
Integrated endoscopic treatment of primitive unresectable tracheal tumor: the INTACT retrospective cohort study
Highlight box
Key findings
• The integration of interventional bronchoscopy with radiotherapy to relieve airway obstruction, may achieve long-term survival benefits in patients suffering from unresectable tracheal tumor with adenoid cystic carcinoma (ACC) histology.
What is known and what is new?
• Primitive tracheal tumors represent a rare entity whose management remains challenging. Interventional pulmonology techniques are usually considered to have a role in palliation for patients suffering from unresectable tumors, or in emergency therapy prior to definitive treatment in patients with acute respiratory distress due to central airway obstruction.
• However, the real impact on patients’ survival of an integration of interventional pulmonology technique with adjuvant radiotherapy in unresectable patients is not known.
What is the implication, and what should change now?
• Our study suggests that the integration of interventional bronchoscopy with radiotherapy to relieve airway obstruction may achieve long-term survival benefits in patients suffering from unresectable tracheal tumor with ACC histology (80% 5-year survival rate).
Introduction
Primary tracheal tumors are rare entity, constituting approximately 0.1–0.4% of malignant diseases (1). Although most of tracheal tumors affecting adults are malignant (90%), in children these tumors appear even rarer and mostly consist of benign diseases (only 10–30% of malignant tumors). Malignant disease of the trachea can arise from different structures such as respiratory epithelium, salivary glands, connective tissue and others, but from a histological point of view, squamous cell carcinoma (SCC) and adenoid cystic carcinoma (ACC) account for about two-thirds of all the adult primary tracheal tumors (2). Symptoms are typically due to central airway obstruction, and appear late, when the tumor has already involved most of the tracheal lumen (3). Timely diagnosis remains a major clinical challenge to improve patients’ survival. Indeed, symptoms are often mistaken for those of other respiratory diseases such as asthma or chronic obstructive pulmonary disease (COPD), resulting in diagnosis delay, when the disease is at an advanced stage and sometimes beyond the scope of curative treatment (4). Surgical resection supplemented by postoperative radiotherapy are the treatment of choice to achieve long-term survival and relieve airway obstruction (5). Patients not suitable for surgery are managed with definitive radiation therapy, although ACCs are typically less radiosensitive as compared with other primitive tracheal tumors (namely SCCs) (6). Interventional pulmonology techniques, such as intraluminal debulking of the tumor supported by laser therapy with or without stent, are usually considered to have a role in palliation for patients suffering from unresectable tumors, or in a setting of emergency therapy prior to definitive treatment in patients with acute respiratory distress due to significant central airway obstruction (3). Retrospective national analysis conducted in England on management and prognosis of primary tracheal cancer, suggest that performing interventional bronchoscopy by itself is an independent and significant survival variable (7). However, due to the lack of significant data on this topic, due to different biological behavior across the different histotypes, the real impact on patients’ survival of an integration of interventional pulmonology technique with adjuvant radiotherapy in unresectable patients is not known. Being that the extension of the disease to adjacent organs, the size of the tumor at the time of diagnosis, and the health of the patients not rarely rule out the possibility of surgery, data that can define the role of operative bronchoscopy integrated with radiotherapy in the definitive treatment of tracheal cancer are needed. The primary explorative aim of this retrospective analysis was to report the 5-year survival rate of patients with primitive tracheal tumor undergoing interventional bronchoscopy plus chemotherapy/radiotherapy (integrated treatment). Factors influencing prognosis were further investigated as secondary aim. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-738/rc).
Methods
Study design
INTACT is a retrospective, monocentric cohort study carried out at the Diagnostic and Interventional Bronchoscopy Unit of the University Hospital of Modena (Italy). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Local Ethics Committee “Area Vasta Emilia Nord” (Prot. AOU 0013040/19 and 276/2019/OSS/AOUMO) and consent to publish data were obtained from all the patients.
Population and measures
To build the study population we queried the local registry of the Bronchoscopy Unit of the University Hospital of Modena (Italy) where clinical, endoscopic, and radiological data of patients consecutively referred since January 2010 are collected. Inclusion criteria were applied to the time frame January 2010–2022 and were as follows: age >18 years, cytologic and/or histologic diagnosis of primitive tracheal tumor. All patients had undergone flexible bronchoscopy whose reports were considered, alongside computed tomography (CT) scan images, in order to confirm primitive tracheal tumors. Patients were excluded if aged >80 years, performance status ≤2 and/or with end-stage chronic obstructive pulmonary disease, interstitial lung disease, life-threatening stenosis requiring urgent endoscopy, open surgery treatment received.
Chart review, health record, medical record, archival data analysis was further performed. The following data were collected: demographic data, Charlson Index for comorbidity assessment, histopathology, genetic analysis of the tumor (EGFR and KRAS mutations, ALK translocations), programmed cell death ligand 1 (PD-L1) expression, type of anticancer treatment received (chemotherapy, radiotherapy, tyrosine kinase inhibitors, immunotherapy), complications of endoscopic treatment, 5-year survival. All interventional procedures were performed in the operating room with a Dumon rigid bronchoscope (Efer Medical, La Ciotat, Cedex, France) under general anesthesia. Neodymium-doped yttrium aluminium garnet (Nd-YAG) laser photoresection (KLS Martin, Diode-pumped Nd: YAG laser Limax®, Germany) was performed at 15–30 watts and pulse duration of 0.5–1.0 s. Whenever indicated, a silicone stent (NOVATECH Doumon stents, Boston Medical Products, Inc., Westborough, MA, USA) was placed. The choice of silicon stents instead of self-expandable metal stents (SEMS) was due to the expertise of our center. Indeed, the use of SEMS has been associated with higher complication rate when removal is required (8). All patients with primitive tracheal tumor with airway stenosis >50% were treated with interventional bronchoscopy before radiotherapy treatment (60 Gy) independently from the onset of symptoms. All disease recurrences with airway stenosis >50% were treated with endoscopic re-intervention. Performance status was assessed by Eastern Cooperative Oncology Group (ECOG) score (9).
Statistical analysis
Baseline and clinical characteristics of the enrolled patients were described as a whole and according to the 5-year survival status. Continuous variables were described by means of median and interquartile range (IQR), whereas categorical variables were described as number and percentage.
The 5-year survival analysis was performed with participants’ follow-up accrued from the date of diagnosis until death. The association between demographic and clinical characteristics with 5-year survival was tested using univariable and multivariable logistic regression model. Significance was set for P values <0.05. In post-hoc sensitivity analyses, the 5-year survival was assessed according to histology, development of distal metastasis, silicone stent positioning and the onset of relapse and displayed by means of Kaplan-Meier curves. In another sensitivity analysis the impact of cystic-adenoid histology and stent positioning on 5-year relapse free survival was assessed through Kaplan-Meier estimates. Statistics were performed using SPSS version 25.0 with PSMATCHING3 R Extension command (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 8.0 (GraphPad Software, Inc., La Jolla, CA, USA) unless otherwise indicated.
Results
Population
A total amount of 13,792 patients were referred to the Bronchoscopy Unit of the University Hospital of Modena (Italy) within the pre-specified period of interest. Out of them, 20 (0.15%) presented with primitive tracheal tumor and 12 (0.09%). resulted eligible. Study flow-chart is shown in Figure 1. The median follow-up from diagnosis was 27 (IQR, 4–60) months.
Demographics, clinical features, and oncological therapies are presented in Table 1. At 5 years, 5 patients (42%) were alive while 7 (58%) were dead. Two out of 5 patients who survived presented expression of mTOR (one associated to androgen receptors) while one patient expressed c-KIT. Among those who were dead at 5 years, 2 patients expressed PD-L1 (one associated to ROS1), one patient showed the expression of Ki67/MIB and one patient had K-RAS mutated. The two groups did not show differences in terms of demographic characteristics and modality of standard treatment (Table 1). Survivors showed a higher prevalence of cystic-adenoid histology (80% vs. 14%), while patients who were dead at 5 years were those with a more advanced T (prevalence of T2: 71% vs. 0%) and a lower response to first line treatment (57% vs. 0%). Treatment complications accounted for stent dislocation (33%) and the onset of granuloma (18%) and were not different among groups. Non major complications (i.e., massive bleeding and/or stent occlusion and/or fistulas) were reported.
Table 1
| Variable | Overall (n=12) | Alive at 5 years (n=5) | Death at 5 years (n=7) |
|---|---|---|---|
| Age at diagnosis, years | 62 [49–67.3] | 50 [46–61] | 63 [55–71] |
| Performance status, ECOG | 1 [1–2] | 1 [1–2] | 1 [1–2] |
| Male sex | 6 [50] | 2 [40] | 4 [57] |
| Smoker status | |||
| Never | 5 [42] | 3 [60] | 2 [29] |
| Former | 5 [42] | 2 [40] | 3 [43] |
| Active | 2 [17] | 0 | 3 [43] |
| Histology | |||
| Cystic-adenoid carcinoma | 5 [42] | 4 [80] | 1 [14] |
| Squamous cell carcinoma | 3 [24] | 1 [20] | 2 [29] |
| Muco-epidermoid carcinoma | 2 [17] | 0 | 2 [29] |
| Sarcomatous | 1 [8] | 0 | 1 [14] |
| Epithelioid angiosarcoma | 1 [8] | 0 | 1 [14] |
| cT | |||
| cT0 o cTIS | 0 | 0 | 0 |
| cT1 | 4 [33] | 3 [60] | 1 [14] |
| cT2 | 5 [42] | 0 | 5 [71] |
| cT3 | 3 [25] | 2 [40] | 1 [14] |
| E | |||
| E1 | 3 [25] | 2 [40] | 1 [14] |
| E2 | 2 [17] | 0 | 2 [29] |
| E3 | 7 [58] | 3 [60] | 4 [57] |
| cN | |||
| N0 | 4 [33] | 3 [60] | 1 [14] |
| N1 | 4 [33] | 1 [20] | 3 [43] |
| N2 | 4 [33] | 1 [20] | 3 [43] |
| M | |||
| M0 | 9 [76] | 3 [60] | 6 [86] |
| M1 | 3 [25] | 2 [40] | 1 [14] |
| Carinal involvement | 4 [33] | 1 [20] | 3 [43] |
| Treatment | |||
| Stent placement | 9 [75] | 3 [60] | 6 [86] |
| RT | 12 [100] | 5 [100] | 7 [100] |
| CHT | 10 [83] | 4 [80] | 6 [86] |
| Radiotherapy | |||
| Total dosage, Grey | 62 [40–70] | 60 [40–68] | 62 [50–70] |
| Application | 2 [1–2] | 2 [1–2] | 2 [1–2] |
| No. of endoscopic treatment | 2 [1–3] | 3 [2–5] | 2 [1–2.5] |
| Complications | |||
| Granuloma | 2 [17] | 1 [20] | 1 [14] |
| Stent dislocation | 4 [33] | 2 [40] | 2 [29] |
| Stent occlusion | 0 | 0 | 0 |
| Major endotracheal bleeding | 0 | 0 | 0 |
| Tracheal injury/fistulation | 0 | 0 | 0 |
| CT response after treatment | |||
| Disease reduction | 4 [33] | 3 [60] | 1 [14] |
| Stable disease | 4 [33] | 2 [40] | 2 [29] |
| Disease progression | 4 [33] | 0 | 4 [57] |
| Relapse | 10 [83] | 3 [60] | 7 [100] |
| Time to relapse, months | 3 [2.3–16.8] | 18 [15.5–20] | 3 [1.5–3] |
| Survival time, months | 26.7 [4.1–82] | 91.3 [78.3–93] | 4.6 [2.7–8.1] |
Data are presented as number and percentage for dichotomous values or median and IQR for continuous values. IQR, interquartile range; CT, computed tomography; E, extension; RT, radiotherapy; CHT, chemotherapy; ECOG, Eastern Cooperative Oncology Group.
Survival analysis
Five-year survival for the whole population is illustrated in Figure S1 by means of Kaplan-Meier curves. Five-year survival rate was 42% (5/12). Table 2 reports the demographic and clinical features associated with 5-years mortality. Among analysed variables, only the presence of cystic-adenoid histology resulted independently associated with survival [odds ratio (OR) =0.1, P=0.04].
Table 2
| Parameter | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age at diagnosis, years | 0.9 | 0.98–1.28 | 0.15 | ||||
| Male sex | 2 | 0.25–16.3 | 0.99 | ||||
| Smoker status | |||||||
| Never | 0.33 | 0.03–1.97 | 0.29 | ||||
| Former | 1.1 | 0.12–7.49 | 0.99 | ||||
| Active | 8 | 0.56–43.1 | 0.23 | ||||
| Histology | |||||||
| Cystic-adenoid carcinoma | 0.04 | 0.003–0.88 | 0.07 | 0.1 | 0.019–0.98 | 0.04 | |
| Squamous cell carcinoma | 1.6 | 0.14–28.4 | 0.90 | ||||
| Other | 12 | 1.1–72 | 0.08 | ||||
| cT | |||||||
| cT0 o cTIS | – | – | – | ||||
| cT1 | 0.1 | 0.01–1.36 | 0.22 | ||||
| cT2 | 21 | 1.6–154 | 0.03 | ||||
| cT3 | 0.25 | 0.02–3.19 | 0.52 | ||||
| E | |||||||
| E1 | 1.25 | 0.02–3.19 | 0.52 | ||||
| E2 | 4.5 | 0.34–21.3 | 0.47 | ||||
| E3 | 0.89 | 0.1–7.11 | 0.99 | ||||
| cN | |||||||
| N0 | 0.11 | 0.01–1.36 | 0.22 | ||||
| N1 | 3 | 0.29–47.4 | 0.58 | ||||
| N2 | 3 | 0.29–47.4 | 0.58 | ||||
| M | |||||||
| M0 | 4 | 0.31–66 | 0.52 | ||||
| M1 | 0.25 | 0.02–3.19 | 0.52 | ||||
| Carinal involvement | 3 | 0.29–47.4 | 0.58 | ||||
| Treatment | |||||||
| Stent placement | 4 | 0.31–66 | 0.52 | ||||
| RT | – | – | – | ||||
| CHT | 1.5 | 0.06–32.7 | 0.99 | ||||
| CT response after treatment | |||||||
| Disease reduction | 0.11 | 0.01–1.36 | 0.22 | ||||
| Stable disease | 0.6 | 0.07–5.69 | 0.99 | ||||
| Disease progression | 14 | 1.1–72 | 0.08 | ||||
| Relapse | 4.5 | 0.72–51 | 0.15 | ||||
| Time to relapse | 0.75 | 0.25–0.92 | 0.01 | ||||
CT, computed tomography; RT, radiotherapy; CHT, chemotherapy; OR, odds ratio; CI, confidence interval.
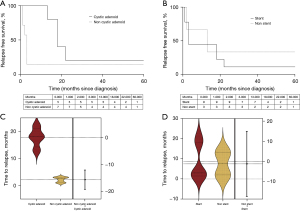
Figure 2 illustrates the 5-year survival according to histology (Figure 2A), the development of distal metastasis (Figure 2B), the endoscopic placement of stent (Figure 2C) and the disease relapse within follow-up (Figure 2D). The presence of cystic-adenoid histology resulted in significantly improved 5-year survival rate (80% versus 14%). The onset of distal metastasis and the placement of tracheal stent did not result significantly associated with lower survival. The survival rate among those who did not experience disease relapse was 100%.

Figure 3 illustrates the relapse-free survival time (Figure 3A,3B) and the median time to the first relapse (Figure 3C,3D) according to histology and endotracheal stent placement. Since diagnosis, the relapse-free survival was not different between patients with cystic adenoid tracheal tumor and those with other histology while the time to the first disease relapse was higher in those with cystic adenoid histology [18 (IQR, 14.3–21) vs. 2.5 (IQR, 1–3) months]. Stent placement did not affect relapse-free survival at 5 years nor the time to the first disease relapse [3 (IQR, 1.5–18) vs. 7.5 (IQR, 2–13) months].
Discussion
Despite limitations, this study suggests that the integration of interventional bronchoscopy with radiotherapy may achieve long-term survival benefits in patients suffering from unresectable tracheal tumor with ACC histology (80% 5-years survival rate). Furthermore, endoscopic tumor resection, can be repeated in case of local recurrence to maintain airway patency without significant side effects.
Treatment options for primitive tracheal tumors: rationale for interventional endoscopic techniques
Primary tracheal cancers are rare subset of tumors, therefore studies investigating the management of this disease are retrospective and analyze small patient populations (10). The low incidence of tracheal tumors has obviated prospective studies to evaluate and compare therapy. However, tracheal cancer surgical resection constitutes the gold standard to achieve long term survival. A retrospective study by Gaissert et al. showed that resection of trachea or carina is associated with long-term survival when compared to primary radiotherapy and/or palliation, particularly for patients with complete resection, negative airway margins, and ACC (11). The reported overall operative mortality was 7.3% and improved each decade from 21% to 3%, probably due to improvements in surgical techniques (11). The benefit of surgical treatment is less clear when resection is incomplete, while the presence of lymph nodes metastases did not seem to decrease survival after surgery (12,13). Furthermore, in ACC for its peculiar behavior characterized by more indolent but progressive local growth, resection provides excellent palliation even in patients with distant metastases (14). However, tumor size at the time of diagnosis, patient’s clinical conditions and comorbidities may limit the possibility of surgery, thus a considerable proportion of patients cannot benefit from the treatment of choice and are treated with definitive radiotherapy (15). Definitive radiotherapy showed a survival benefit when compared to palliative treatment, especially in patients with SCC histology (6). However, study by Xie et al. showed a 5-year overall survival (OS) of 22.7% in patients treated with definitive radiotherapy for unresectable tracheal tumor versus 55% in patients underwent surgery plus radiotherapy (6). Studies focused only on unresectable ACCs, a histotype considered poorly radiosensitive, showed 5-year OS ranged between 17% to 56% after definitive radiotherapy, with significant worse outcome when compared to patients underwent surgery (16-18). More recently, retrospective data from French and Germany studies show no significant difference between operated and non-operated patients with ACCs, with 5-year OR of 82–92% after definitive radiotherapy (19,20). However, local relapse was observed mainly in non-operated patients, raises the question of combining radiosensitising agent such as platinum-based combination, or interventional endoscopic technique to control intraluminal tumor growth. In our series, none of the patients died due to tracheal obstruction. This result might be due to the tight follow-up and early re-intervention.
Interventional bronchoscopy in patients with ACC histology
Given that ACCs are generally considered chemoresistant, and target therapies currently have no indication in localized tumor, interventional bronchoscopy with endoscopic resection can be a rational treatment to obtain a debulking of the endocanalicular tumor, both before definitive radiotherapy and in case of local recurrence. In our series, interventional bronchoscopy techniques (with or without stent placement) were performed in all patients with >50% obstruction of the airway lumen before definitive radiotherapy regardless of symptoms, therefore outside palliative proposal. Being considered palliative, endoscopic intervention is often performed when significant symptoms are present, and the degree of obstruction is very severe; thus, the risks of the intervention can be relevant. Indeed, computational fluid dynamic study showed that the pressure drops trough airway stenosis, the physical mechanism behind the dyspnea, increase dramatically only if >70% of the tracheal lumen was occluded (21). The present study suggests that integration of interventional bronchoscopy plus radiotherapy may have a significant impact on survival in patients suffering from ACCs compared to other primary tracheal cancer treated with the same modality. This result can certainly be attributed to the biological features of ACC, which is generally considered a low-grade malignant tumor despite its ability to metastasize and recur locally. However, all patients with ACC enrolled in the present study required multiple endoscopic procedures for local recurrence during the 5-year follow-up, despite definitive radiotherapy treatment with radiation doses over 60 Gy. This finding may suggest that local control of recurrence by interventional bronchoscopic technique may play a significant role in the survival gain in ACC patients, justifying the high OS found in our study. Furthermore, maintain airway patency may play a key role in life expectancy also in ACC patients affected by distal metastasis, considering the slowly progression of the extra-tracheal spread of the disease, which occurs often over several years. It could be surprising that patients of our series with distal metastasis displayed longer survival time. However, we should consider that 2 out of 3 (66%) of them presented ACC histology that is associated with slow progression even when distal metastases occur. Another result suggested of this study concerns the low impact of tracheal stenting both on 5-year survival and on disease recurrence. SPOC trial was the first randomized controlled trial investigated the potential benefit of silicone stent insertion in symptomatic malignant airway obstruction due to non-small cell lung cancer without extrinsic compression (22). In the SPOC trial silicon stent clearly reduced relapses by offering a “barrier effect”, but there was no significant impact on survival. The results found in our series suggest that the poor effect of stent placement on recurrence may be due to the different biological behavior of primary tracheal tumors. Indeed, ACC spread through direct extension, submucosal or perineural invasion, often recurring even after surgery. Thus, it is probable that through these local spreading mechanisms ACC may invalidate the “barrier effect” of stent placement. Furthermore, in more aggressive histotypes of primary tracheal tumors, stent may not be sufficient to stem the rapid growth of the tumor within the airways.
Complications and side effects
Our series showed that the onset of complications after multimodal treatment was not significant. The main side effect was represented by stent dislocation (33%) that was not associated with respiratory failure or tracheal occlusion and was successfully managed with re-intervention. Moreover, major complications such as massive tracheal bleeding, fistulas or tracheal injury were not reported.
Limitations
Our study has several limits. First, the retrospective design, the reduced sample and the lack of a control group represent major limitations and definitive conclusions on the analyzed treatment cannot be drawn. Second, patients have been treated in center with high expertise in interventional pulmonology, therefore the validity of data cannot be extrapolated for all. Notwithstanding, our results suggest that interventional bronchoscopy should be considered early as an integral part of management of patients suffering from unresectable primitive tracheal tumors; and should be also re-propose in case of recurrence, being burdened by very few side effects. However, considering the rarity of tracheal tumors, multicenter or nationwide study is warranted to validate these finding in non-resectable tracheal tumors.
Conclusions
Multimodal treatment including interventional bronchoscopy and associated radiotherapy for unresectable primary tracheal tumors seems not burdened by significant complication and may provide benefits in terms of survival for those patients with ACC histology. These results require confirmation in larger series.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-738/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-738/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-738/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-738/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Local Ethics Committee “Area Vasta Emilia Nord” (Prot. AOU 0013040/19 and 276/2019/OSS/AOUMO) and consent to publish data were obtained from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Macchiarini P. Primary tracheal tumours. Lancet Oncol 2006;7:83-91. [Crossref] [PubMed]
- Junker K. Pathology of tracheal tumors. Thorac Surg Clin 2014;24:7-11. [Crossref] [PubMed]
- Sherani K, Vakil A, Dodhia C, et al. Malignant tracheal tumors: a review of current diagnostic and management strategies. Curr Opin Pulm Med 2015;21:322-6. [Crossref] [PubMed]
- Bhattacharyya N. Contemporary staging and prognosis for primary tracheal malignancies: a population-based analysis. Otolaryngol Head Neck Surg 2004;131:639-42. [Crossref] [PubMed]
- Behringer D, Könemann S, Hecker E. Treatment approaches to primary tracheal cancer. Thorac Surg Clin 2014;24:73-6. [Crossref] [PubMed]
- Xie L, Fan M, Sheets NC, et al. The use of radiation therapy appears to improve outcome in patients with malignant primary tracheal tumors: a SEER-based analysis. Int J Radiat Oncol Biol Phys 2012;84:464-70. [Crossref] [PubMed]
- Nouraei SM, Middleton SE, Nouraei SA, et al. Management and prognosis of primary tracheal cancer: a national analysis. Laryngoscope 2014;124:145-50. [Crossref] [PubMed]
- Jeong BH, Ng J, Jeong SH, et al. Clinical Outcomes of Complications Following Self-Expandable Metallic Stent Insertion for Benign Tracheobronchial Stenosis. Medicina (Kaunas) 2020;56:367. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Marchioni A, Andrisani D, Tonelli R, et al. Integrated intErventional bronchoscopy in the treatment of locally adVanced non-small lung cancER with central Malignant airway Obstructions: a multicentric REtrospective study (EVERMORE). Lung Cancer 2020;148:40-7. [Crossref] [PubMed]
- Gaissert HA, Grillo HC, Shadmehr MB, et al. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg 2004;78:1889-96; discussion 1896-7. [Crossref] [PubMed]
- Grillo HC, Mathisen DJ. Primary tracheal tumors: treatment and results. Ann Thorac Surg 1990;49:69-77. [Crossref] [PubMed]
- Regnard JF, Fourquier P, Levasseur P. Results and prognostic factors in resections of primary tracheal tumors: a multicenter retrospective study. The French Society of Cardiovascular Surgery. J Thorac Cardiovasc Surg 1996;111:808-13; discussion 813-4. [Crossref] [PubMed]
- Maziak DE. Biology of Adenoid Cystic Carcinoma of the Tracheobronchial Tree and Principles of Management. Thorac Surg Clin 2018;28:145-8. [Crossref] [PubMed]
- He J, Shen J, Huang J, et al. Prognosis of primary tracheal tumor: A population-based analysis. J Surg Oncol 2017;115:1004-10. [Crossref] [PubMed]
- Mendenhall WM, Morris CG, Amdur RJ, et al. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck 2004;26:154-62. [Crossref] [PubMed]
- van Weert S, Bloemena E, van der Waal I, et al. Adenoid cystic carcinoma of the head and neck: a single-center analysis of 105 consecutive cases over a 30-year period. Oral Oncol 2013;49:824-9. [Crossref] [PubMed]
- Le Péchoux C, Baldeyrou P, Ferreira I, et al. Thoracic adenoid cystic carcinomas. Cancer Radiother 2005;9:358-61. [PubMed]
- Levy A, Omeiri A, Fadel E, et al. Radiotherapy for Tracheal-Bronchial Cystic Adenoid Carcinomas. Clin Oncol (R Coll Radiol) 2018;30:39-46. [Crossref] [PubMed]
- Högerle BA, Lasitschka F, Muley T, et al. Primary adenoid cystic carcinoma of the trachea: clinical outcome of 38 patients after interdisciplinary treatment in a single institution. Radiat Oncol 2019;14:117. [Crossref] [PubMed]
- Brouns M, Jayaraju ST, Lacor C, et al. Tracheal stenosis: a flow dynamics study. J Appl Physiol (1985) 2007;102:1178-84. [Crossref] [PubMed]
- Dutau H, Di Palma F, Thibout Y, et al. Impact of Silicone Stent Placement in Symptomatic Airway Obstruction due to Non-Small Cell Lung Cancer - A French Multicenter Randomized Controlled Study: The SPOC Trial. Respiration 2020;99:344-52. [Crossref] [PubMed]



