
Interest of the Leicester Cough Questionnaire in predicting postoperatives complications after lung resection: LCQ-SURGE
Highlight box
Key findings
• The Leicester Cough Questionnaire (LCQ) is not associated with post-operatives complications after lung resection.
What is known and what is new?
• The LCQ assesses the quality of life related to chronic cough and consists of 19 questions distributed across 3 domains.
• Our findings indicate that a low LCQ score does not result in any more postoperative complications than a high score.
What is the implication, and what should change now?
• A decline in quality of life associated with chronic cough before surgery was observed in 21.4% of patients.
• Patients scheduled for surgery with preoperative chronic cough could undergo assessment using this questionnaire to anticipate potential postoperative challenges, including aspects such as quality of life and pain.
Introduction
Lung cancer, with approximately 2.2 million new cases and 1.8 million deaths, was the second most commonly diagnosed cancer and the leading cause of cancer-related deaths in 2020, accounting for about one in 5 (18%) deaths worldwide (1).
Lung resection surgeries such as segmentectomy, and lobectomy, are considered the best curative options for non-small cell lung cancer (NSCLC) at clinical stages I and II (Grade 1B recommendation) (2,3). The 5-year survival rate is estimated to be 40% with surgery compared to 17% without surgery (4).
Despite improvements in procedures, there is still a high rate of morbidity and mortality due to postoperative cardiopulmonary complications, with a 13% incidence of pulmonary complications (5,6).
Various tools allow for preoperative risk stratification, with the current gold standard being cardiopulmonary exercise testing (CPET), which assesses respiratory, cardiovascular, and muscular function during exercise (7,8). However, other more accessible field tests are also available to predict postoperative complications, such as the 6-minute stepper test (8).
Patients undergoing lung resection often have multiple comorbidities, such as respiratory disorders and chronic obstructive pulmonary disease (COPD), accompanied by chronic cough (9). Chronic cough, lasting more than 8 weeks, is a common symptom that can significantly impact patients’ quality of life. Chronic cough can be associated with underlying conditions such as asthma, gastroesophageal reflux, COPD, or bronchiectasis (10).
The evaluation of chronic cough can be done through self-administered questionnaires. There are several subjective cough assessment tools, such as the Visual Analogue Scale (VAS) and the Cough Symptom Score (CSS) (11). In 2003, an English team developed the Leicester Cough Questionnaire (LCQ), which takes into account the various aspects of cough impact, particularly on quality of life (12). The LCQ is a user-friendly, quick-to-complete, low-cost, and reproducible self-administered questionnaire that captures the impact of cough on quality of life over the previous 2 weeks (12). Studies have shown that the LCQ is valid, reliable, and sensitive in patients with chronic cough (12). Its reproducibility, internal reliability, and sensitivity have been demonstrated in various respiratory conditions, including bronchiectasis (13), COPD (14), and acute cough (15). In 2022, Wu et al. demonstrated the sensitivity of the LCQ to predict the onset of cough in patients after pulmonary resection (16). Cough is not systematically assessed in a pre-operative setting of lung surgery and has not been used in multivariable risk prediction scores such as the Thoracoscore.
The main objective of this study was to evaluate the relevance of preoperative LCQ to predict postoperative complications after major lung resection. We also assessed the utility of the Quality of Life Questionnaire for Lung Cancer 13 (QLQ-LC13), peak flow (PF) and cough peak flow (CPF) to predict postoperative complications as they are objective measurements of the obstructive syndrome that may be correlated to the subjective cough. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1324/rc).
Methods
Patient consent & ethics
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was conducted in accordance with the ethical standards of the institutional and national research committee: Cerni (Ethics committee for non-interventional research) (E2023-36). As this study was retrospective, patients were not informed that they were included in this specific study but had been informed that all their health data recorded in Rouen University Hospital health records could be re-used for future research studies, and had the right to oppose this data re-use, according to French law.
Study design/settings
This was a retrospective cohort study conducted in the Thoracic Surgery Department of Rouen University Hospital from November 21st, 2022, to June 2nd, 2023. We analyzed the association between the LCQ score and postoperative complications ≥2 at 30 days according to the Clavien-Dindo classification (16). We also collected the following data: QLQ-LC13 score, PF and CPF, cardiorespiratory history, forced expiratory volume in one second (FEV1), and World Health Organization (WHO) performance score (17). Data were collected after the reference test.
Eligibility
The inclusion criteria were patients aged ≥18 years who underwent major lung resection for any indications, including segmentectomy, lobectomy, bi-lobectomy, or pneumonectomy, and completed an LCQ self-administered questionnaire. The exclusion criterion was patients lost to follow-up after surgery discharge. Consecutive patients were identified during their hospitalization period, which took place from November 2022 to June 2023 in the Thoracic Surgery Department of the University Hospital Center of Rouen.
LCQ
The LCQ consists of 19 items divided into three domains: physical (8 questions), psychological (7 questions), and social (4 questions), with 7 response options for each question. The mean score is calculated for each domain, and the total score ranges from 3 to 21, with a higher score indicating better health status (12). The validated French version of the questionnaire was used in this study (18). The questionnaire was given to the patient before the surgical intervention, on the day before or the day of the operation, filled out manually, and collected by a physiotherapist. The choice of this questionnaire was based on its overall assessment of the impact of cough on quality of life as well as its reproducibility. Studies have demonstrated that this questionnaire is valid, reliable, and sensitive in patients with chronic cough (12). Other studies have focused on its reproducibility, internal reliability, and sensitivity in various respiratory conditions, including bronchiectasis (13), COPD (14), and acute cough (15). In their 2023 study, Reynolds et al. established normative values for the LCQ in a healthy population, with a score above 17.68 indicating no impairment in quality of life related to chronic cough (19).
QLQ-LC13
The QLQ-LC13 is an extension of the Quality of Life Questionnaire QLQ C30, specifically designed for lung cancer patients. It was published in 1994 by the European Organization for Research and Treatment of Cancer (EORTC) (20). It consists of 13 questions covering typical symptoms experienced by lung cancer patients, such as cough, pain, dyspnea, mouth sores, peripheral neuropathy, and hair loss, a low score indicates a better quality of life. In clinical research, the QLQ-LC13 is considered a standard instrument for measuring the quality of life in lung cancer patients (21). The French version of the questionnaire was used in this study. The QLQ-LC13 questionnaire was given to the patient along with the LCQ questionnaire on the day before the surgery in their hospital room. For patients admitted on the day of their surgery, the questionnaires were completed approximately two or three hours before the procedure in the outpatient surgery department. Patients filled out the questionnaires independently.
PF and CPF
PF is a simple measurement of the maximum flow that can be achieved during a forced expiration after a full inspiration. The PF meter used in this study was the Mini-Wright® (CCIL, Essex, United Kingdom) Peak Flow Meter with an adult cardboard mouthpiece. The measurement was taken preoperatively, on the day before or the day of the operation, with the assistance of a physiotherapist. The patient was placed in a seated position, and three consecutive measurements were taken, with the best of the three recorded. CPF was measured under the same conditions using the same equipment. The PF measurements were taken before the patient completed the questionnaires.
Data sources/measurement
The primary outcome measure was the severity of complications according to the Clavien-Dindo classification (22), collected at 30±10 days postoperatively. For each complication, we recorded the functional outcome and treatment administered to classify the morbidity. The same complication could have a different grade depending on the treatment administered. We also recorded postoperative complications occurring between the day of the operation and the 30-day follow-up visit. Data were collected from the patients’ electronic medical records.
Bias
To limit the risk of bias, patients filled the questionnaires by themselves the day before or a few hours before their operation. PF and CPF measurements were performed by a single physiotherapist at the time that the questionnaires were collected. Three measurements were taken, and the best of the three was recorded. The data from the electronic medical records were retrieved and analyzed by another physiotherapist.
Sample size calculation
No formal sample size calculation was performed before the study, but a power calculation was performed at the end of the study. Assuming a complication rate at 30%, a two-sided type I error rate at 5% for comparison of the area under the curve (AUC) at 0.50, a moderate area under the receiver operating characteristic (ROC) curve (AUC =0.70), the statistical power was estimated at 83%; for a weak area under the ROC curve (AUC =0.65), the statistical power was estimated at 55%.
Statistical analysis
Continuous data were presented as mean ± standard deviation, and categorical data were presented as frequency and percentage (%). The primary statistical analysis involved estimating the area under the ROC curve, using the De Long method with a 95% confidence interval (CI), for the LCQ score to predict postoperative complications of grade ≥2 within 30 days after surgery. Secondary analyses were also conducted to estimate the ROC curves for the associations between QLQ-LC13 scores, PF, CPF, and the prediction of postoperative complications of grade ≥2 within 30 days following the surgery. Associations between PF and LCQ score, as well as CPF and LCQ score, were analyzed with Pearson correlation coefficient. Associations between LCQ score and pleural drainage duration, as well as LCQ score and length of stay in the thoracic surgery department, were analyzed with the Spearman correlation coefficient.
Results
Patients’ characteristics and clinical data
Among 210 patients who had major lung resection at Rouen University Hospital between November 21st, 2022, and June 2nd, 2023, 71 were eligible for inclusion in the study. Finally, 70 patients were included because one patient was lost to follow-up upon hospital discharge (Figure 1). The measurement of PF was performed on the 70 patients in the cohort.
Continuous data are presented as mean ± standard deviation, and categorical data are presented as frequency and percentage (%).
Patients’ characteristics and clinical data are presented in Table 1. The mean age of patients at the time of surgery was 67.15 years (±8.76) (Table 1). Men accounted for 61.4% of the cohort (n=43). Among the patients, 70% were former smokers, and 14.3% were active smokers at the time of surgery. Approximately 51.4% of patients had a body mass index (BMI) greater than 25 kg/m2, with 24.3% falling into the category of moderate obesity. A minimally invasive approach was used in 94.3% of cases, with 25 patients undergoing video-assisted thoracoscopic surgery (VATS) and 41 patients undergoing robotic-assisted thoracoscopic surgery (RATS). Only 4 patients required conversion to thoracotomy. The mean forced expiratory volume in one second (FEV1) was 87.73% (±27.37%), and the mean diffusing capacity of the lung for carbon monoxide (DLCO) was 70.85% (±17.03%). The main comorbidities observed were hypertension 58.6% (n=41), previous history of cancer 42.9% (n=30), COPD (28.6%) and cardiac disease 28.6% (Table 2).
Table 1
| Variables | Value (N=70) |
|---|---|
| Sex | |
| Female | 27 (38.6) |
| Male | 43 (61.4) |
| Age (years) | 67.15±8.76 |
| Smoking status | |
| Active | 10 (14.3) |
| Former | 49 (70.0) |
| BMI (kg/m2) | |
| <18.5 | 2 (2.9) |
| 18.5–24.9 | 32 (45.7) |
| 25–30 | 19 (27.1) |
| >30 | 17 (24.3) |
| Disease (final pathology) | |
| Non-small cell lung cancer | 60 (85.7) |
| Benign tumor | 9 (12.9) |
| Emphysema | 1 (1.4) |
| Surgical approach | |
| VATS | 25 (35.7) |
| RATS | 41 (58.6) |
| Conversion | 4 (5.7) |
| Type of resection | |
| Segmentectomy | 32 (45.7) |
| Lobectomy | 38 (54.3) |
| Bi-lobectomy | 0 (0.0) |
| Pneumonectomy | 0 (0.0) |
| pTNM staging | |
| Non-cancer | 10 (14.3) |
| IA | 9 (12.9) |
| IB | 13 (18.6) |
| IC | 10 (14.3) |
| IIA | 19 (27.1) |
| IIB | 1 (1.4) |
| IIIA | 6 (8.6) |
| IIIB | 0 (0.0) |
| IV | 2 (2.9) |
| FEV1 (%) | 87.73±27.37 |
| DLCO (%) | 70.85±17.03 |
| Performance status WHO | |
| 0 | 60 (85.7) |
| 1 | 8 (11.4) |
| 2 | 2 (2.9) |
Values are expressed in n (%) or mean ± standard deviation. BMI, body mass index; VATS, video-assisted thoracoscopic surgery; RATS, robotic-assisted thoracoscopic surgery; pTNM, pathological tumor-node-metastasis; FEV1, forced expiratory volume in one second; DLCO, diffusing capacity of the lung for carbon monoxide; WHO, World Health Organization.
Table 2
| Variables | Value (N=70) |
|---|---|
| Hypertension | 41 (58.6) |
| History of cancer | 30 (42.9) |
| COPD | 20 (28.6) |
| Gold 1 | 2 (2.9) |
| Gold 2 | 11 (15.7) |
| Gold 3 | 6 (8.6) |
| Gold 4 | 1 (1.4) |
| Cardiac disease | 20 (28.6) |
| Hypercholesterolemia | 17 (24.3) |
| PAOD | 12 (17.1) |
| NIDD | 8 (11.4) |
| Other | 16 (22.9) |
Values are expressed in n (%). COPD, chronic obstructive pulmonary disease; PAOD, peripheral arterial occlusive disease; NIDD, non-insulin-dependent diabetes.
Variables of interest
Nineteen (27.1%) postoperative complications of grade ≥2 according to the Clavien-Dindo (21) classification were observed (Table 3). The results of the two questionnaires and PFs are reported in Table 3. The mean LCQ total score was 18.11±2.56, and the mean QLQ-LC13 score was 17.39±4.61.
Table 3
| Variables | Value (N=70) |
|---|---|
| Postoperative complications ≥2 | |
| No | 51 (72.9) |
| Yes | 19 (27.1) |
| LCQ score | |
| Total | 18.11±2.56 |
| Physical domain | 5.8±0.78 |
| Psychological domain | 5.98±0.91 |
| Social domain | 6.33±1.16 |
| QLQ-LC13 score | 17.39±4.61 |
| PF (L/min) | 349.71±123.34 |
| CPF (L/min) | 313.77±125.21 |
Values are expressed in n (%) or mean ± standard deviation. LCQ, Leicester Cough Questionnaire; QLQ-LC13, Quality of Life Questionnaire-Lung Cancer 13; PF, peak flow; CPF, cough peak flow.
The distribution of complications is reported in Table 4.
Table 4
| Grade | No. of subjects | Nature of complications |
|---|---|---|
| 0 | 35 | – |
| I | 16 | 7 pleural effusion, 5 prolonged air leak, 4 other |
| II | 7 | 6 pneumonia, 1 empyema |
| IIIa | 6 | 3 subcutaneous emphysema, 2 pneumothorax, 1 pleuresia |
| IIIb | 2 | 2 hemothorax |
| Iva | 2 | 2 acute respiratory distress |
| IVb | 2 | 1 acute multivisceral failure, 1 acute respiratory distress |
LCQ
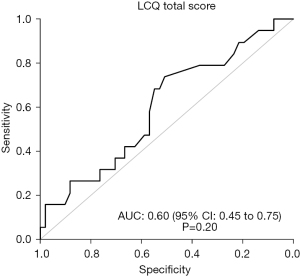
The area under the ROC curve of the LCQ total score to predict postoperative complications of grade ≥2 within 30 days after surgery was 0.60 (95% CI: 0.45, 0.75, P=0.20), indicating a rather low accuracy, not statistically significant (Figure 2). The physical domain of the LCQ score was not significantly predictive of postoperative complications of grade ≥2 (AUC =0.58, 95% CI: 0.42, 0.74, P=0.31).
The Pearson correlation coefficient between CPF and the LCQ total score was estimated at 0.02 (95% CI: −0.22, 0.25), indicating a very weak relationship between the two variables.
The Spearman correlation coefficients (used to limit the influence of outliers) between the LCQ total score and duration of pleural drainage, and duration of hospitalization, were estimated at −0.10 (95% CI: −0.33, 0.17) and −0.20 (95% CI: −0.42, 0.03), respectively, indicating a weak correlation between LCQ score and the two variables.
QLQ-LC13, PF and CPF
The area under the ROC curve of the QLQ-LC13 score to predict postoperative complications of grade ≥2 within 30 days after surgery was estimated at 0.69 (95% CI: 0.54, 0.83, P=0.02). The areas under the ROC curve for PF and CPF to predict postoperative complications were estimated at 0.66 (95% CI: 0.50, 0.81, P=0.04) and 0.61 (95% CI: 0.46, 0.76, P=0.16), respectively (Figure 3).

Since the areas were between 0.50 and 0.70, the predictive performances of these variables were considered weak.
Multivariable model
The multivariate model was a logistic regression explaining the risk of grade ≥2 complications based on the WHO performance status, age, FEV1, DLCO, and the physical component of the LCQ dichotomized as (> median vs. < median with median =6.05). In this model, the effect of the physical component (> median) on the risk of complication was estimated to have an odds ratio of 0.95 (95% CI: 0.78, 1.17, P=0.66). For this multivariate model, the two missing DLCO values were imputed by the mean value (70.85); there were no other missing data on the variables involved.
Discussion
To our knowledge, there is currently no study examining LCQ as a predictive tool for postoperative complications following major lung resection surgery. This study did not show a significant predictive performance of the LCQ total score, QLQ-LC13 score, PF or CPF to predict postoperative complications of grade ≥2 according to the Clavien-Dindo classification.
Our results remain consistent with secondary analyses; we do not observe significant predictive capacity between QLQ-LC13 outcomes, PF or CPF and postoperative complications of grade ≥2 according to the Clavien-Dindo classification. However, the statistical precision was insufficient to show a moderate or even strong predictive capacity, given the CIs.
In study, the mean LCQ total score was high (18.1) indicating a good quality of life related to cough in patients. In the study by Reynolds et al., the objective was to establish the normal threshold of LCQ using data from a healthy population (19). Based on their results, it is suggested that the following scores be considered as the threshold for normal scores on the LCQ—total score: 17.68; physical domain: 5.36; psychological domain: 5.81; social domain: 6.06 (19). In this study, 21.4% of our patients had an LCQ total score below the threshold of 17.68, indicating impaired quality of life related to chronic cough (Table 5). This data was not available in the literature until now.
Table 5
| LCQ total score | Value (N=70) |
|---|---|
| ≥17.68 | 55 (78.6) |
| <17.68 | 15 (21.4) |
Values are expressed in n (%). LCQ, Leicester Cough Questionnaire.
In a retrospective study, Xie et al. evaluated the relationship between chronic cough and clinical-pathological characteristics in patients with NSCLC who had lung resection. These authors reported a mean LCQ total score of 19.79±0.53 preoperatively (23), similar to our score. Despite the pulmonary involvement in this patient population, the prevalence of chronic cough was similar to that observed in the healthy population. Our sample included few patients with chronic cough, which could explain the high scores obtained.
Furthermore, in this study, only 28.5% of our patients had COPD, and only seven of these patients (9.9%) had severe or very severe disease. The study of Berkhof et al., which validated the sensitivity to changes of the English version of LCQ in COPD patients, included a majority of patients with severe and very severe disease (92.5%), and the mean FEV1 was 47%±13% (14). In comparison, o patients had better respiratory function, which could also explain the absence of chronic cough and the LCQ scores.
The French version we used in this study has not been validated in a population of patients with NSCLC. Reychler et al. validated a version in French in their 2015 study in a sample of patients with severe or very severe COPD, with a mean FEV1 of 39.1%±11.9%, which was much lower than our score (18). To date, no study has validated a French version of the LCQ for patients with NSCLC undergoing major lung resection. However, in 2018, a Mandarin-Chinese version of the LCQ (LCQ-MC) was validated in patients with NSCLC diagnosed after VATS lung resection (24). This version of the questionnaire was subsequently widely used in this patient population. Multiple studies have shown that the resection of subcarinal, para-tracheal, and lower lymph nodes, postoperative acid reflux, and anesthesia duration are independent risk factors related to cough in NSCLC patients after VATS lung resection, using LCQ-MC as an outcome measure (11,16,25).
PF was shown to predict postoperative pulmonary complications in lung cancer patients after surgery. Authors showed significantly lower preoperative PF values in the group with pulmonary complications (280.93±88.99 L/min) than in the group without pulmonary complications, (358.38±93.69 L/min) (P<0.001) (26). Our results were different for two reasons: Firstly, the mean PF in our sample was similar to that of their patients without pulmonary complications. Secondly, we investigated all postoperative complications, not just those related to the lungs.
Regarding CPF, no studies were found on the preoperative use of CPF in patients with NSCLC. CPF has primarily been studied in patients with neuromuscular diseases and to predict the success of extubation in intensive care unit patients (27). Patients undergoing lung resection surgery experience a restrictive syndrome in the postoperative period due to the involvement of both the thoracic wall and lung parenchyma (28). Additionally, unilateral left vocal cord paralysis is a relatively common comorbidity in lung surgery (29). This surgery-related paralysis is associated with a higher incidence of aspiration pneumonia and extended hospitalization (29). It would be interesting to evaluate a worsening of preexisting cough in the postoperative period.
Our study has several limitations; notably it is retrospective and monocentric. The reason for including only 71 of 210 patients in the study was not due to patient characteristics but rather to the organization within the department. These patients did not fill the questionnaire. Only one caregiver was responsible for collecting the questionnaires, and some outpatients were difficult to reach. The small sample size limits the power of the study, so we cannot draw a definitive conclusion from these negative results. As a retrospective analysis, there may be distortion in the recording of medical data and recall bias. The measurement of CPF was performed with a circular mouthpiece, similar to PF. However, directing a cough through this type of mouthpiece is challenging, and a nasobuccal mask might have yielded different results, possibly higher than PF (27). However, we had minimal data loss, with only one patient lost to follow-up. To limit the risk of bias, all patients completed the questionnaires independently, and PF and CPF measurements were done by a single physiotherapist, while data from the electronic medical record were retrieved and analyzed by another physiotherapist. Furthermore, our study allowed us to assess the prevalence of chronic cough preoperatively in patients undergoing major lung resection surgery, which has not been evaluated before.
For future research, a larger sample and the inclusion of patients with chronic cough should be considered.
The overall prevalence of chronic cough in adults worldwide is 9.6% (95% CI: 7.6–11.7%; I2=99%), and in Europe, it is 12.7% (95% CI: 10.4–15.2%) (30). The likelihood of cough occurrence after lung surgery is 25% to 50% (3). The incidence rate is over 50% within the year following the operation and 18% within 5 years. Furthermore, 25% of long-term surviving patients may suffer from cough after lung resection (3). Therefore, it would be pertinent to investigate the impact of this post-operative cough in patients undergoing major lung resection, on post-operative complications such as the duration of pleural drainage, the occurrence of subpleural emphysema, and the length of stay.
Conclusions
This study failed to demonstrate the relevance of preoperative LCQ to predict postoperative complications after major lung surgery. However, the statistical precision was insufficient to show a moderate predictive capacity. Currently, other tools are available to predict postoperative complications, such as CPET and the 6-minute stepper test (8). Nevertheless, the LCQ is a simple and reproducible test that warrants further study in a larger population of patients with NSCLC and major lung surgery.
Acknowledgments
The authors are grateful to Nikki Sabourin-Gibbs, CHU Rouen, for her help in editing the manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1324/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1324/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1324/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1324/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was conducted in accordance with the ethical standards of the institutional and national research committee: Cerni (Ethics committee for non-interventional research) (E2023-36). As this study was retrospective, patients were not informed that they were included in this specific study but had been informed that all their health data recorded in Rouen University Hospital health records could be re-used for future research studies, and had the right to oppose this data re-use, according to French law.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Sarna L, Evangelista L, Tashkin D, et al. Impact of respiratory symptoms and pulmonary function on quality of life of long-term survivors of non-small cell lung cancer. Chest 2004;125:439-45. [Crossref] [PubMed]
- Li X, Li X, Zhang W, et al. Factors and potential treatments of cough after pulmonary resection: A systematic review. Asian J Surg 2021;44:1029-36. [Crossref] [PubMed]
- de Leyn P, Decker G. Surgical treatment of non-small cell lung cancer. Rev Mal Respir 2004;21:971-82. [Crossref] [PubMed]
- Benzo R, Kelley GA, Recchi L, et al. Complications of lung resection and exercise capacity: a meta-analysis. Respir Med 2007;101:1790-7. [Crossref] [PubMed]
- Agostini P, Lugg ST, Adams K, et al. Postoperative pulmonary complications and rehabilitation requirements following lobectomy: a propensity score matched study of patients undergoing video-assisted thoracoscopic surgery versus thoracotomy†. Interact Cardiovasc Thorac Surg 2017;24:931-7. [Crossref] [PubMed]
- Brunelli A, Charloux A, Bolliger CT, et al. The European Respiratory Society and European Society of Thoracic Surgeons clinical guidelines for evaluating fitness for radical treatment (surgery and chemoradiotherapy) in patients with lung cancer. Eur J Cardiothorac Surg 2009;36:181-4. [Crossref] [PubMed]
- Boujibar F, Gillibert A, Bonnevie T, et al. The 6-minute stepper test and the sit-to-stand test predict complications after major pulmonary resection via minimally invasive surgery: a prospective inception cohort study. J Physiother 2022;68:130-5. [Crossref] [PubMed]
- Mu T, Li J, Huang Q, et al. Characteristics and Risk Factors for Persistent Cough After Pulmonary Resection. Ann Thorac Surg 2023;115:1337-43. [Crossref] [PubMed]
- Nguyen AM, Schelfhout J, Muccino D, et al. Leicester Cough Questionnaire validation and clinically important thresholds for change in refractory or unexplained chronic cough. Ther Adv Respir Dis 2022;16:17534666221099737. [Crossref] [PubMed]
- Lin R, Che G. Risk factors of cough in non-small cell lung cancer patients after video-assisted thoracoscopic surgery. J Thorac Dis 2018;10:5368-75. [Crossref] [PubMed]
- Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003;58:339-43. [Crossref] [PubMed]
- Murray MP, Turnbull K, MacQuarrie S, et al. Validation of the Leicester Cough Questionnaire in non-cystic fibrosis bronchiectasis. Eur Respir J 2009;34:125-31. [Crossref] [PubMed]
- Berkhof FF, Boom LN, ten Hertog NE, et al. The validity and precision of the Leicester Cough Questionnaire in COPD patients with chronic cough. Health Qual Life Outcomes 2012;10:4. [Crossref] [PubMed]
- Yousaf N, Lee KK, Jayaraman B, et al. The assessment of quality of life in acute cough with the Leicester Cough Questionnaire (LCQ-acute). Cough 2011;7:4. [Crossref] [PubMed]
- Wu X, Xing H, Chen P, et al. Lymph Node Dissection Is a Risk Factor for Short-Term Cough after Pulmonary Resection. Curr Oncol 2022;29:294-307. [Crossref] [PubMed]
- West HJ, Jin JO. JAMA Oncology Patient Page. Performance Status in Patients With Cancer. JAMA Oncol 2015;1:998. [Crossref] [PubMed]
- Reychler G, Schinckus M, Fremault A, et al. Validation of the French version of the Leicester Cough Questionnaire in chronic obstructive pulmonary disease. Chron Respir Dis 2015;12:313-9. [Crossref] [PubMed]
- Reynolds JE, Jetté ME, Wright ML, et al. Normative Values for the Leicester Cough Questionnaire in Healthy Individuals. Ann Otol Rhinol Laryngol 2023;132:705-8. [Crossref] [PubMed]
- Bergman B, Aaronson NK, Ahmedzai S, et al. The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer 1994;30A:635-42. [Crossref] [PubMed]
- Koller M, Warncke S, Hjermstad MJ, et al. Use of the lung cancer-specific Quality of Life Questionnaire EORTC QLQ-LC13 in clinical trials: A systematic review of the literature 20 years after its development. Cancer 2015;121:4300-23. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Xie MR, Zhu YF, Zhou MQ, et al. Analysis of factors related to chronic cough after lung cancer surgery. Thorac Cancer 2019;10:898-903. [Crossref] [PubMed]
- Lin R, Che G. Validation of the Mandarin Chinese version of the Leicester Cough Questionnaire in non-small cell lung cancer patients after surgery. Thorac Cancer 2018;9:486-90. [Crossref] [PubMed]
- Pan LY, Peng LP, Xu C, et al. Predictive factors of cough after uniportal video-assisted thoracoscopic pulmonary resection. J Thorac Dis 2020;12:5958-69. [Crossref] [PubMed]
- Zhou K, Wu YM, Su JH, et al. Can Preoperative Peak Expiratory Flow Predict Postoperative Pulmonary Complications in Lung Cancer Patients Undergoing Lobectomy? Chinese Journal of Lung Cancer 2017;20:603-9. [PubMed]
- Brennan M, McDonnell MJ, Duignan N, et al. The use of cough peak flow in the assessment of respiratory function in clinical practice- A narrative literature review. Respir Med 2022;193:106740. [Crossref] [PubMed]
- Liu GX, Su JH, Wang X, et al. Value of Peak Expiratory Flow Rate in Evaluating Cough Ability in Patients Undergoing Lung Surgery. Can Respir J 2021;2021:5888783. [Crossref] [PubMed]
- Chang TL, Fang TJ, Wong AMK, et al. Clinical and functional characteristics of lung surgery-related vocal fold palsy. Biomed J 2021;44:S101-9. [Crossref] [PubMed]
- Song WJ, Chang YS, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J 2015;45:1479-81. [Crossref] [PubMed]



