
Feasibility of the modified mini-bronchoalveolar lavage techniques using a nasogastric suction catheter and polytetrafluoroethylene bronchoscopic suction catheter in diagnosing bilateral pneumonia: a pilot study
Highlight box
Key findings
• In this pilot study, mini-bronchoalveolar lavage (BAL) using different catheters is superior to endotracheal aspirate according to microbial yield and diagnosis of polymicrobial bilateral pneumonia. Mini-BAL catheter with larger diameter such as nasogastric (NG) tube yield more return fluid than polytetrafluoroethylene (PTFE) catheter but no difference in diagnostic yield.
What is known and what is new?
• Mini-BAL is an alternative technique in collecting lower respiratory tract specimen and has shown higher diagnostic yield than endotracheal aspirate in diagnosing ventilator associated pneumonia (VAP). However, different properties of mini-BAL catheter have never been compared.
• Our pilot study showed that mini-BAL using PTFE catheter could reach to the lung periphery better than NG tube. Larger diameter of mini-BAL using NG tube yield more return fluid. However, the diagnostic yield was not significantly different. Mini-BAL has potential to diagnose more cases of polymicrobial VAP, leading to appropriate antibiotic prescription.
What is the implication, and what should change now?
• According to the high mortality rate in VAP, using mini-BAL as an initial diagnostic test could guide appropriate antibiotic prescriptions. Moreover, modified mini-BAL is easy to perform, cost-effective, and has few complications.
Introduction
Pneumonia is a condition commonly encountered in medical practice, particularly among patients requiring mechanical ventilation. Approximately 5–40% of patients on mechanical ventilation for >48 h develop this condition (1). Its diagnosis is based on the criteria established by Johanson, which include clinical suspicion of pneumonia accompanied by an infiltrate on chest radiography and at least one of the following: leukocytosis (>12,100 cells/cumm3) or leukopenia (<4,100 cells/cumm3), fever (>38.3 ℃) or hypothermia (36 ℃), or the presence of purulent tracheobronchial secretions (1,2). Currently, according to the International European Respiratory Society (ERS)/European Society of Intensive Care Medicine (ESICM)/European Society of Clinical Microbiology and Infectious Diseases (ESCMID)/Asociación Latinoamericana del Tórax (ALAT) 2017 guidelines, specimens must be collected from the lower respiratory tract before antibiotic treatment. This then allows for an appropriate adjustment of antibiotic therapy based on the culture results, thereby reducing the unnecessary use of antibiotics (3). The Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) 2014 guideline recommends that collecting a high-quality Gram stain from a respiratory specimen can diagnose pneumonia and facilitate testing for antibiotic susceptibility (4). There are several methods for collecting respiratory tract samples, including invasive techniques [e.g., bronchoscopic bronchoalveolar lavage (BAL)] and noninvasive ones (i.e., blind mini-BAL) (1,4). However, the current medical practice guidelines recommend using noninvasive sampling with semiquantitative cultures to diagnose pneumonia. In addition, several research studies have shown no significant difference between the sampling results obtained via bronchoscopy compared with noninvasive techniques such as mini-BAL (5-9). In Thailand, the use of bronchoscopy for diagnosing pneumonia is limited due to the shortage of pulmonologists. Hence, endotracheal aspiration is commonly used. However, the procedure is associated with a risk of bacterial contamination in the upper respiratory tract (10,11). Thus, data interpretation and antibiotic therapy adjustment becomes challenging, and this phenomenon eventually affects treatment efficacy.
The mini-BAL catheter comprises a suction catheter that can be inserted into the distal bronchus. Thus, an actual lower respiratory tract specimen can be collected. This type of device is commercially available as a prefabricated set product (Combi Cath), which is expensive and not available in Thailand. In the past, a modified mini-BAL catheter has been developed and used as an alternative (12,13). However, small-scale studies have used nasogastric (NG) tubes and bronchoscopic suction catheters. Their outcomes were compared with those of bronchoscopic BAL, and results showed no significant differences (13,14).
The properties of the mini-BAL catheter may directly affect the quality of the collected specimen. NG tubes or in-line suction catheters are relatively soft. Thus, there is an increased risk of bending in the proximal airway, and the catheter cannot reach the distal bronchus. Therefore, a polytetrafluoroethylene (PTFE) catheter, which is a bronchoscopic suction catheter that is more rigid, has a smaller diameter, and is longer, was applied. This device can help access the distal airway during mini-BAL procedures to obtain a specimen from the lower respiratory tract with more confidence. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1573/rc).
Methods
Study design
This prospective open-label pilot study enrolled patients with pneumonia on mechanical ventilators in the internal medicine ward and intensive care unit (ICU) from April 2021 to November 2021. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Vajira Hospital (COA 094/2565). An informed consent was obtained from patients’ legal representatives.
Participants
The inclusion criteria were as follows: (I) patients aged over 18 years old, (II) those who require mechanical ventilation, and (III) those clinically suspected of pneumonia and abnormal chest radiography images consistent with bilateral pneumonia. The exclusion criteria were as follows: (I) patients who required bronchoscopy examination; (II) those with a high intracranial pressure; (III) those with an increased risk of bleeding, such as low platelet count (<50,000 cells/cumm3) or coagulopathy with an international normalized ratio of >1.5; (IV) those with pneumothorax; and (V) those with confirmed coronavirus disease 2019 infection. The criteria for discontinuing the procedures were (I) severe hypoxemia with oxygen saturation levels of <90% during the procedure and (II) severe tachycardia or arrhythmia with a heart rate of >130 beats per minute during the procedure.
Procedures
Prior to the study session, physicians were already familiar with mini-BAL using NG tube while the PTFE catheter was improvised technique. The researcher was trained to perform mini-BAL using both catheters. We do portable chest X-ray immediately after catheter insertion in some patients to assure proper catheter position placed in the distal airway (Figures S1,S2).
Patients diagnosed with bilateral pneumonia requiring mechanical ventilation were included in this study. Initially, endotracheal aspiration was collected for ventilator associated pneumonia (VAP) diagnosis according to the discretion of the attending physician.
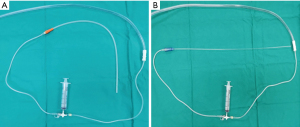
Mini-BAL was performed within 24 h after endotracheal aspiration using both methods in a randomized sequence. After completing the first technique, the second technique was immediately used unless there were any complications. The mini-BAL was performed using a NG tube suction catheter with a diameter of 4×4 mm and a length of 50 cm. The catheter was inserted gently until resistance was felt. Then, 20 mL of sterile 0.9% NaCl solution was instilled through the catheter five times. Then, aspiration of the lavage fluid was collected for laboratory analysis. The process of performing mini-BAL using a PTFE catheter is similar, except that a catheter made of polytetrafluoroethylene with a diameter of 2×2.7 mm and a length of 60 cm (Figure 1) is used. Each technique was performed for no more than 2 min. All samples were submitted to our microbiology laboratory. Samples were cultured and were used for quantifying bacterial load. Bacterial identification was performed using standard microbiologic techniques. The growths were expressed as a number of colony-forming units (CFU)/mL. The threshold applied to quantitative cultures for diagnosing VAP was 104 CFU/mL for non-bronchoscopic BAL.
During the procedure, vital signs such as blood pressure, heart rate, and oxygen saturation were monitored. The termination criteria are described above. The retrieved fluid was sent for cell count and differential count. Bacterial culture and other additional microbiologic studies were performed according to the discretion of the attending physician.
Outcomes
The primary outcomes were characteristics of return fluid, return fluid volume, white blood cell (WBC) count, and culture results using the modified mini-BAL techniques using an NG tube and PETF bronchoscopic suction catheter for diagnosing bilateral pneumonia compared with endotracheal aspiration. The secondary outcomes were the safety and complications of the modified mini-BAL techniques.
Statistical analysis
Quantitative data such as baseline characteristic of the participants including age, body mass index (BMI), Acute Physiology and Chronic Health Evaluation II score, mechanical ventilation duration before mini-BAL, partial pressure of oxygen in arterial blood/fraction of inspiratory oxygen concentration ratio, volume of return fluid, cell count, and cell differentiation were expressed as either means, standard deviations, or medians and interquartile ranges, based on data appropriateness. Qualitative data including sex, underlying diseases, and identified pathogens were expressed as frequency distributions and percentages. The cut-off point for significant growths was 104 CFU/mL in both procedures.
The accuracy of endotracheal aspiration and mini-BAL for diagnosing pneumonia was evaluated based on their sensitivity, specificity, accuracy, positive predictive value, and negative predictive value, with corresponding 95% confidence intervals (CIs). Cohen’s Kappa coefficient could be used to assess the agreement between the two methods for diagnosing pneumonia. Complications from both endotracheal aspiration and mini-BAL were expressed as frequency distribution and percentages. Statistical analyses were performed using Stata version 13.0 (StataCorp, College Station, TX, USA), and a P value of 0.05 was considered statistically significant.
Results
The study included a sample group with 40 patients. All patients provided written informed consent. The patients were randomly assigned at a 1:1 ratio to the mini-BAL using an NG tube and then to the mini-BAL with a PTFE catheter (Figure 2).

Baseline characteristics of the participants
Approximately 52.5% of patients were men, and their average age was 72.32±14.51 years. The average BMI was 23.09±5.18 kg/m2. The coexisting conditions included hypertension (72.5%), hyperlipidemia (50%), diabetes mellitus (35%), chronic kidney disease (17.5%), old cerebrovascular accident (CVA) (17.5%), coronary artery disease (10%), chronic obstructive pulmonary disease (7.5%), liver disease (5%), asthma (2.5%), and other comorbidities (57.5%). The median Acute Physiology and Chronic Health Evaluation II score was 11 [interquartile range (IQR): 10–18]. The median mechanical ventilation duration before mini-BAL was 8 days (IQR: 3–16 days), and the median partial pressure of oxygen in arterial blood/fraction of inspiratory oxygen concentration ratio was 301.5 (IQR: 215–401). Area of involvement on chest radiography was observed in Q1 at 70%, Q2 at 47.5%, Q3 at 95%, and Q4 at 97.5% (see Figure S3). The sputum characteristics were as follows: purulent (60%), watery (22.5%), mucoid (12.5%), and blood-stained (5%).
Clinical and laboratory findings
The mini-BAL lavage using an NG tube and the mini-BAL lavage using a PETF catheter were successful during the first attempt in 100% of cases. The median return fluid volume was 50 mL (IQR: 40–70 mL) in the mini-BAL lavage using an NG tube and 40 mL (IQR: 30–60 mL) in the mini-BAL lavage using an PETF catheter (P=0.001). The median WBC counts from the mini-BAL lavage using an NG tube and PTFE catheter were 245 cells/cumm3 (IQR: 67–2,060 cells/cumm3) and 305 cells/cumm3 (IQR: 52–610 cells/cumm3), respectively (P=0.03). The median polymorphonuclear cell (PMN)% from the mini-BAL lavage using an NG tube and PTFE catheter were 83.5% (IQR: 62–95%) and 89% (IQR: 75–96%), respectively (P=0.70). The retrieved fluid volume from the mini-BAL lavage using an NG tube and PTFE catheter was subjected to Gram staining, with 45.0% and 47.5% of positive Gram stain, respectively. In terms of culture results, the specimens obtained from the mini-BAL lavage using an NG tube and PTFE catheter tested positive for 77.5% and 75.0% bacterial growths, respectively (P=0.56) (Table 1).
Table 1
| Variables | NG | PTFE | P value |
|---|---|---|---|
| Attempt | |||
| First | 40 (100.0) | 40 (100.0) | NA |
| Returned fluid (mL) | 50 (40–70) | 40 (30–60) | 0.001† |
| WBC count (cells/cumm3) | 245 (67–2,060) | 305 (52–610) | 0.03† |
| PMN (%) | 84 (62–95) | 89 (75–96) | 0.70† |
| Lymphocyte (%) | 13 (3.5–31.5) | 5 (3–13) | 0.11† |
| Eosinophil (%) | 0 (0–0) | 0 (0–0) | 0.66† |
| Mononuclear (%) | 0 (0–2.5) | 0 (0–2) | 0.98† |
| Gram stain | 18 (45.0) | 19 (47.5) | >0.99‡ |
| AFB | 1 (2.5) | 1 (2.5) | >0.99‡ |
| mAFB | 0 (0.0) | 0 (0.0) | NA |
| Bacterial culture | 0.56‡ | ||
| Positive | 31 (77.5) | 30 (75.0) | |
| Negative | 7 (17.5) | 8 (20.0) | |
| Normal flora | 2 (5.0) | 2 (5.0) |
Data are presented as number (%) or mean (interquartile range). †, Wilcoxon-signed rank test; ‡, McNemar’s test. BAL, bronchoalveolar lavage; NG, nasogastric; PTFE, polytetrafluoroethylene; NA, data not applicable; WBC, white blood cell; PMN, polymorphonuclear cell; AFB, acid fast bacilli; mAFB, modified acid fast bacilli.
Microorganism growth in mini-BAL
Endotracheal aspiration revealed a positive culture in 70% of cases, with monomicrobial growth in 50% of cases and polymicrobial growth with ≥2 microorganisms in 20% of cases. The microorganisms cultured included Acinetobacter baumannii (37.5%), Burkholderia cepacia (5%), Candida albicans (5%), Candida tropicalis (2.5%), Enterobacter cloacae (2.5%), Escherichia coli (5%), Klebsiella pneumoniae (5%), Pseudomonas aeruginosa (17.5%), Serratia marcescens (2.5%), Staphylococcus aureus (2.5%), and Stenotrophomonas maltophilia (7.5%).
Mini-BAL using an NG tube had a positive culture rate of 77.5%, with 30% of the cultures being monomicrobial and 47.5% being polymicrobial. The microorganisms identified included Acinetobacter baumannii (40%), Burkholderia cepacia (10%), Candida albicans (12.5%), Candida tropicalis (10%), Chryseobacterium gleum (2.5%), Elizabethkingia meningoseptica (2.5%), Enterobacter cloacae (2.5%), Escherichia coli (2.5%), Klebsiella pneumoniae (10%), Providencia stuartii (2.5%), Pseudomonas aeruginosa (20%), Serratia marcescens (2.5%), Staphylococcus aureus (2.5%), and Stenotrophomonas maltophilia (15%). Mini-BAL using a PTFE bronchoscopic suction catheter was found to be positive in 75% of cases. Approximately 30% of positive cases were identified as monomicrobial infections. Meanwhile, 45% were polymicrobial. The identified microorganisms included Acinetobacter baumannii (40%), Burkholderia cepacia (10%), Candida albicans (10%), Candida tropicalis (12.5%), Chryseobacterium gleum (2.5%), Elizabethkingia meningoseptica (2.5%), Enterobacter cloacae (5%), Escherichia coli (5%), Klebsiella oxytoca (2.5%), Klebsiella pneumoniae (7.5%), Providencia stuartii (2.5%), Pseudomonas aeruginosa (17.5%), Serratia marcescens (2.5%), Staphylococcus aureus (2.5%), and Stenotrophomonas maltophilia (15%) (see Table S1). There were six cases with discordant results across mini-BAL methods (see Table S2).
There were 6 patients (15%) that endotracheal aspiration were not grow but showed significant growth in mini-BAL specimens. Three of them were Candida species, one with P.aeruginosa, one with A.baumanii, and one with methicillin sensitive S.aureus. However, three patients with Candida spp. were considered to be colonized. The rest were already prescribed broad spectrum antibiotic which are active against identified organism. So, this rarely led to the change of the antibiotic regimen.
Accuracy of pneumonia diagnosis
Using endotracheal aspiration as the current standard for VAP diagnosis, the sensitivity values of the NG tube and PETF groups were 89.3% (95% CI: 71.8–97.7%) and 89.3% (95% CI: 71.8–97.7%). Further, the specificity values were 44.4% (95% CI: 13.7–78.8%) in the NG tube group and 55.6% (95% CI: 21.2–86.3%) in the PTFE group. The positive predictive value of the NG tube group was 83.3% (95% CI: 65.3–94.4%), and the negative predictive value of the NG tube group was 57.1% (95% CI: 18.4–90.1%). The positive predictive value and the negative predictive value of the PTFE group were 86.2% (95% CI: 68.3–96.1%) and 62.5% (95% CI: 24.5–91.5%), respectively (Table 2).
Table 2
| Test | Sensitivity (95% CI) (%) |
Specificity (95% CI) (%) |
PPV (95% CI) (%) |
NPV (95% CI) (%) |
Kappa (95% CI) |
Agreement (%) |
|---|---|---|---|---|---|---|
| NG tube | 89.3 (71.8–97.7) | 44.4 (13.7–78.8) | 83.3 (65.3–94.4) | 57.1 (18.4–90.1) | 0.365 (0.012–0.718) | 78.4 |
| PTFE | 89.3 (71.8–97.7) | 55.6 (21.2–86.3) | 86.2 (68.3–96.1) | 62.5 (24.5–91.5) | 0.466 (0.129–0.803) | 81.1 |
CI, confident interval; PPV, positive predictive value; NPV, negative predictive value; NG, nasogastric; PTFE, polytetrafluoroethylene.
To evaluate consistency in bacterial growth with endotracheal aspirate, the concordance rates of the NG tube and PETF groups were 78.4% and 81.1%, with a Kappa coefficient index of 0.365 (95% CI: 0.012–0.718) and 0.466 (95% CI: 0.129–0.803), respectively.
Safety
Only 8 of 40 participants experienced mild complications, which included arrhythmia (2.5%), mild hypoxemia (2.5%), and mild bleeding (5.0%). All these complications did not cause procedure termination. The use of NG tube resulted in mild hypoxemia in 2.5% of cases. Meanwhile, the use of a PTFE catheter resulted in arrhythmia in 2.5% of cases and mild bleeding in 5.0% of cases (Table 3).
Table 3
| Variables | All, n (%) | Endotracheal aspiration, n (%) | NG tube, n (%) | PTFE, n (%) | P value† | P value‡ | P value§ |
|---|---|---|---|---|---|---|---|
| Complication | 4 (10.0) | 0 (0.0) | 1 (2.5) | 3 (7.5) | >0.99 | 0.24 | 0.61 |
| Arrhythmia | 1 (2.5) | 0 (0.0) | 0 (0.0) | 1 (2.5) | NA | >0.99 | >0.99 |
| Mild hypoxemia | 1 (2.5) | 0 (0.0) | 1 (2.5) | 0 (0.0) | >0.99 | NA | >0.99 |
| Mild bleeding | 2 (5.0) | 0 (0.0) | 0 (0.0) | 2 (5.0) | NA | 0.49 | 0.49 |
P value corresponds to Fisher’s exact test. †, endotracheal aspiration versus NG; ‡, endotracheal aspiration versus PTFE; §, NG versus PTFE. BAL, bronchoalveolar lavage; NG, nasogastric; PTFE, polytetrafluoroethylene; NA, data not applicable.
Discussion
Previous study used bronchoscopic BAL as the gold standard (15). Mini-BAL is safe and effective in diagnosing VAP and is considered an alternative to bronchoscopic BAL particularly in resource-limited settings. Nowadays, there was no standardized protocol for performing mini-BAL, and the techniques varied among different studies. We hypothesized that the use of a catheter that can go further to the distal part of the tracheobronchial tree such as the PTFE catheter, rather than the modified mini-BAL using an NG tube, can collect actual lower respiratory tract specimens. This hypothesis was confirmed based on the placement of the catheter tip on immediate chest radiography after catheter insertion (see Figures S1,S2). Our study was based on the recommendations of the ATS/IDSA guidelines. That is, endotracheal aspiration, a noninvasive method for microbial investigation in VAP, should be used as a standard reference for VAP diagnosis. Results showed that the mini-BAL technique is safe and easy to perform. This can be used widely and is not necessarily limited to pulmonologists. Furthermore, the smaller diameter of mini-BAL catheter compared to standard bronchoscope (4.9 mm) can avoid high airway pressure and patient distress. There was no need for additional sedative medication. The mini-BAL technique using an NG tube catheter had a greater return fluid volume than the mini-BAL using a PTFE catheter, probably due to the larger diameter of the NG tube. The PTFE group had a significantly higher WBC count and absolute neutrophil count. Hence, actual lower respiratory tract specimens represent the inflammatory process in the pneumonia-affected lung. However, there was no significant difference in terms of microbial yield. Of note, the number of polymicrobial organisms detected via mini-BAL is relatively higher than that of endotracheal aspiration isolates. Based on the study of Natarajan, the incidence of polymicrobial VAP was 62.9% (15). This could influence the appropriate selection of empirical antibiotics in VAP. The mini-BAL techniques had discordant microbial results. This could be explained by the sampling error for the catheter tip that was not confirmed via imaging before BAL. However, most participants had bilateral dependent infiltrates that the catheter could frequently go into. Our study showed that the catheter used in mini-BAL affected the quality of specimens in terms of return fluid volume. The catheter with a greater diameter, such as the NG tube, could yield a higher return fluid volume. Moreover, the catheter with a smaller diameter that could get to a more distal tracheobronchial tree was not superior to the NG tube in terms of microbial diagnosis. With multilobular involvement in VAP, a broad sampling technique such as mini-BAL with the NG tube may be more appropriate than sampling in small and selected areas (16). Additionally, the effectiveness of gram staining in mini-BAL is typically lower than endotracheal aspirate due to dilution with water. Indeed, in this study, the detection rate of gram staining for mini-BAL was 45% of NG tube group and 47.5% in PTFE group which was much higher than endotracheal aspirate (17). Our study had several limitations. First, bronchoscopic BAL was not used as the reference standard as bronchoscopy is rarely performed in diagnosing VAP in our settings. Previous research has found that bronchoscopy and mini-BAL have a similar yield in diagnosing bilateral pneumonia (5-7,9,14,17). We used endotracheal aspirates in the initial microbial investigation and guidance of empirical antibiotic. Our study was in accordance with a prior preliminary study showing that mini-BAL was used to diagnose a significantly higher percentage of bacterial pneumonias than nasotracheal suctioning (12). Second, we do not ascertain laterality of the mini-BAL catheter in each patient. As the subjects in this study had bilateral pneumonia, though desired, it may not be very important to know the laterality of the sampling site. However, it implies that our study cannot be extrapolated to patients with unilateral pneumonia. Third, mini-BAL was not immediately collected after endotracheal aspiration, and the microbial results could be affected after the initiation of antibiotics. However, despite the lag time between specimen collection and antibiotics, the microbial results showed a significant growth of organisms, and most of them were correlated with endotracheal aspirates. Fourth, we cannot rule out proximal bronchial contamination since we do not use protected brush specimen and the initial return fluid was not discarded. These proximal secretions might have grown on the culture due to airway colonization and even increased the proportion of neutrophils compared with an actual BAL sample (17). Fifth, both mini-BAL methods were sequentially performed in the same patient, which could affect the results such as the volume of return fluid or the growth of microorganisms. To address this, a randomized crossover design was used, and no significant difference was found. Sixth, this was a pilot study with a small sample size.
However, it highlights the role of mini-BAL in the initial microbial investigation to improve VAP diagnosis particularly in polymicrobial VAP. Hence, our results should be applied to other studies with larger sample sizes in the future. In terms of safety, approximately 10% of patients experienced complications related to the mini-BAL procedure. Given that fewer risks are generally associated with the endotracheal aspiration technique, this risk in mini-BAL cannot be overlooked. However, the complications of mini-BAL are much less common compared to bronchoscopic BAL in critically ill patients.
Conclusions
Since the use of endotracheal aspirates can provide guidance in empirical antibiotic therapy, it is recommended in the initial microbial investigation of VAP based on the current guidelines. However, it has several limitations. Mini-BAL is less invasive, can provide a higher diagnostic yield, and can detect more polymicrobial VAP. Further, the modified mini-BAL catheter is flexible and noninjurious, has an appropriate diameter, and can be guided to distal tracheobronchial tree to cover multilobular area.
Acknowledgments
We thank ENAGO for the linguistic editing and proofreading of the manuscript.
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1573/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1573/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1573/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1573/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Vajira Hospital (COA 094/2565). Informed consent was obtained from all subjects involved in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Papazian L, Klompas M, Luyt CE. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med 2020;46:888-906. [Crossref] [PubMed]
- Fàbregas N, Ewig S, Torres A, et al. Clinical diagnosis of ventilator associated pneumonia revisited: comparative validation using immediate post-mortem lung biopsies. Thorax 1999;54:867-73. [Crossref] [PubMed]
- Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur Respir J 2017;50:1700582. [Crossref] [PubMed]
- Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-e111. [Crossref] [PubMed]
- Gurlevik A, Urkmez S, Utku T, et al. Comparison of Bronchoalveolar Lavage and Protected Mini- Bronchoalveolar Lavage in Diagnosis of Pneumonia in Intensive Care Unit. J Anesth Crit Care Open Access 2017;7:00256.
- Waghray P, Tummuru VR, Koteshwara Rao ANV, et al. Mini BAL vs bronchoscopic BAL in intubated patients in a tertiary care centre, Mahabubnagar, AP: Our experience. Apollo Medicine 2015;12:15-7. [Crossref]
- Tasbakan MS, Gurgun A, Basoglu OK, et al. Comparison of bronchoalveolar lavage and mini-bronchoalveolar lavage in the diagnosis of pneumonia in immunocompromised patients. Respiration 2011;81:229-35. [Crossref] [PubMed]
- Khilnani GC, Arafath TK, Hadda V, et al. Comparison of bronchoscopic and non-bronchoscopic techniques for diagnosis of ventilator associated pneumonia. Indian J Crit Care Med 2011;15:16-23. [Crossref] [PubMed]
- Afify MH, Shaheen EA, El-Dahdouh SS, et al. Comparison between bronchoscopic BAL and non-bronchoscopic BAL in patients with VAP. Egyptian Journal of Chest Diseases and Tuberculosis 2016;65:113-9. [Crossref]
- Koenig SM, Truwit JD. Ventilator-associated pneumonia: diagnosis, treatment, and prevention. Clin Microbiol Rev 2006;19:637-57. [Crossref] [PubMed]
- Meyer P, Rousseau H, Maillet JM, et al. Evaluation of blind nasotracheal suctioning and non-bronchoscopic mini-bronchoalveolar lavage in critically ill patients with infectious pneumonia: a preliminary study. Respir Care 2014;59:345-52. [Crossref] [PubMed]
- Schverdfinger S, Carboni Bisso I, Famiglietti R, et al. Tracheal aspirate with closed suction device: A modified technique developed during the COVID-19 pandemic. Acta Colombiana de Cuidado Intensivo 2021;21:292-7. [Crossref]
- Leo A, Galindo-Galindo J, Folch E, et al. Comparison of bronchoscopic bronchoalveolar lavage vs blind lavage with a modified nasogastric tube in the etiologic diagnosis of ventilator-associated pneumonia. Med Intensiva 2008;32:115-20. [Crossref] [PubMed]
- Hussain SM, Abubaker J, Ali M, et al. Comparison of quantitative bronchoscopic lavage cultures (B-BAL) with blind NG tube lavage (N-BAL) cultures in the diagnosis of ventilator associated pneumonia (VAP). J Coll Physicians Surg Pak 2009;19:245-8. [PubMed]
- Natarajan R, Ramanathan V, Sistla S. Poor Sensorium at the Time of Intubation Predicts Polymicrobial Ventilator Associated Pneumonia. Ther Clin Risk Manag 2022;18:125-33. [Crossref] [PubMed]
- Ioanas M, Ferrer R, Angrill J, et al. Microbial investigation in ventilator-associated pneumonia. Eur Respir J 2001;17:791-801. [Crossref] [PubMed]
- Morris AJ, Tanner DC, Reller LB. Rejection criteria for endotracheal aspirates from adults. J Clin Microbiol 1993;31:1027-9. [Crossref] [PubMed]


