation in the control group and since the number of patients was too low to report any relevant findings, we decided to exclude these patients from our study. When we enrolled patients in the Sinefuma cessation program, one patient’s insurance did not cover the costs of the smoking cessation programme after which the patient continued smoking. We considered any form of smoking cessation at T3 to be feasible, because we believe that financial imbursements should not play a role in smoking cessation.
The last limitation of this study was the COVID-19 pandemic, resulting in a temporary stop in inclusion. During the COVID-19 pandemic the hospitals aimed on acute care, resulting in barriers for conducting clinical research and restrictions on activities that potentially expose patients to a risk of contracting COVID-19. Also, all elective care was postponed, resulting in two delayed surgery dates in ASz, thereby exceeding the DLCA-S criteria.
Conclusions
We found a completion rate of 95.8% for a six-pillar multimodal prehabilitation programme prior to surgical resection of NSCLC without compromising the DLCA-S criteria. Furthermore, our programme may improve preoperative functional capacity and score high on patient satisfaction and usefulness. We strongly advise the appointment of a coordinator for a structured planning of the lung perioperative pathway, including prehabilitation. In the future, large cohort studies into multimodal prehabilitation in lung surgery are necessary to provide evidence on optimal programme design, functional capacity, complication reduction, and shortened LoS.
Acknowledgments
This work was supported by the Dutch Prehab Lung Research Group. All authors are members of the Dutch Prehab Lung Research Group. Also, the following co-authors are members of the research group: Goof Schep, PhD (sports physician, MMC); Magdolen Youssef-El Soud, MSc (pulmonologist, MMC); Loes Janssen, PhD (research coordinator, MMC); Loes van de Voort, BSc (physiotherapist, MMC); Nicky Rademakers, MSc (physiotherapist, MMC); Chris de Jongh, MSc (physiotherapist, FYSIOOOO); Cathrin van Erven, MSc (dietitian, MMC); Carlijn de Betue, PhD (surgeon, ASz); Eric van Thiel, MSc (pulmonologist, ASz); Fleur van Tour (research nurse, ASz); Sanne Hoornweg (physiotherapist, ASz); Frank de Kort (physiotherapist, ASz); Mirjam Staffeleu-Noodelijk (dietitian, ASz); Els Driessen, MSc (clinical psychologist, ASz); Marieke van de Wal, MSc (clinical psychologist, MMC); Netty de Graaff, MSc (master advanced nursing practice, ASz); and Anouk van Limpt, MSc (nurse specialist lung cancer, MMC).
Funding: This work was supported by FrieslandCampina by providing protein supplements. ASz used a hospital subsidy received to support scientific research, to finance aspects of this trial.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1929/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1929/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1929/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1929/coif). M.S.B. received stipendium, speerpuntengelden from the Albert Schweitzer ziekenhuis. E.M.v.M. received speaker fees and financial support for the Organization of Educational Events from Johnson & Johnson. G.M.H.M. received the stipendium from the Albert Schweitzer Hospital Fund. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Central Committee on Research Involving Human Subjects (CCMO) (number NL70578.015.19, reference number of the Medical Ethical Review Committee of Maxima Medical Centre: W19.045). Informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Salati M, Brunelli A, Decaluwe H, et al. Report from the European Society of Thoracic Surgeons Database 2017: patterns of care and perioperative outcomes of surgery for malignant lung neoplasm. Eur J Cardiothorac Surg 2017;52:1041-8. [Crossref] [PubMed]
- Wang S, Li X, Li Y, et al. The long-term impact of postoperative pulmonary complications after video-assisted thoracic surgery lobectomy for lung cancer. J Thorac Dis 2017;9:5143-52. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Molenaar CJL, Minnella EM, Coca-Martinez M, et al. Effect of Multimodal Prehabilitation on Reducing Postoperative Complications and Enhancing Functional Capacity Following Colorectal Cancer Surgery: The PREHAB Randomized Clinical Trial. JAMA Surg 2023;158:572-81. [Crossref] [PubMed]
- Barberan-Garcia A, Ubré M, Roca J, et al. Personalised Prehabilitation in High-risk Patients Undergoing Elective Major Abdominal Surgery: A Randomized Blinded Controlled Trial. Ann Surg 2018;267:50-6. [Crossref] [PubMed]
- West MA, Asher R, Browning M, et al. Validation of preoperative cardiopulmonary exercise testing-derived variables to predict in-hospital morbidity after major colorectal surgery. Br J Surg 2016;103:744-52. [Crossref] [PubMed]
- Mahendran K, Naidu B. The key questions in rehabilitation in thoracic surgery. J Thorac Dis 2018;10:S924-30. [Crossref] [PubMed]
- Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database Syst Rev 2017;6:CD012020. [Crossref] [PubMed]
- Ten Berge M, Beck N, Heineman DJ, et al. Dutch Lung Surgery Audit: A National Audit Comprising Lung and Thoracic Surgery Patients. Ann Thorac Surg 2018;106:390-7. [Crossref] [PubMed]
- Voorn MJJ, Franssen RFW, Hoogeboom TJ, et al. Evidence base for exercise prehabilitation suggests favourable outcomes for patients undergoing surgery for non-small cell lung cancer despite being of low therapeutic quality: a systematic review and meta-analysis. Eur J Surg Oncol 2023;49:879-94. [Crossref] [PubMed]
- Ferreira V, Minnella EM, Awasthi R, et al. Multimodal Prehabilitation for Lung Cancer Surgery: A Randomized Controlled Trial. Ann Thorac Surg 2021;112:1600-8. [Crossref] [PubMed]
- Molenaar CJL, Von Meyenfeldt EM, de Betue CTI, et al. Multimodal prehabilitation in patients with non-small cell lung cancer undergoing anatomical resection: protocol of a non-randomised feasibility study. Perioper Med (Lond) 2023;12:41. [Crossref] [PubMed]
- ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. [Crossref] [PubMed]
- Meyer K, Samek L, Schwaibold M, et al. Physical responses to different modes of interval exercise in patients with chronic heart failure--application to exercise training. Eur Heart J 1996;17:1040-7. [Crossref] [PubMed]
- Reynolds JM, Gordon TJ, Robergs RA. Prediction of one repetition maximum strength from multiple repetition maximum testing and anthropometry. J Strength Cond Res 2006;20:584-92. [PubMed]
- Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996;12:S15-9. [Crossref] [PubMed]
- Weggemans RM, Kromhout D, van Weel C. New dietary reference values vitamin D. Ned Tijdschr Geneeskd 2012;156:A5565. [PubMed]
SineFuma - Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Slankamenac K, Graf R, Barkun J, et al. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1-7. [Crossref] [PubMed]
- van Wijk L, van der Snee L, Buis CI, et al. A prospective cohort study evaluating screening and assessment of six modifiable risk factors in HPB cancer patients and compliance to recommended prehabilitation interventions. Perioper Med (Lond) 2021;10:5. [Crossref] [PubMed]
- Bojesen RD, Jørgensen LB, Grube C, et al. Fit for Surgery-feasibility of short-course multimodal individualized prehabilitation in high-risk frail colon cancer patients prior to surgery. Pilot Feasibility Stud 2022;8:11. [Crossref] [PubMed]
- Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract 2017;23:377-81. [Crossref] [PubMed]
- Kushibe K, Kawaguchi T, Kimura M, et al. Changes in ventilatory capacity, exercise capacity, and pulmonary blood flow after lobectomy in patients with lung cancer--which lobectomy has the most loss in exercise capacity? Interact Cardiovasc Thorac Surg 2008;7:1011-4. [Crossref] [PubMed]


