
Cryptogenic organizing pneumonia: clinical outcomes of 60 consecutive cases
Highlight box
Key findings
• Pulmonary fibrosis and high serum levels of Krebs von den Lungen (KL)-6 suggest non-improvement of cryptogenic organizing pneumonia (COP). Blood test results at the diagnosis of COP indicating an inflammatory reaction are correlated with its relapse.
What is known and what is new?
• Corticosteroid treatment results in rapid improvement in patients with COP. However, relapse is common.
• Inflammatory parameters, including high serum levels of C-reactive protein and a high percentage of neutrophils in peripheral blood, are significantly correlated with a shorter time to the first relapse. Fibrosis observed on computed tomography images of the chest and high serum levels of KL-6 suggest non-improvement of COP.
What is the implication, and what should change now?
• Patients with COP who present with inflammatory findings, as well as radiological and laboratory findings of fibrosis, at the time of diagnosis must be followed up to prevent relapse and disease progression.
Introduction
Background
Organizing pneumonia (OP) is characterized by the presence of intra-alveolar buds of granulation tissue comprising fibroblasts and myofibroblasts intermixed with the connective tissue matrix (1,2). Idiopathic bronchiolitis obliterans organizing pneumonia (BOOP) is synonymous with cryptogenic organizing pneumonia (COP) (3). Bronchiolitis obliterans with endobronchial polyps (i.e., idiopathic BOOP/COP) rarely show clinical evidence of airflow obstruction; consequently, idiopathic BOOP/COP is clinically recognized as an interstitial lung disease rather than an airflow obstructive disease (4). The differential diagnoses for idiopathic BOOP/COP include infectious pulmonary diseases, localized organizing pneumonia, usual interstitial pneumonia, diffuse alveolar damage, chronic eosinophilic pneumonia, hypersensitivity pneumonitis, collagen vascular disease, granulomatosis with polyangiitis, pulmonary lesions due to drugs, and pulmonary lymphoproliferative disorders (2,5,6). Fibrotic lesions without normal alveolar structures are observed in fibrosing nonspecific interstitial pneumonia (NSIP); however, idiopathic BOOP/COP lacks this feature (7).
Rationale and knowledge gap
Idiopathic BOOP/COP is included in idiopathic interstitial pneumonias (IIPs) as COP (8). The clinical symptoms of COP improve rapidly following corticosteroid treatment. However, this improvement is followed by relapse in some cases (relapse rates: 9–58%) (9-15).
Fulminant COP may be associated with a poor prognosis in some patients (16,17). Beardsley and Rassl (18) proposed a new entity, “fibrosing organizing pneumonia (FOP)”, based on a report on steroid-resistant idiopathic BOOP/COP (19). Similarly, a rapidly progressing type of FOP, which may respond to immunosuppressive drugs, has also been reported (20).
Objective
This study aimed to clarify the clinical outcomes of COP after treatment and follow-up and identify the prognostic factors associated with such outcomes and relapse. Data were retrieved from the pathology database of the NHO Kinki Chuo Chest Medical Center (KCCMC), and the clinical course of COP, including improvement, worsening, and relapse, was analyzed. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-225/rc).
Methods
Patients and COP diagnosis
The OP pattern is characterized by the presence of polypoid plugs of granulation tissue within the lumen of terminal bronchioles, alveolar ducts, and peribronchiolar alveoli (18). The American Thoracic Society (ATS)/European Respiratory Society (ERS) International Multidisciplinary Consensus Classification of IIPs (2002 and 2013) (8,21) recommends diagnosing COP based on the presence of pathological and radiological findings suggestive of OP and the exclusion of possible causes of secondary OP (1).
Histopathologically confirmed consecutive cases of OP diagnosed between January 2007 and December 2013 were retrieved from the database using the keywords “OP”, “organizing”, and “organization”. Among the 624 cases retrieved, 161 cases with overlap were excluded, and 139 cases without OP pattern were excluded after reviewing the pathology reports (presence of notes such as “OP pattern was absent” or “pleuritis with organization”). Furthermore, 23 patients with OP and eosinophilic infiltration were also excluded. Thus, 301 consecutive patients with histopathologically confirmed OP were identified.
The cases were classified as idiopathic (n=92) or non-idiopathic (secondary; n=209) histopathologically confirmed OP after reviewing the medical records. Patients with pathological and clinical findings indicating COP but without the characteristic radiological OP pattern (n=32) were excluded. Sixty patients with pathological, clinical, and radiological findings consistent with those of COP were identified and classified into three groups: patients showing improvement without relapse (Group 1), patients with at least one episode of relapse (Group 2), and patients without improvement (Group 3).
This retrospective observational study was approved by the Institutional Review Board of the KCCMC (No. 541; approval date, February 15, 2016) and adhered to the Declaration of Helsinki (as revised in 2013). The requirement for obtaining informed consent was waived owing to the retrospective study design.
Radiological assessment
A pulmonologist (T.A.) and a chest radiologist (M.A.) independently reviewed the chest computed tomography (CT) images of 92 patients with clinically and histopathologically confirmed idiopathic OP. Disagreements between the reviewers regarding the presence of the OP pattern were resolved by reaching a consensus.
The images acquired using the HiSpeed Advantage or Light-Speed 16 CT scanner (GE Healthcare, Milwaukee, WI, USA) were assessed to detect the following features: consolidation, ground-glass opacity, traction bronchiectasis, bronchodilation in consolidation, reticular opacity, honeycomb lung, nodular opacity, and OP pattern. Consolidation was defined as an increase in the attenuation of the lung parenchyma compared with that of the pulmonary vessels. The distribution of consolidation was also analyzed. The radiological OP pattern was defined as the presence of bilateral/unilateral patchy consolidation or nodules with/without ground-glass opacities exhibiting a predominantly subpleural and peribronchiolar distribution (8,13,21). Traction bronchiectasis was defined as the dilation and beading of the bronchi within areas showing radiological fibrosis (22). Bronchodilation in consolidation was defined as the presence of dilated airways within the consolidation.
Improvement and relapse of COP
Improvement was defined as an improvement in the clinical and radiological findings of COP occurring spontaneously or after corticosteroid treatment that was observed at least once. Relapse was defined as the appearance of characteristic new infiltrates on chest CT images and the presence of compatible clinical, bronchoalveolar lavage (BAL), and/or lung biopsy findings with no identified cause (10,13). Histopathological evidence was not required for diagnosing relapse if typical clinical and radiological features of COP were present (11).
Clinical data
The following laboratory data were retrieved: the serum C-reactive protein (CRP; normal level, <0.3 mg/dL), Krebs von den Lungen-6 protein (KL-6; normal level, <500 U/mL), and surfactant protein-D (SP-D; normal level, <110 ng/mL) levels; arterial blood gas measurements; pulmonary function test results; and BAL findings. The serum KL-6 and SP-D levels were measured via enzyme-linked immunosorbent assay using commercial kits [Nanopia KL-6 (SEKISUI MEDICAL Co., Ltd., Tokyo, Japan) and SP-D ELISA (Yamasa Co., Tokyo, Japan)] (23).
BAL was performed as previously reported. Briefly, three aliquots of 50 mL of saline (total volume, 150 mL) were infused chiefly into the right middle lobe or left lingular-lobe bronchus, if OP suspected lesions were present in these lobes. BAL from other bronchus was avoided if possible because recovery of bronchoalveolar lavage fluid (BALF) might be low. The supernatants were separated from cell pellets via centrifugation at 800 ×g for 5 min at 4 ℃. Slides were prepared by cytocentrifugation and stained using the Wright-Giemsa staining method. A differential count of 500 nucleated cells was performed (24). If infection or aspiration pneumonia was suspected, bronchial washing was performed by infusing 20 mL saline into the bronchi leading to the most important lesions.
All pulmonary function tests were performed at the same laboratory using the CHESTAC-8800 or 8900 spirometry system (CHEST M.I., Inc., Tokyo, Japan). The findings were evaluated in accordance with the recommendations of the ATS and ERS (25).
Statistical analysis
Data are presented as the median (range) or number (percentage). The differences in continuous and categorical variables were analyzed using the Mann-Whitney U test and Fisher’s exact tests, respectively. Significant variables for predicting the time to the first episode of relapse were identified using Cox proportional hazards regression analysis. The inter-relationships between multiple elements were analyzed using Spearman’s rank correlation coefficient analysis. Relapse-free survival curves were plotted using the Kaplan-Meier method, and the predictive significance of relapse was evaluated using the log-rank test. SP-D-ref and KL-6-ref were defined as the serum SP-D and KL-6 levels divided by each reference value (SP-D: 110 ng/mL and KL-6: 500 U/mL), respectively. The SP-D-ref/KL-6-ref ratio was calculated by dividing the SP-D-ref by the KL-6-ref. SP-D-ref/KL-6ref >1 means SP-D is predominantly elevated. Statistical significance was set at a P value of <0.05. All statistical analyses were performed using SPSS version 29 for Macintosh (IBM Corp., Armonk, NY, USA).
Results
Patient demographics
COP was histologically diagnosed by transbronchial lung biopsy (TBLB) (n=58), CT-guided lung biopsy (n=1), and video-assisted thoracoscopic surgery (n=1). Median number [range] of TBLB specimens was 3 [1–9]. COP lesions were observed in the upper lobe (n=43), the middle lobe (n=43), and the lower lung lobe (n=51) of the right lungs and in the upper lobe (n=34) and the lower lobe (n=47) of the left lungs. COP lesions were present in either of the lingular segments or middle lobes in all patients.
Among the 60 patients with COP identified via a database search, 41 patients showed improvement without relapsing (Group 1), whereas thirteen patients showed improvement before relapsing at least once (Group 2). Six patients did not show any improvement (Group 3; Figure 1).

The median age at the time of diagnosis was 69.5 years (range, 27–88 years) in Groups 1 and 2 (n=54; male, 34; female, 20). Twenty-seven patients (50.0%) in Groups 1 and 2 had received corticosteroids or immunosuppressive agents. Starting dose of prednisolone (PSL), 0.5 or 1.0 mg/day, and introduction of immunosuppressive agents was not different between Group 1 and 2 (Fisher’s exact test; P=0.66 and P>0.99, respectively). Immunosuppressive agents were administered in 5 patients: cyclosporine A (n=3), azathioprine (n=1) and intravenous cyclophosphamide (n=1) (Table 1). PSL treatment period until relapse of patients in Group 2 was similar to the whole PSL treatment period of patients in Group 1 (P=0.22, Mann-Whitney U test). In Group 2, nine out of ten PSL treated patients relapsed before the end of the PSL treatment. Hence, relapse of Group 2 did not depend on the early reduction of PSL.
Table 1
| Parameters | Improvement (+) | Improvement (−) (n=6) | ||
|---|---|---|---|---|
| Group 1 [relapse (−) (n=41)] |
Group 2 [relapse (+) (n=13)] |
Group 3 | ||
| Male/female | 25/16 | 9/4 | 5/1 | |
| Age (years) | 69 [43–88] | 71 [27–82] | 65.5 [44–71] | |
| History of smoking | ||||
| NS/ES/CS | 16/17/8 | 5/4/4 | 2/4/0 | |
| Pack years | 22.5 [0–90] | 15 [0–60] | 37 [0–144] | |
| Diagnostic method | ||||
| Transbronchial lung biopsy | 40 (95.2) | 12 (92.3) | 6 (100.0) | |
| Surgical lung biopsy | 0 (0.0) | 1 (7.7) | 0 (0.0) | |
| CT guided lung biopsy | 1 (2.4) | 0 (0.0) | 0 (0.0) | |
| Symptoms | ||||
| Any symptoms | 33 (80.4) | 13 (100.0) | 4 (66.7) | |
| Cough | 19 (46.3) | 3 (23.1) | 2 (33.3) | |
| Sputum | 9 (22.0) | 2 (15.4) | 0 (0.0) | |
| Fever | 13 (31.7) | 6 (46.2) | 0 (0.0) | |
| Dyspnea | 13 (31.7) | 4 (30.8) | 3 (50.0) | |
| Treatment | 17 (41.5) | 10 (76.9) | 1 (16.7) | |
| PSL ≤0.5 mg/kg/day¶ | 11 (26.8) | 8 (61.5) | 0 (0.0) | |
| PSL >0.5 mg/kg/day¶ | 6 (14.6) | 2 (15.4) | 1 (16.7) | |
| PSL treatment period until relapse (days)§ | 154 [24–1,525] | 310 [92–831] | 238 [238] | |
| Methylprednisolone pulse# | 4 (9.8) | 4 (30.8) | 1 (16.7) | |
| Immunosuppressive agents* | 3 (7.3) | 2 (15.4) | 0 (0.0) | |
| Treatment period (days) | 154 [24–1,525] | 839 [281–1,903] | 0 [0–238] | |
| Observation period (days) | 497 [7–2,330] | 1,099 [151–2,777] | 644 [140–1,219] | |
| Days from biopsy to relapse | NE | 242 [92–1,033] | NE | |
| Died before the last observation | 1 (2.4) | 0 (0.0) | 1 (16.7) | |
Values are presented as median [range] or n (%). Relapse (+) = patients with relapse; relapse (−) = patients without relapse. Improvement (+) = patients with improvement; improvement (−) = patients without improvement. All patients with COP of this study (n=60) were classified into three groups: patients showing improvement without relapse (Group 1), patients with at least one episode of relapse (Group 2), and patients without improvement (Group 3). ¶, dose of prednisolone was not different between Group 1 and 2 (Fisher’s exact test, P=0.66). §, PSL treatment period of Group 1, 2, and 3 was defined as whole treatment period, treatment period until relapse, and whole treatment period, respectively. There was no significant difference in PSL treatment period between Group 1 and 2 (P=0.22, Mann-Whitney U test). #, Methylprednisolone pulse: methylprednisolone 500–1,000 mg/day for three successive days. *, cyclosporine A (n=2) and intravenous cyclophosphamide (n=1) was administered in Group 1 and cyclosporine A (n=1) and azathioprine (n=1) in Group 2. There was no significant difference in frequency of immunosuppressive drugs administration between Group 1 and 2. NS, never smoker; ES, ex-smoker; CS, current smoker; CT, computed tomography; PSL, prednisolone; NE, not evaluated; COP, cryptogenic organizing pneumonia.
The median age at the time of diagnosis was 65.5 years (range, 44–71 years) in Group 3 (n=6; male, 5; female, 1). One patient (16.7%) had received corticosteroid treatment (Table 1).
Figure 2 presents the typical radiological features of each clinical course.

Intergroup comparison according to improvement
The clinical characteristics of Group 3 were compared with those of Groups 1 and 2. The serum levels of KL-6 in Group 3 were significantly higher than those in Groups 1 and 2 (468 vs. 988 U/mL; P=0.004). Similarly, the incidence of traction bronchiectasis (24.1% vs. 66.7%; P=0.048) and reticular opacity (23.5% vs. 83.3%; P=0.006) in Group 3 was significantly higher than that in Groups 1 and 2 (Table 2). The SP-D-ref/KL-6-ref ratio in Group 3 was significantly lower than that in Groups 1 and 2 (P=0.03). However, KL-6 predominant increase, suggested by SP-D-ref/KL-6-ref <1 could not predict no improvement (P=0.10). No significant differences were observed among the groups in terms of the findings of arterial blood gas analysis, pulmonary function tests, and BAL (Table S1).
Table 2
| Parameters | Groups 1 and 2 [improvement (+) (n=54)] |
Group 3 [improvement (−) (n=6)] |
P value |
|---|---|---|---|
| Laboratory data (n=60) | |||
| WBC (cells/μL) | 7,250 [3,300–15,600] | 7,850 [3,700–13,600] | 0.87 |
| Neutrophils (%) | 70.75 [48.5–90.8] | 67.4 [35.3–84.5] | 0.19 |
| Lymphocytes (%) | 19.8 [5.3–46.2] | 24.2 [8.9–52.2] | 0.40 |
| Eosinophils (%) | 2.1 [0–8.6] | 3.1 [1–5.1] | 0.63 |
| CRP (mg/dL) | 2.7 [0.01–32.01]* | 0.2 [0.08–9.66] | 0.09 |
| LDH (IU/L) | 205 [78–370]** | 218 [183–294] | 0.39 |
| CPK (IU/L) | 68 [13–420]# | 97 [62–316] | 0.10 |
| KL-6 (IU/mL) | 468 [118–3,450]¶ | 988 [657–4,715] | 0.004 |
| SP-D (ng/mL) | 116 [17–1,640]¶ | 160 [88–371] | 0.40 |
| SP-D-ref/KL-6-ref‡ | 1.13 [0.24–9.54]¶ | 0.61 [0.36–1.11] | 0.03 |
| SP-D-ref/KL-6-ref‡, <1 | 21 (44.6) | 5 (83.3) | 0.10 |
| IgE (IU/mL) | 211 [10–4,396]§ | 130 [37–1,266]† | 0.82 |
| Radiological findings (n=60) | |||
| Consolidation | 54 (100.0) | 5 (83.3) | 0.10 |
| Unilateral consolidation | 10 (18.5) | 1 (16.7) | >0.99 |
| Bilateral multifocal consolidation | 43 (79.6) | 5 (83.3) | >0.99 |
| Ground-glass opacity | 54 (100.0) | 6 (100.0) | – |
| Traction-bronchiectasis | 13 (24.1) | 4 (66.7) | 0.048 |
| Bronchodilation in consolidation | 36 (66.7) | 4 (66.7) | >0.99 |
| Honeycomb lung | 0 (0.0) | 0 (0.0) | – |
| Reticular opacity | 12 (22.2) | 5 (83.3) | 0.006 |
Values are presented as median [range] or n (%). All patients with COP of this study (n=60) were classified into three groups: patients showing improvement without relapse (Group 1), patients with at least one episode of relapse (Group 2), and patients without improvement (Group 3). Improvement (+) = patients with improvement; improvement (−) = patients without improvement. *, n=52; **, n=51; #, n=49; ¶, n=47; §, n=43; †, n=3; ‡, serum levels of SP-D and KL-6, divided by each reference value (110 ng/mL and 500 U/mL, respectively), were called SP-D-ref and KL-6-ref. The SP-D-ref/KL-6-ref ratio was calculated by dividing the SP-D-ref by the KL-6-ref. COP, cryptogenic organizing pneumonia; WBC, white blood cell; CRP, C-reactive protein; LDH, lactate dehydrogenase; CPK, creatine phosphokinase; KL-6, Klebs von den Lungen-6; SP-D, surfactant protein-D; Ig, immunoglobulin; COP, cryptogenic organizing pneumonia.
Relapse of COP
Thirteen (24.1%) of the 54 patients who showed improvement experienced relapse (Figure 1). Among them, 5 (38.5%), 7 (53.8%), and 1 (7.7%) experienced one, two, and four episodes of relapse, respectively. Three of the 13 improved patients experienced relapse (1,033, 148, and 103 days after diagnosis) after the almost complete disappearance of COP suspected shadows within two months without treatment. The median duration from the day of biopsy to the first episode of relapse was 242 days (range, 92–1,033 days; Table 1). The percentage of lymphocytes (%lymphocytes) in peripheral blood leukocytes (PBLs) was significantly lower, and the CRP level in the patients who experienced relapse was significantly higher than that in those who did not relapse (P=0.01 and P=0.003, respectively; data not shown). No significant differences were observed between patients who did and did not experience relapse in terms of the radiological findings (data not shown).
Predictive factors for relapse
Univariate Cox proportional hazards regression analysis revealed high serum CRP levels (P<0.002) and low lymphocyte percentage (%lymphocytes) (P=0.049) in Groups 1 and 2. These factors showed significant correlations with a shorter time from biopsy to the first relapse (Table 3). A high percentage of neutrophils in PBLs (%neutrophils) (P=0.06) tended to suggest a shorter time from biopsy to first relapse (Table 3). When continuous variables with P<0.10 were classified by each median value, %lymphocyte, %neutrophils, CRP were significant predictors of relapse. No significant association was observed between the CT findings and the time from biopsy to the first episode of relapse. However, this interval was shorter in patients with reticular opacity than in those without reticular opacity (P=0.07; Table 3). The arterial blood analysis, pulmonary function test, and BAL fluid analysis findings were not evaluated owing to missing data.
Table 3
| Parameters | HR | 95% CI | P value |
|---|---|---|---|
| Laboratory data (n=54) | |||
| WBC (×100 cells/μL) | 1.013 | 0.994–1.033 | 0.19 |
| Neutrophils (%) | 1.047 | 0.997–1.099 | 0.06 |
| Neutrophils, >70.75/≤70.75 (%)‡ | 4.050 | 1.111–14.762 | 0.03 |
| Lymphocytes (%) | 0.941 | 0.886–0.999 | 0.049 |
| Lymphocytes, >19.8/≤19.8 (%)‡ | 0.319 | 0.075–0.999 | 0.049 |
| Eosinophils (%) | 0.998 | 0.779–1.278 | 0.98 |
| CRP (mg/dL)* | 1.093 | 1.037–1.153 | <0.001 |
| CRP, >2.7/≤2.7 (mg/dL)‡ | 4.214 | 1.157–15.344 | 0.02 |
| LDH (IU/L)** | 0.997 | 0.987–1.008 | 0.63 |
| CPK (IU/L)# | 0.988 | 0.973–1.004 | 0.14 |
| KL-6 (×100 IU/mL)¶ | 1.007 | 0.876–1.158 | 0.91 |
| SP-D (ng/mL)¶ | 1.001 | 0.999–1.002 | 0.44 |
| SP-D-ref/KL-6-ref† | 1.131 | 0.906–1.412 | 0.27 |
| IgE (IU/mL)§ | 1.000 | 0.999–1.000 | 0.64 |
| Radiological findings (n=54) | |||
| Consolidation | NE | ||
| Unilateral consolidation | 0.676 | 0.087–5.240 | 0.70 |
| Bilateral multifocal consolidation | 1.480 | 0.191–11.478 | 0.70 |
| Ground-glass opacity | NE | ||
| Traction-bronchiectasis | 1.557 | 0.507–4.783 | 0.43 |
| Bronchodilation in consolidation | 3.323 | 0.733–15.069 | 0.11 |
| Reticular opacity | 2.910 | 0.902–9.386 | 0.07 |
‡, continuous variables, whose P value less than <0.10, were classified into two groups by each median value; *, n=52; **, n=51; #, n=49; ¶, n=47; †, serum levels of SP-D and KL-6, divided by each reference value (110 ng/mL and 500 U/mL, respectively), were called SP-D-ref and KL-6-ref. The SP-D-ref/KL-6-ref ratio was calculated by dividing the SP-D-ref by the KL-6-ref; §, n=43. Honeycomb lung was not evaluated because of absence in all cases. COP patients showing improvement without relapse (Group 1) and COP patients with at least one episode of relapse (Group 2) were included in this examination. COP, cryptogenic organizing pneumonia; WBC, white blood cell; CRP, C-reactive protein; LDH, lactate dehydrogenase; CPK, creatine phosphokinase; KL-6, Klebs von den Lungen-6; SP-D, surfactant protein-D; Ig, immunoglobulin; HR, hazard ratio; CI, confidence interval; NE, not evaluated.
Receiver operating characteristic analysis to predict the relapse of COP
The cut-off values were determined via receiver operating characteristic analysis using predictive factors of relapse with a P value of <0.10 (Table 3). The cut-off values for CRP, %neutrophils, and %lymphocytes were 6.84 mg/dL (1 − specificity =0.179, sensitivity =0.769), 68.7% (1 – specificity =0.463, sensitivity =0.923), and 14.1% (1 – specificity =0.308, sensitivity =0.829), respectively. The time from biopsy to the first episode of relapse was compared between patients with higher and lower parameters of serum CRP, %neutrophils, and %lymphocytes using each cutoff level (Figure 3). Higher serum CRP levels (>6.84 mg/dL), higher %neutrophils (>68.7%), and lower %lymphocytes (≤14.1%) were significant predictors of the first relapse by log-rank test (P<0.001, P=0.003, and P=0.006, respectively).
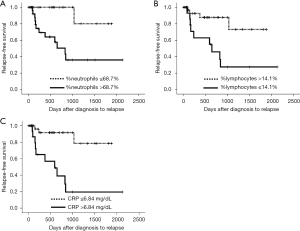
Discussion
Key findings
Sixty consecutive patients with COP were reviewed and classified into three groups based on the clinical course, and the clinical characteristics of each group were evaluated: patients with COP showing improvement without relapsing (Group 1; n=41; 68.3%), patients with COP showing improvement followed by relapse(s) (Group 2; n=13; 21.7%), and patients with COP showing no improvement (Group 3; n=6; 10%). The relapse rate observed in the present study (24.1%) is consistent with those reported in previous studies (9-15).
Strengths and limitations
The present study highlights the importance of neutrophil-mediated inflammation in predicting COP relapse. In addition to elevated serum CRP levels, higher %neutrophils and lower %lymphocytes in PBLs at the time of diagnosis can also be used to predict relapse. Patients with no improvement (Group 3) showed higher KL-6 and lower SP-D-ref/KL-6-ref ratio, higher incidence of traction bronchiectasis and reticular opacity.
The present study had some limitations. First, the histological findings were largely derived from small lung tissue samples collected via TBLB. Eosinophilic pneumonia could not be completely ruled out; however, <25% of eosinophils were detected in the BAL fluid in all cases (5,9,26,27). Second, this was a retrospective study, and some data were missing. Consequently, the association between neutrophils in BAL fluid and relapse could not be evaluated. Third, number of patients with no improvement included in Group 3 was limited. Hence, characteristics of COP patients with no improvement during the clinical course of COP should be further elucidated by future studies. Lastly, this study was conducted at a single center.
Comparison with similar researches
Reticular opacity, NSIP-like patterns on CT images, and histological findings of scarring and remodeling of the background lung parenchyma are related to steroid resistance and disease progression (13,18,19,26,28). Such cases of OP with fibrosis are now classified as FOP (18) or a fibrosing variant of organizing pneumonia (FVOP) (21). Most patients in the present study were diagnosed based on the histopathological findings observed in small tissue samples collected via TBLB. Thus, definitively distinguishing OP from NSIP, FOP, and FVOP was difficult. FVOP is often associated with anti-aminoacyl tRNA synthetase (ARS) antibody (29); however, the anti-ARS antibody levels could not be evaluated as anti-ARS antibody tests were not commercially available during the study period (30). The diagnosis of cicatricial OP, defined as dense collagenized scar tissue associated with the preservation of the underlying lung tissue, cannot be ruled out (31). Some patients with cicatricial OP show a progressive clinical course (31).
Explanations of findings
The serum KL-6 levels of Group 3 were higher in the present study, and traction bronchiectasis and reticular opacities, suggestive of pulmonary fibrosis, were observed in five patients in Group 3 (Table 2). Cox proportional hazard regression analysis revealed that patients showing improvement in reticular opacity tended to relapse (Table 3). Thus, patients with OP and fibrotic opacities on CT will show a progressive clinical course or relapse after improvement. Saito et al. reported that the incidence of traction bronchiectasis in the relapse group (60%) was significantly higher than that in the non-relapse group (17.3%) (32). Okada et al. reported an association between KL-6 levels and traction bronchiectasis on chest CT in patients with COP (33). High serum levels of KL-6 could be a predictor of complications such as fibrosis and poor outcomes. The results of these studies are consistent with those of the present study. Moreover, SP-D was elevated in the present study in Groups 1 and 2, as indicated by the higher SP-D-ref/KL-6-ref ratio than that in Group 3 in the present study (Table 2). The relationship between biomarkers, including KL-6 and SP-D, and the clinical course of COP has rarely been reported.
Inflammatory processes may be predictors of relapse (34,35). High CRP levels show a significant association with relapse within 6 months to 1 year in patients with OP, including patients with secondary OP. Moreover, high CRP levels were associated with intra-alveolar fibrin deposition (33). High %neutrophils in the BAL fluid and high fibrin deposition levels in lung biopsy specimens are predictive factors for relapse in patients with OP, including secondary OP. The increase in the neutrophil count in BAL fluid and neutrophil-mediated inflammation may be correlated with disease severity and prognosis, possibly due to neutrophil-mediated injury to the alveolar wall (35). Although a significant difference between the CRP levels of patients who did and did not experience relapse was not observed, these two studies suggested that inflammatory processes may be associated with relapse in patients with OP. Recently, Zhou et al. reported patients who experienced relapse have higher CRP levels and experience fever more frequently than the patients who did not experience relapse (36). Some parameters except for inflammatory findings were reported to be predictors of relapse. The partial pressure of oxygen (10), forced vital capacity (37), and serum γ-glutamyl-transferase levels (11,38) are significant predictors for relapse; however, unlike the serum CRP levels, their reproducibility remains unconfirmed.
Implications and actions needed
Recently cryobiopsy has been introduced and histological findings of acute or subacute ILDs can be examined in detail than before (39,40). We would like to investigate not only clinical and radiological parameters, but also pathological parameters to predict relapse in the future studies. Reduction method of steroid could be associated with relapse of COP although there was no significant difference between Group 1 and 2. Prospective studies might be needed to draw definite conclusions dose of steroid (41) and its reduction method.
Conclusions
CT findings depicting pulmonary fibrosis and high serum levels of KL-6 were correlated with the non-improvement of COP. Furthermore, blood test results indicating an inflammatory reaction were correlated with relapse in patients showing improvement.
Acknowledgments
We thank Ms. Tomomi Homma and the radiological technologists at KCCMC for collaborating on the data collection. We are grateful to Dr. Masanori Kitaichi (Department of Pathology, NHO Minami Wakayama Medical Center) for the pathological diagnosis during routine clinical evaluation.
Funding: The authors were partly supported by grants from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-225/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-225/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-225/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-225/coif). Y.I. is a consultant or steering/advisory committee member of Boehringer Ingelheim, Roche, SAVARA, and Taiho (not related to this study). Y.I. received lecture fees from Boehringer Ingelheim, Shionogi, Kyorin, Thermo Fisher, and GSK (not related to this study). T.A. received lecture fees from Boehringer Ingelheim and Shionogi for activities not connected to the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of the KCCMC (No. 541; approval date, February 15, 2016). The requirement for obtaining informed consent was waived owing to the retrospective study design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cottin V, Cordier JF. Cryptogenic organizing pneumonia. Semin Respir Crit Care Med 2012;33:462-75. [Crossref] [PubMed]
- Cherian SV, Patel D, Machnicki S, et al. Algorithmic Approach to the Diagnosis of Organizing Pneumonia: A Correlation of Clinical, Radiologic, and Pathologic Features. Chest 2022;162:156-78. [Crossref] [PubMed]
- Epler GR, Colby TV, McLoud TC, et al. Bronchiolitis obliterans organizing pneumonia. N Engl J Med 1985;312:152-8. [Crossref] [PubMed]
- Colby TV. Pathologic aspects of bronchiolitis obliterans organizing pneumonia. Chest 1992;102:38S-43S. [Crossref] [PubMed]
- Kitaichi M. Differential diagnosis of bronchiolitis obliterans organizing pneumonia. Chest 1992;102:44S-9S. [Crossref] [PubMed]
- Kitaichi M. Bronchiolitis Obliterasn Organizing Pneumonia (BOOP). In: Takishima T. Basic and Clinical Aspects of Pulmonary Fibrosis. Boca Raton: RC Press; 1994;463-88.
- Katzenstein AL, Myers JL. Nonspecific interstitial pneumonia and the other idiopathic interstitial pneumonias: classification and diagnostic criteria. Am J Surg Pathol 2000;24:1-3. [Crossref] [PubMed]
- American Thoracic Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. Erratum in: Am J Respir Crit Care Med 2002;166:426. [Crossref] [PubMed]
- Raghu G, Meyer KC. Cryptogenic organising pneumonia: current understanding of an enigmatic lung disease. Eur Respir Rev 2021;30:210094. [Crossref] [PubMed]
- Watanabe K, Senju S, Wen FQ, et al. Factors related to the relapse of bronchiolitis obliterans organizing pneumonia. Chest 1998;114:1599-606. [Crossref] [PubMed]
- Lazor R, Vandevenne A, Pelletier A, et al. Cryptogenic organizing pneumonia. Characteristics of relapses in a series of 48 patients. The Groupe d'Etudes et de Recherche sur les Maladles "Orphelines" Pulmonaires (GERM"O"P). Am J Respir Crit Care Med 2000;162:571-7. [Crossref] [PubMed]
- Oymak FS, Demirbaş HM, Mavili E, et al. Bronchiolitis obliterans organizing pneumonia. Clinical and roentgenological features in 26 cases. Respiration 2005;72:254-62. [Crossref] [PubMed]
- Lee JW, Lee KS, Lee HY, et al. Cryptogenic organizing pneumonia: serial high-resolution CT findings in 22 patients. AJR Am J Roentgenol 2010;195:916-22. [Crossref] [PubMed]
- Yoo JW, Song JW, Jang SJ, et al. Comparison between cryptogenic organizing pneumonia and connective tissue disease-related organizing pneumonia. Rheumatology (Oxford) 2011;50:932-8. [Crossref] [PubMed]
- Drakopanagiotakis F, Paschalaki K, Abu-Hijleh M, et al. Cryptogenic and secondary organizing pneumonia: clinical presentation, radiographic findings, treatment response, and prognosis. Chest 2011;139:893-900. [Crossref] [PubMed]
- Iannuzzi MC, Farhi DC, Bostrom PD, et al. Fulminant respiratory failure and death in a patient with idiopathic bronchiolitis obliterans. Arch Intern Med 1985;145:733-4. [Crossref] [PubMed]
- Koinuma D, Miki M, Ebina M, et al. Successful treatment of a case with rapidly progressive Bronchiolitis obliterans organizing pneumonia (BOOP) using cyclosporin A and corticosteroid. Intern Med 2002;41:26-9. [Crossref] [PubMed]
- Beardsley B, Rassl D. Fibrosing organising pneumonia. J Clin Pathol 2013;66:875-81. [Crossref] [PubMed]
- Yousem SA, Lohr RH, Colby TV. Idiopathic bronchiolitis obliterans organizing pneumonia/cryptogenic organizing pneumonia with unfavorable outcome: pathologic predictors. Mod Pathol 1997;10:864-71. [PubMed]
- Kobayashi T, Kitaichi M, Tachibana K, et al. A Cryptogenic Case of Fulminant Fibrosing Organizing Pneumonia. Intern Med 2017;56:1185-91. [Crossref] [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Müller NL, Silva IS, Hansell DM, et al. Bronchial Abnormalities. In: Sujal RD. Imaging of the Chest. Expert Radiology Series. Philadelphia: Saunders/Elsevier; 2008;1030.
- Inoue Y, Trapnell BC, Tazawa R, et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med 2008;177:752-62. [Crossref] [PubMed]
- Oyama Y, Fujisawa T, Hashimoto D, et al. Efficacy of short-term prednisolone treatment in patients with chronic eosinophilic pneumonia. Eur Respir J 2015;45:1624-31. [Crossref] [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- Cordier JF, Loire R, Brune J. Idiopathic bronchiolitis obliterans organizing pneumonia. Definition of characteristic clinical profiles in a series of 16 patients. Chest 1989;96:999-1004. [Crossref] [PubMed]
- Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012;185:1004-14. [Crossref] [PubMed]
- Lee JS, Lynch DA, Sharma S, et al. Organizing pneumonia: prognostic implication of high-resolution computed tomography features. J Comput Assist Tomogr 2003;27:260-5. [Crossref] [PubMed]
- Zhan X, Yan W, Wang Y, et al. Clinical features of anti-synthetase syndrome associated interstitial lung disease: a retrospective cohort in China. BMC Pulm Med 2021;21:57. [Crossref] [PubMed]
- Nakashima R, Imura Y, Hosono Y, et al. The multicenter study of a new assay for simultaneous detection of multiple anti-aminoacyl-tRNA synthetases in myositis and interstitial pneumonia. PLoS One 2014;9:e85062. [Crossref] [PubMed]
- Yousem SA. Cicatricial variant of cryptogenic organizing pneumonia. Hum Pathol 2017;64:76-82. [Crossref] [PubMed]
- Saito Z, Kaneko Y, Hasegawa T, et al. Predictive factors for relapse of cryptogenic organizing pneumonia. BMC Pulm Med 2019;19:10. [Crossref] [PubMed]
- Okada F, Ando Y, Honda K, et al. Comparison of pulmonary CT findings and serum KL-6 levels in patients with cryptogenic organizing pneumonia. Br J Radiol 2009;82:212-8. [Crossref] [PubMed]
- Nagata N, Wakamatsu K, Kumazoe H, et al. Clinical significance of intra-alveolar fibrin deposition in transbronchial lung biopsy in patients with organizing pneumonia. Lung 2015;193:203-8. [Crossref] [PubMed]
- Onishi Y, Kawamura T, Nakahara Y, et al. Factors associated with the relapse of cryptogenic and secondary organizing pneumonia. Respir Investig 2017;55:10-5. [Crossref] [PubMed]
- Zhou Y, Wang L, Huang M, et al. A long-term retrospective study of patients with biopsy-proven cryptogenic organizing pneumonia. Chron Respir Dis 2019;16:1479973119853829. [Crossref] [PubMed]
- Kim M, Cha SI, Seo H, et al. Predictors of Relapse in Patients with Organizing Pneumonia. Tuberc Respir Dis (Seoul) 2015;78:190-5. [Crossref] [PubMed]
- Barroso E, Hernandez L, Gil J, et al. Idiopathic organizing pneumonia: a relapsing disease. 19 years of experience in a hospital setting. Respiration 2007;74:624-31. [Crossref] [PubMed]
- Shintani R, Oda T, Niwa T, et al. Transbronchial lung cryobiopsy in idiopathic acute fibrinous and organizing pneumonia. Respir Med Case Rep 2019;28:100888. [Crossref] [PubMed]
- Troy LK, Grainge C, Corte TJ, et al. Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): a prospective, comparative study. Lancet Respir Med 2020;8:171-81. [Crossref] [PubMed]
- Atsumi K, Hisakane K, Mikami E, et al. Minimal effective dose of maintenance steroid therapy for relapse of cryptogenic organizing pneumonia. Respir Med 2023;218:107390. [Crossref] [PubMed]


