
Atezolizumab and nintedanib in patients with non-small cell lung cancer and interstitial lung disease
Introduction
The excellent results achieved in trials assessing immune checkpoint inhibitors (ICIs)—especially the anti-programmed cell death protein 1 (PD-1) and anti-programmed death-ligand 1 (PD-L1) inhibitors—have established a strong position for those agents in the treatment of advanced non-small cell lung cancer (NSCLC) (1-4). However, ICIs can occasionally cause unique side effects known as immune-related adverse events affecting multiple organs, including the skin, endocrine system, gastrointestinal tract, lung, liver, nervous system, and muscular system (5,6). Pneumonitis induced by ICIs is an especially serious and sometimes life-threatening adverse event whose risk has been reported to be elevated in patients with pre-existing interstitial lung disease (ILD) (7-10).
Nintedanib is a multi-kinase inhibitor that blocks vascular endothelial growth factor receptors 1–3, fibroblast growth factor receptors 1–3, and platelet-derived growth factor receptors α and β, targeting the proangiogenic pathway downstream of vascular endothelial growth factor. It has been approved by the US Food and Drug Administration for the treatment of IPF, systemic sclerosis-associated ILD, and chronic fibrosing ILDs with a progressive phenotype, that is called progressive fibrosing ILD (PF-ILD) (11-13). Treatment with nintedanib reduced both the rate of forced vital capacity (FVC) decline in patients with the foregoing conditions and the incidence of acute exacerbation of IPF. The anti-fibrotic effects of nintedanib might also reduce the potential for pneumonitis to be induced by cytotoxic anticancer drugs, molecularly targeted drugs, and ICIs.
Furthermore, nintedanib also inhibits tumor angiogenesis (14). Recently, the randomized phase III J-SONIC trial demonstrated significantly prolonged overall survival after treatment with nintedanib, carboplatin, and nanoparticle albumin-bound (nab)-paclitaxel compared with carboplatin and nab-paclitaxel alone in patients with advanced non-squamous NSCLC and IPF (15). Although few publications have reported the combined use of an ICI and nintedanib, that treatment strategy might enhance safety by protecting against ICI-induced pneumonitis and promoting the antitumor effect of ICIs in lung cancer. Hence, we are reporting a series of patients with NSCLC complicated by progressive fibrosing ILDs treated with both atezolizumab and nintedanib. We present this article in accordance with the STROBE and AME Case Series reporting checklists (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-45/rc).
Methods
We retrospectively reviewed consecutive 140 patients who were diagnosed with NSCLC and treated with atezolizumab at Aichi Cancer Center Hospital in Japan between April 2018 and December 2021. The inclusion criteria included patients with histologically or cytologically confirmed NSCLC, those with investigator-diagnosed PF-ILD (including IPF), and those receiving nintedanib before or concurrently with atezolizumab treatment. Among those patients, four with pre-existing ILDs who also received nintedanib treatment were analyzed. One diagnostic radiologist (T.H.) and one pulmonologist (J.S.) evaluated baseline chest computed tomography (CT) findings according to the clinical practice guideline jointly published by the American Thoracic Society, the European Respiratory Society, the Japanese Respiratory Society, and the Latin American Thoracic Association with discrepancies being resolved by consensus discussion (16). Pneumonitis was graded based on the US National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 5.0 (17). All CT scans performed before the initiation of first-line systemic treatment to the end of the observation period were reviewed. Based on the CT image findings and pulmonary function test results, a comprehensive judgment was made by one diagnostic radiologist (T.H.) and two pulmonologists (J.S. and T.Y.) to determine whether the patient was compatible with IPF or PF-ILD. All four patients were treated with atezolizumab monotherapy at 1,200 mg every 3 weeks and nintedanib 150 mg twice daily. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Aichi Cancer Center Hospital Review Board (approval No. 2022-0-059) and individual consent for this retrospective analysis was waived. The data cut off was 30 April 2022.
Results
Table 1 summarizes the characteristics, treatment profiles, and outcomes of the 4 patients. All patients were diagnosed with pre-existing ILD at least before the initiation of first-line systemic treatment and had never received any treatment for ILD, such as corticosteroids, other immunosuppressants, and anti-fibrotic agents. All patients had no history of autoimmune disease or previous chest radiation therapy. The clinical course of the four patients is shown in Figure 1 and chest CT image before atezolizumab treatment is shown in Figure 2A-2D.
Table 1
| Case | Sex | Age (years) | Histology | UIP pattern on CT | IPF/ PF-ILD |
Autoimmune disease | History of chest RT | Smoking status | Pack- years |
PD-L1 TPS (%) | Atezolizumab treatment | ICI-induced pneumonitis | Response | PFS (months) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lines | Cycles | ||||||||||||||
| 1 | Female | 86 | AdSq | Indeterminate for UIP | PF-ILD | None | None | Former | 7.5 | ≥75 | 2 | 10 | No | PR | 8.1 |
| 2 | Male | 64 | Sq | UIP | IPF | None | None | Former | 43 | 40 | 2 | 1 | No | PD | 0.9 |
| 3 | Male | 78 | Sq | Indeterminate for UIP | PF-ILD | None | None | Former | 50 | ≥75 | 3 | 2 | No | PR | 7.6 |
| 4 | Male | 71 | Sq | Probable UIP | PF-ILD | None | None | Former | 76 | 40 | 2 | 2 | Grade 1 | PD | 2.0 |
AdSq, adenosquamous carcinoma; Sq, squamous cell carcinoma; CT, computed tomography; UIP, usual interstitial pneumonia; IPF, idiopathic pulmonary fibrosis; PF-ILD, progressive fibrosing interstitial lung disease; RT, radiation therapy; PD-L1, programmed death-ligand 1; TPS, tumor proportion score; ICI, immune checkpoint inhibitor; PR, partial response; PD, progressive disease PFS, progression-free survival.
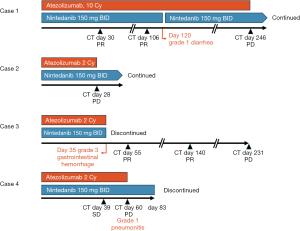
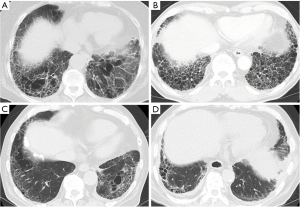
Case 1 was an 86-year-old woman, a postoperative recurrence of adenosquamous lung carcinoma with recurrent lesions in the left upper lobe was complicated by ILD being treated with home oxygen therapy. On CT, her usual interstitial pneumonia (UIP) pattern was classified as indeterminate for UIP (Figure 2A). The PD-L1 tumor proportion score (TPS) using the IHC 22C3 pharmDx assay (Dako North America, Carpinteria, CA, USA) was ≥75%. After this patient progressed on first-line vinorelbine therapy, intravenous atezolizumab 1,200 mg every day 1 of a 3-week cycle and oral nintedanib 150 mg twice daily every day were started concurrently. Because of a partial response on day 30 of atezolizumab treatment, treatment with atezolizumab and nintedanib was continued. CT images on day 106 showed that the tumor in the left upper lobe had further shrunk compared with previous imaging (Figure 3A). At that time, her respiratory condition improved, and home oxygen therapy was discontinued. The patient developed grade 1 diarrhea at approximately day 120 and stopped nintedanib for one week. On day 246, the tumor in the upper lobe of the left lung was found to be enlarged, and treatment with atezolizumab was terminated. Meanwhile, nintedanib was continued for managing ILD. Table 2 shows the changes in pulmonary function before and after atezolizumab plus nintedanib treatment. The percent predicted FVC increased from 103.0% to 118.5%, and the percent predicted diffusing capacity of the lungs for carbon monoxide increased from 38.0% to 47.0%. Furthermore, her oxygenation ability improved, and home oxygen was discontinued. This improvement in pulmonary function may be influenced by tumor shrinkage caused by atezolizumab plus nintedanib treatment.
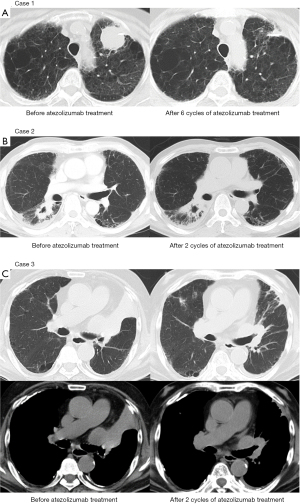
Table 2
| Case | Before atezolizumab plus nintedanib treatment | After atezolizumab plus nintedanib treatment | |||
|---|---|---|---|---|---|
| Percent predicted FVC (%) | Percent predicted DLCO (%) | Percent predicted FVC (%) | Percent predicted DLCO (%) | ||
| 1 | 103.0 | 38.0 | 118.5 | 47.0 | |
| 2 | 67.4 | 44.7 | 62.8 | 49.3 | |
FVC, forced vital capacity; DLCO, diffusing capacity of the lungs for carbon monoxide.
Case 2 was a 64-year-old male smoker diagnosed with IPF continued to smoke for one pack-year until subsequently diagnosed with right upper lobe squamous cell lung cancer and lung metastases. A UIP pattern was evident on chest CT (Figure 2B). The PD-L1 TPS using the immunohistochemistry (IHC) 22C3 pharmDx assay was 40%. In the first-line, carboplatin and nab-paclitaxel were administered for 4 cycles; however, the tumor progressed. Oral nintedanib 150 mg twice daily was then started, followed 3 weeks later by intravenous atezolizumab 1,200 mg in 3-week cycles as second-line treatment. On day 28 of atezolizumab treatment, CT images showed tumor progression; atezolizumab was therefore discontinued (Figure 3B). Regarding his pulmonary function, the percent predicted FVC decreased from 67.4% to 62.8%, but no decrease in the percent predicted diffusing capacity of the lungs for carbon monoxide was observed (Table 2). Tumor growth might have influenced the decrease in the percent predicted FVC. In the third line, cisplatin plus S-1 was started while nintedanib treatment continued. After 4 cycles, tumor progression again occurred, but no treatment-related pneumonitis developed at any point during active treatment. The focus of this patient’s treatment then shifted to palliative care.
Case 3 was a 78-year-old man had stage IIB locally advanced squamous cell lung cancer in the upper lobe of the left lung. His PD-L1 TPS using the IHC 22C3 pharmDx assay was ≥75%. He had comorbidities of ILD, atrial fibrillation, sleep apnea syndrome, hypertension, and previously untreated hepatocellular carcinoma. Surgery and definitive radiation therapy were not indicated given the deterioration of his lung function. After first-line treatment with carboplatin plus nab-paclitaxel for 2 cycles, the tumor improved, but grade 2 drug-induced pneumonitis was observed. Treatment was therefore discontinued. Eight months later, re-challenge with the same treatment was tried in the second-line. After 3 cycles, although the patient achieved a partial response, he also experienced another bout of grade 1 drug-induced pneumonitis. The carboplatin and nab-paclitaxel were therefore terminated. Six months later, the lung cancer progressed again, resulting in left lower lobe atelectasis because of swelling of a lesion in the left hilar lymph node. On chest CT, the finding was indeterminate for UIP (Figure 2C). Oral nintedanib 150 mg twice daily was started, and one week later, atezolizumab 1,200 mg on day 1 of a 3-week cycle was started as third line treatment. After 2 cycles of atezolizumab (day 35), the patient experienced a grade 3 lower gastrointestinal hemorrhage for which he was urgently hospitalized. Upper and lower gastrointestinal endoscopy revealed an ulcer in the descending colon, which was thought to be the cause of the gastrointestinal hemorrhage. Atezolizumab and nintedanib were both discontinued. Thoracic CT on day 55 from the start of atezolizumab treatment showed a partial response and release of the atelectasis of the left lung (Figure 3C). On the other hand, growth of the hepatocellular carcinoma was observed. Nintedanib was permanently discontinued because of toxicity, and atezolizumab was not restarted. After 6 months (day 231 from the start of atezolizumab), thoracic CT revealed progression of the left lung tumor.
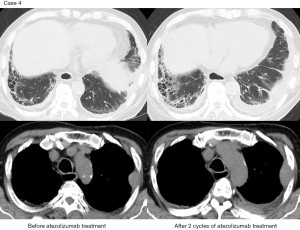
Case 4 was a 71-year-old man with multiple bone metastases, who had undergone right upper lobectomy for lung squamous cell carcinoma 4 years ago. Besides, he had a history of previous laryngectomy for laryngeal cancer (squamous cell carcinoma) 11 years ago. Based on the clinical course, it was determined that the patient had lung squamous cell carcinoma recurrence. Carboplatin and nab-paclitaxel were administered as first-line treatment, but after 3 cycles, the tumor progressed. The IHC 22C3 pharmDx assay resulted in a PD-L1 TPS of 40%, and on chest CT, a finding of probable UIP pattern was reported (Figure 2D). Oral nintedanib 150 mg twice daily was started, and 6 days later, treatment with intravenous atezolizumab 1,200 mg every day 1 of a 3-week cycle was administered. Chest CT on day 39 showed stable disease and no evidence of pneumonitis. A repeat chest CT on day 60 showed an enlarged tumor in the chest wall and ground-glass opacities in the lower lobe of the right lung (Figure 4). Because of a lack of respiratory symptoms, grade 1 drug-induced pneumonitis was diagnosed, and treatment with atezolizumab was discontinued. Corticosteroids were not administered, and the patient experienced no dyspnea. Nintedanib was discontinued 3 weeks after the onset of pneumonitis at the attending physician’s discretion.
Discussion
Several retrospective studies have reported that, compared with other patients having NSCLC, those with pre-existing ILD more often develop ICI-induced pneumonitis (7,8). In a meta-analysis examining the onset of ICI-induced pneumonitis, pneumonitis was found to occur significantly less frequently with PD-L1 inhibitors than with PD-1 inhibitors (18). On the other hand, in a phase II study of atezolizumab for pretreated patients with NSCLC and ILD, pneumonitis was observed in 29.4%, 23.5% of whom experienced grade 3 or higher events, including one case (5.9%) of grade 5; as a result, the study was terminated early (19). Meanwhile, several other studies demonstrated that the onset of ICI-induced pneumonitis did not worsen the prognosis of patients with NSCLC (20,21). ICI treatment in patients with NSCLC and pre-existing ILD therefore remains controversial.
Nintedanib has shown a substantial clinical benefit of a reduced rate of FVC decline in patients with IPF, systemic sclerosis-associated ILD, and progressive fibrosing ILDs. However, whether nintedanib also reduces the risk for the development of drug-induced pneumonitis remains unclear. The J-SONIC trial, a Japanese intergroup study of nintedanib in patients with NSCLC and IPF, analyzed the safety and efficacy of nintedanib in combination with carboplatin and nab-paclitaxel (15). Although that study did not meet its primary endpoint of exacerbation-free survival, defined as the time from randomization to the date of acute IPF exacerbation or death from any cause, the incidence of acute IPF exacerbation was nevertheless much lower than expected in both the chemotherapy group and the nintedanib plus chemotherapy group. Carboplatin plus nab-paclitaxel carries less risk for pneumonitis, with a 4.3% frequency of acute ILD exacerbation having been reported in a multicenter phase II study of patients with NSCLC and pre-existing ILD (22). Although the anti-fibrotic effect of nintedanib suggests that the drug could be promising when used in combination with ICIs, little evidence has been developed to show that nintedanib reduces the risk of pneumonitis in that scenario. In the one case of the combined use of nintedanib and an ICI that has been reported, a patient who had developed ICI-induced pneumonitis multiple times was subsequently treated with nintedanib and atezolizumab, resulting in avoidance of drug-induced pneumonitis (23).
In our study, all four patients with NSCLC and ILD received atezolizumab in combination with nintedanib, and just 1 patient experienced pneumonitis as a symptomless grade 1 event. The fact that the ICI could be administered without any patient developing symptomatic grade 2 or higher pneumonitis is a promising demonstration of the inhibitory effect of nintedanib on the onset of pneumonitis. A single-arm phase II trial of atezolizumab in patients with NSCLC with pre-existing ILD showed that 23.5% developed severe pneumonitis of grade 3 or higher, and the study was discontinued (19). Although the results showed that nintedanib could be combined with atezolizumab while suppressing symptomatic pneumonitis, safety cannot be overemphasized based on a case series of only four patients. Another limitation of this case series is the fact that atezolizumab and nintedanib were administered for a short time, except in 1 patient who eventually experienced tumoral exacerbation. The effect of any long-term concomitant administration of atezolizumab with nintedanib is not clear. Further case series are therefore needed to clarify the ameliorating effect of nintedanib for the development or exacerbation of ICI-induced pneumonitis.
Adding nintedanib to ICI treatment increases the incidences of several adverse events. In the J-SONIC trial, leukocytopenia, neutropenia, increased aspartate aminotransferase, increased alanine aminotransferase, febrile neutropenia, diarrhea, and proteinuria occurred more often in the nintedanib plus chemotherapy group than in the chemotherapy group (15). In case 2 in the present study, lower gastrointestinal hemorrhage led to the complete discontinuation of nintedanib, and in case 4, grade 1 diarrhea led to the discontinuation of nintedanib for several days. When the potential adverse events attributable to nintedanib are added to the potential immune-related adverse events attributable to atezolizumab, care must be taken to manage the combined risks. Older patients with IPF have been reported to have significantly higher rates of adverse events, such as diarrhea, nausea, and elevated liver enzymes (24). Conversely, other studies have reported a decrease in FVC in patients who discontinued nintedanib treatment but not in those who reduced its dose (25). Therefore, adverse events should be appropriately managed to obtain better clinical outcomes.
In this case series, two patients achieved a partial response. In the randomized phase III OAK trial, which compared atezolizumab with docetaxel in patients with previously treated NSCLC, the objective response rate in the intention-to-treat population was 14% (4). To date, several antiangiogenic compounds—bevacizumab, ramucirumab, and nintedanib—have demonstrated antitumor effects when combined with chemotherapy in NSCLC (26-28). Atezolizumab combined with bevacizumab has also been shown to be effective in a variety of solid tumors (28-31). In general, antiangiogenic compounds enhance the antitumor effects of chemotherapy or immunotherapy. The combination of nintedanib and ICIs might therefore be considered to be another promising treatment strategy.
Conclusions
Although ICIs have transformed the treatment landscape of NSCLC, patients with pre-existing ILD are not able to gain full clinical benefits from ICIs because of the risk of developing pneumonitis. Combined treatment with nintedanib and an ICI—for example, atezolizumab—has the potential to be effective in both ameliorating the risk of drug-induced pneumonitis and enhancing the antitumor effect of the ICI. In this study, the clinical courses of four patients with NSCLC and pre-existing ILD treated with both nintedanib and atezolizumab at our institution were investigated. Despite having a small sample size, this case series is the first to report the co-administration of nintedanib and atezolizumab in patients with both NSCLC and pre-existing ILD. In this study, a partial response was observed in two patients who received nintedanib combined with atezolizumab. However, the effect of such combination treatment on the incidence of pneumonitis remains unclear, and data about the incidence and management of adverse events are lacking. Prospective clinical trials of this combination treatment are therefore needed.
Acknowledgments
We thank Enago Group (www.enago.jp) for editing a draft of this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE and AME Case Series reporting checklists. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-45/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-45/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-45/coif). T.Y. reported receiving personal fees from Taiho Pharmaceutical, Chugai Pharmaceutical, MSD, Ono Pharmaceutical, AstraZeneca, Merck, Bristol-Meyers Squibb, Boehringer Ingelheim, Daiichi Sankyo, and Eli Lilly. J.S. reported receiving personal fees from MSD, Bristol-Myers Squibb, Chugai Pharmaceutical, AstraZeneca, and Ono Pharmaceutical. Y.F. reported receiving a grant from Chugai Pharmaceutical, and personal fees from Novartis, Yakult, Ono Pharmaceutical, Astra Zeneca, Otsuka Pharmaceutical, and Daiichi Sankyo. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Aichi Cancer Center Hospital Review Board (approval No. 2022-0-059) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Sun X, Roudi R, Dai T, et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: a PRISMA systematic review and meta-analysis. BMC Cancer 2019;19:558. [Crossref] [PubMed]
- Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 2018;360:k793. [Crossref] [PubMed]
- Yamaguchi T, Shimizu J, Hasegawa T, et al. Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: A retrospective analysis. Lung Cancer 2018;125:212-7. [Crossref] [PubMed]
- Kanai O, Kim YH, Demura Y, et al. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac Cancer 2018;9:847-55. [Crossref] [PubMed]
- Shimoji K, Masuda T, Yamaguchi K, et al. Association of Preexisting Interstitial Lung Abnormalities With Immune Checkpoint Inhibitor-Induced Interstitial Lung Disease Among Patients With Nonlung Cancers. JAMA Netw Open 2020;3:e2022906. [Crossref] [PubMed]
- Yamaguchi T, Shimizu J, Hasegawa T, et al. Pre-existing interstitial lung disease is associated with onset of nivolumab-induced pneumonitis in patients with solid tumors: a retrospective analysis. BMC Cancer 2021;21:924. [Crossref] [PubMed]
- Distler O, Highland KB, Gahlemann M, et al. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. N Engl J Med 2019;380:2518-28. [Crossref] [PubMed]
- Wells AU, Flaherty KR, Brown KK, et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med 2020;8:453-60. [Crossref] [PubMed]
- Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med 2019;381:1718-27. [Crossref] [PubMed]
- Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res 2008;68:4774-82. [Crossref] [PubMed]
- Otsubo K, Kishimoto J, Ando M, et al. Nintedanib plus chemotherapy for nonsmall cell lung cancer with idiopathic pulmonary fibrosis: a randomised phase 3 trial. Eur Respir J 2022;60:2200380. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-68. [Crossref] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE), Version 5.
0. 2017. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40, accessed 28 Feb 2024. - Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest 2017;152:271-81. [Crossref] [PubMed]
- Ikeda S, Kato T, Kenmotsu H, et al. A Phase 2 Study of Atezolizumab for Pretreated NSCLC With Idiopathic Interstitial Pneumonitis. J Thorac Oncol 2020;15:1935-42. [Crossref] [PubMed]
- Yamaguchi O, Kaira K, Shinomiya S, et al. Pre-existing interstitial lung disease does not affect prognosis in non-small cell lung cancer patients with PD-L1 expression ≥50% on first-line pembrolizumab. Thorac Cancer 2021;12:304-13. [Crossref] [PubMed]
- Fujimoto D, Yoshioka H, Kataoka Y, et al. Efficacy and safety of nivolumab in previously treated patients with non-small cell lung cancer: A multicenter retrospective cohort study. Lung Cancer 2018;119:14-20. [Crossref] [PubMed]
- Kenmotsu H, Yoh K, Mori K, et al. Phase II study of nab-paclitaxel + carboplatin for patients with non-small-cell lung cancer and interstitial lung disease. Cancer Sci 2019;110:3738-45. [Crossref] [PubMed]
- Yamakawa H, Oba T, Ohta H, et al. Nintedanib allows retreatment with atezolizumab of combined non-small cell lung cancer/idiopathic pulmonary fibrosis after atezolizumab-induced pneumonitis: a case report. BMC Pulm Med 2019;19:156. [Crossref] [PubMed]
- Komatsu M, Yamamoto H, Ichiyama T, et al. Tolerability of nintedanib in the elderly with idiopathic pulmonary fibrosis: A single-center retrospective study. PLoS One 2022;17:e0262795. [Crossref] [PubMed]
- Ruaro B, Salotti A, Reccardini N, et al. Functional Progression after Dose Suspension or Discontinuation of Nintedanib in Idiopathic Pulmonary Fibrosis: A Real-Life Multicentre Study. Pharmaceuticals (Basel) 2024;17:119. [Crossref] [PubMed]
- Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665-73. [Crossref] [PubMed]
- Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014;15:143-55. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. [Crossref] [PubMed]
- Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019;393:2404-15. [Crossref] [PubMed]
- Seto T, Nosaki K, Shimokawa M, et al. Phase II study of atezolizumab with bevacizumab for non-squamous non-small cell lung cancer with high PD-L1 expression (@Be Study). J Immunother Cancer 2022;10:e004025. [Crossref] [PubMed]


