Erythroblast transformation-specific 2 correlates with vascular smooth muscle cell apoptosis in rat heterotopic heart transplantation model
Introduction
There have been over 60,000 heart transplants performed worldwide since the first successful heart transplantation by Christian Barnard in 1967 (1). Cardiac transplantation has been the most effective operation for patients with end-stage heart failure for over five decades (2-5). Short-term survival has improved significantly with better immunosuppressive therapy and improved surgical techniques. However, recent findings have also revealed that the major determinants of patient long-term survival are malignancy and cardiac allograft vasculopathy (CAV), which is also known as transplant coronary artery disease or cardiac transplant vasculopathy (6,7). Multiple experimental and clinical studies have shown that vascular smooth muscle cell (VSMC), especially its migration, proliferation and apoptosis (8-10), plays an important role in the formation of CAV. Until now, the molecular mechanism of VSMC is little understood in rat heterotopic heart transplantation model.
The erythroblast transformation-specific (Ets) family includes transcription factors involved in signal transduction, cell cycle progression and differentiation (11-13). Each Ets family member contains a conserved DNA-binding domain of 85 amino acids, named the ETS domain (13), which recognizes GGAA/T purine-rich core sequences in promoters of target genes. The erythroblast transformation-specific (Ets) family encompasses a large number of genes such as ets-1, ets-2, elf-1, elk-1, erg-1, fli-1, PU-1 that has been linked with various organ transplantation in fruitflies, worms, fishes, frogs and mice (14-16). Among these members, Ets-2 has attracted researchers’ interest. Ets-2 was first identified by its homology to v-Ets of the erythroid-myeloid transforming E26 avian retrovirus (17), expressed in a variety of tissues and cells. Recently, it was shown that Ets-2 may play an important role in organ transplantation. For example, Ets-2 was increased and co-localized with type I collagen in the pathogenesis of pulmonary fibrosis after lung transplantation (18). Additionally, Ets-2 regulating the expression of miR-223 was highly predictive of acute rejection in renal transplantation (19). Liver injury that could be caused by liver transplant is associated with human fibrinogen-like protein 2 (hfgl2) expression, whose transcription is dependent on Ets-2 and mitogen-activated protein kinase (MAPK) signaling pathways (20). Meanwhile, the expression of Ets-2 is associated with VSMC migration (21), proliferation (22), apoptosis (23), while vasculopathy is associated with VSMC apoptosis (24,25). Therefore we put forward hypotheses that Ets-2 is correlated with VSMC apoptosis in rat heterotopic heart transplantation model.
In this study, we examined the expression of Ets-2 after rat heterotopic heart transplantation and demonstrated whether Ets-2 could regulate VSMC apoptosis to affect CAV process. All data indicated that Ets-2 could inhibit VSMC apoptosis in rat heterotopic heart transplantation model and relieve CAV, which may have therapeutic implications for improving the survival of cardiac transplant patients in clinical practice.
Methods
Animals, experimental protocol and groups
Male (Lew:RT1l and WF:RT1u) rats, 8–12 weeks, were obtained from the Department of Animal Center, Nantong University (26). All animal care and surgical interventions were executed according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (National Research Council, 1996, USA) (27), and were approved by the Chinese National Committee to the Use of Experimental Animals for Medical Purposes, Jiangsu Branch. The number of the ethical approval is 20160606-001.
All efforts were made to minimize the number of animals used and their suffering. Hearts from Wistar-Furth (WF:RT1u) rats were heterotopically transplanted into Lewis (Lew:RT1l) rats without immunosuppression using the previously described technique with modification (28-30). Additional syngeneic heterotopic cardiac transplantations were performed in Lewis rats. The modified surgical procedure connected the recipient external jugular vein to the pulmonary artery (the aorta) using a 18 G cuff and the recipient common carotid artery to the innominate artery using a 20 G cuff. The 2% carbrital (45 mg/kg) was used as anesthetics. Wistar rats to Lewis rats and Lewis rats to Lewis rats heart transplantation was performed, with a surgical success rate of 94.2% (49/52) and 96.2% (50/52). Three deaths were related to anesthetic accidents, and two animals died of bleeding. The cold ischemia time was less than 30 min. Recipients were sacrificed on day 7, 14, 21, 28 post-transplant. The coronary artery and a few tissues around it were removed and processed for histopathology, immunohistochemistry, western blot analyses and double immunofluorescent staining. Normal donor hearts were harvested on day 0 (n=3 in each group/day). The viability of the cardiac allograft was assessed by daily common palpation (31). The day of graft failure was defined by cessation of graft heartbeat.
Western blot
To obtain samples for western blot analysis (32), the hearts were excised and snap frozen at −70 °C until used. To prepare lysates, heart samples were minced with eye scissors in ice. The samples were then homogenized in lysis buffer (1% NP-40, 50 mmol/L Tris, pH =7.5, 5 mmol/L EDTA, 1% SDS, 1% sodium deoxycholate, 1% triton X-100, 1 mmol/L PMSF, 10 mg/mL aprotinin, and 1 mg/mL leupeptin) and clarified by centrifuging for 20 min in a microcentrifuge at 4 °C. After determination of its protein concentration with the Bradford assay (Bio-Rad, Richmond, CA, USA), the resulting supernatant (50 µg of protein) was subjected to SDS-PAGE. The separated proteins were transferred to a PVGF membrane (Millipore, Bedford, MA, USA) by a transfer apparatus at 350 mA for 2.5 h. The membrane was then blocked with 5% nonfat milk and incubated with primary antibodies against Ets-2 (rabbit, 1:500; Santa Cruz Biotech, Santa Cruz, CA, USA), cleaved caspase 3 (mouse, 1:1,000; Santa Cruz Biotech, Santa Cruz, CA, USA), procaspase 3 (mouse, 1:1,000; Santa Cruz Biotech, Santa Cruz, CA, USA), p53 (rabbit, 1:500; Santa Cruz Biotech), Bcl-xl (rabbit, 1:500; Santa Cruz Biotech) or β-actin (rabbit, 1:1,000; Sigma, St. Louis, MO, USA). After the wash with TBST buffer for three 5 min, the membrane was incubated with HRP-conjugated secondary antibody (1:5,000; Santa Cruz Biotech) for 2 h at room temperature (RT). The detection of immunoreactive bands was performed with an enhanced chemiluminescence system (NEN Life Science Products, Boston, MA, USA). Values are responsible for at least three independent reactions.
Histopathology and immunohistochemistry
Heart tissue samples was processed and fixed in 10% formalin, embedded in paraffin and sectioned. The sections were either stained with hematoxylin and eosin (HE) or analyzed for Ets-2 expression by immunohistochemistry (33). Sections were deparaffinized and rehydrated using xylene and ethanol, respectively. Then the sections were boiled at a controlled final temperature of 121 °C for 20 min in 10 mm citrate buffer solution (pH 6.0) for antigen retrieval. Endogenous peroxidase activity was blocked by immersed in 0.3% hydrogen peroxide solution for 10 min. After rinsing in PBS (pH 7.2), 10% goat serum was applied for 1 h at RT to block any nonspecific reactions and incubated overnight at 4 °C with anti-Ets-2 antibody (rabbit, 1:200; Santa Cruz Biotech) or cleaved caspase 3 antibody (mouse, 1:200; Santa Cruz Biotech, Santa Cruz, CA, USA). The sections were washed with PBS three times, incubated with HRP-conjugated secondary antibody (Envision TM Detection Kit, GK500705, Gene Tech) at 37 °C for 30 min, and then washed three times with PBS. Finally, the sections were incubated with DAB in 0.05 mol/L Tris buffer (pH 7.6) containing 0.03% H2O2 for signal development, and the sections were counterstained with 20% hematoxylin. The slides were dehydrated, cleared, cover slipped, and evaluated. Each sample was incubated with an isotypic antibody dilution under the same experimental conditions as the negative control. Samples were then viewed under a light microscope. Cells with strong or moderate brown staining were counted as positive, cells with no staining were counted as negative.
Double immunofluorescent staining
After the sections were prepared, they were first blocked with 10% normal serum blocking solution, with the species matched to the secondary antibody, containing 3% (w/v) BSA, 0.1% Triton X-100, and 0.05% Tween-20 for 2 h at RT to avoid non-specific staining. Then, the sections were incubated with anti-Ets-2 antibody (rabbit, 1:100; Santa Cruz Biotech), α-SMA (alpha smooth muscle actin) antibody (mouse, 1:100; Abcam, Cambridge, MA, USA) or cleaved caspase 3 antibody (mouse, 1:100; Santa Cruz Biotech, Santa Cruz, CA, USA). Briefly, sections were incubated with all primary antibodies overnight at 4 °C, followed by a mixture of FITC and TRITC-conjugated secondary antibodies for 2 h at 4 °C. The stained sections were examined with a Leica fluorescence microscope (Leica DM 5000B; Leica CTR5000).
Cell culture and siRNA transfection
The rat VSMC line A7R5 was purchased from the Shanghai Institute of Cell Biology. The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen Life Technologies, Grand Island, NY, USA) with 10% fetal bovine serum (FBS, Invitrogen, USA) at 37 °C in a humidified chamber containing 5% CO2. The small interfering RNA (siRNA) for Ets-2 knockdown was synthesized by Genechem (Shanghai, China). The Ets-2 siRNA sequence targeted were as follows: SiRNA1: 5'-CAAACCAGUUAUUCCUGCAGCAGUA-3'; SiRNA2: 5'-UAACUGGUUUGCCUUGCUCGACUGG-3'; SiRNA3: 5'-GCAGCAACUUGAAUUUGCUCACCAA-3'. Non-specific siRNA sequence was used as the negative control. Cell transfection was performed using lipofectamine 2,000 (Invitrogen) according to the manufacturer’s instructions.
Apoptosis analysis
The following two methods were used to assess cell apoptosis: western blot analysis of caspase 3 activation and TUNEL staining. Analysis of DNA fragmentation using fluorescent terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) was performed to assess cell apoptosis in vivo, using a commercial kit (Roche, USA) as described previously (34). The percentage of TUNEL positive cells was calculated by dividing the number of TUNEL positive cells by the number of DAPI positive nuclei at 200× magnification for eight fields in each sample.
Immunoprecipitation
For immunoprecipitation assay as described previously (35), 2 mg of rat tissue after heart transplantation was incubated with 1 mg primary antibodies or control rabbit IgG (Bioworld Technology, Louis Park, MN, USA) at 4 °C overnight. Twenty microliters of protein G-Sepharose (Sigma, 1:1 slush in PBS) was then added for 2 h at 4 °C with rocking. The precipitates were washed four times with homogenization buffer and boiled for 5 min with SDS sample buffer.
Statistical analysis
The SPSS19.0 software was used for statistical analysis. All values in the text and figures are presented as the mean ± SEM of at least three independent experiments. ANOVA across all investigated groups was conducted first. Post hoc pairwise tests for certain group pairs, with assessment of statistical significance, were performed after Bonferroni correction of the overall significance level. Although Bonferroni adjustment was performed across groups, it was not performed across variables. P values <0.05 (2-sided) were considered statistically significant. More specific information regarding group comparison is provided in the figure legend for each figure.
Results
The survival time and CAV process of heart allograft and isograft
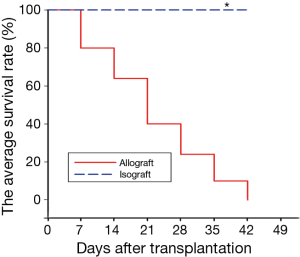
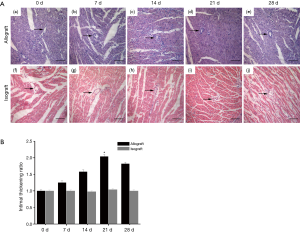
Technically successful transplantation is defined as the forceful beat of the transplanted heart after 72 h. The average survival rate of heterotopic heart transplantation was shown as Figure 1, graft survival times for isografts were significantly longer than those for allografts (isograft median survival time >175 days vs. allograft median survival time of 21±2.0 days). Next, we observed the difference between cardiac allograft and isograft by HE staining (Figure 2A). The staining showed that the vascular intima became thicker and the lumen got narrower from day 0 to day 21 after allograft (Figure 2A, a-d). And the variation became moderate at day 28 postoperatively in cardiac allografts (Figure 2A, e). However, the vessel has no obvious intimal thickening and lumens stenosis in syngeneic hearts (Figure 2A, f-j). The bar chart demonstrated intimal thickness in isograft and allograft groups by densitometry (Figure 2B) and we found these changes were corresponding to the change of survival rate.
Differences of the expression and distribution of Ets-2 in isografts and allografts after cardiac transplantation
Previous researches revealed that transcription factor Ets-2 was associated with vasculopathy (36), therefore we examined Ets-2 expression by western blot in normal heart tissue and graft (Figure 3A-C). Ets-2 protein level was high in normal heart tissue compared with allografts. Ets-2 expression decreased from day 0 after transplantation and hit bottom at day 21, then slowly recovered to the normal level. However, in isograft group, Ets-2 expression remained almost unchanged. Next, we performed immunohistochemistry to identify the tissue location of Ets-2 on transverse paraffin-embedded sections of heart tissues after cardiac transplantation (Figure 3D). Ets-2 staining was observed around the vessel. The positive staining for Ets-2 was relatively high at day 0, then the staining gradually attenuated until 21 day postoperatively in heart allografts. Interestingly, at day 28, Ets-2 expression slightly increased (Figure 3D, a-e). Though Ets-2 expression was found in the isograft group, it had no obvious changes at different time points (Figure 3D, f-j). These data suggested that Ets-2 may play a role in CAV in the allograft group.
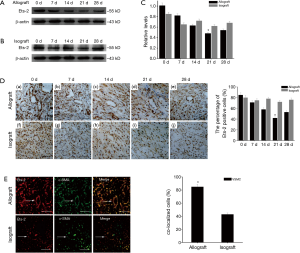
To confirm the expression of Ets-2 is associated with VSMC lesion, double immunofluorescent staining was applied. The co-localization of Ets-2 and α-SMA in allograft group was more obvious than in isograft group at day 21 (Figure 3E). Based on above results, we hypothesized that Ets-2 could influence the biological behavior of VSMC and contributed to CAV process.
Ets-2 is relevant to VSMC apoptosis in allografts
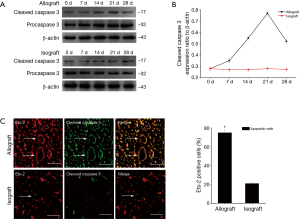
Ets-2 is correlated with VSMC apoptosis (23) that mediates CAV (37,38). To understand whether Ets-2 regulates VSMC apoptosis, the relationship between Ets-2 and cleaved caspase 3, procaspase 3 after heart transplantation was examined. Western blot revealed that cleaved caspase 3 protein level was low at day 0 in allograft, but increased from day 7, reached a peak at day 21, then slightly dropped at day 28. Nevertheless, cleaved caspase 3 expression had no apparent change in isograft group. Meanwhile, the expression of procaspase 3 has no obvious change in allografts and isograft groups (Figure 4A,B). The double labeling immunofluorescent staining showed the co-localization of Ets-2 and cleaved caspase 3 increased more significantly in allografts compared to isografts at day 21 (Figure 4C).
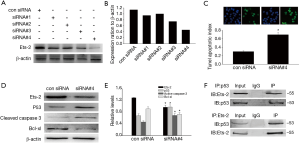
To further identify the function of Ets-2 in VSMC apoptosis, we transfected A7R5 cells with Ets-2 siRNAs to down-regulate Ets-2 expression. Western blot showed a significant reduction of Ets-2 protein level following Ets-2 siRNA3 transfection. Thus, Ets-2 siRNA3 was used for further experiments (Figure 5A,B). Next, TUNEL staining showed that downregulation of Ets-2 could promote VSMC apoptosis (Figure 5C). Meanwhile, cleaved caspase 3 and p53 expression increased, Bcl-XL expression decreased in Ets-2 siRNA transfected A7R5 cells (Figure 5D,E). Furthermore, Ets-2 interacted with p53 in A7R5 cells by immunoprecipitation (Figure 5F). The data suggested that Ets-2 might inhibit VSMC apoptosis via p53 pathway.
Discussion
Heart transplantation remains the only restorative treatment for patients with incurable cardiac diseases and prolonging graft survival is still a subject for intense researches (39). CAV is a challenging long-term complication of heart transplantation and remains a chief cause of graft failure, re-transplantation, and recipient death (40). CAV is a pathological process that affects the vasculature of the transplanted heart, which is characterized by concentric thickening of the vessel wall resulting from intimal hyperplasia in the coronary vessels and the intramyocardial microvasculature (41,42). This thickening leads to narrowing of the vessel lumen detected by angiography (43). In our study, the similar results were observed by HE staining. Then, what causes the vascular wall changes in CAV? The majority of cells in the CAV intima are VSMCs (44), whose dysfunction may lead to pathological processes and cause vascular diseases. VSMC apoptosis is important in the development of CAV (24). In our study, apoptosis of VSMCs was detected in allografts.
Bcl-2 family proteins exert anti-apoptotic role in apoptotic cell death of human malignancies (45), consisting of Bcl-2, Bcl-xl, and Mcl-1 (46). Among them, Bcl-xl is a key anti-apoptotic protein expressed in many tumor types and its overexpression contributes to chemotherapeutic resistance. In previous studies, Bcl-xl expression was found to be influenced by a variety of transcription factors and signal transduction pathways including nuclear factor-kB (NF-kB), signal transducers and activators of transcription (STATs). Additionally, analysis of human Bcl-xl promoter revealed nine potential Ets-binding sites (47).
Ets proteins that bind to DNA can activate transcription alone or in conjunction with other transcription factors. Previous studies have shown that Ets-2 exerts an anti-apoptosis function via Akt pathway in bladder cancer cells (48). In addition, Ets-2 also inhibits apoptosis through up-regulation of Bcl-xl level in in human mesothelioma cells (47). To confirm the effect of Ets-2 on VSMC apoptosis, we examined the expression of cleaved caspase 3, a very specific and sensitive apoptotic marker (49), after rat heterotopic heart transplantation, indicating that cleaved caspase 3 had an inverse expression tendency with Ets-2. Notably, the co-localization of the Ets-2 and cleaved caspase 3 increased significantly in allograft. Moreover, silencing of Ets-2 induced the expression of Bcl-xl, which alleviates apoptotic cell death. Therefore, we speculated that Ets-2 may down-regulate VSMC apoptosis after heart transplantation in rat.
Ets-2 is concomitant with cellular apoptosis, thus it is not surprising that Ets-2/p53 pathway is activated in response to heart transplantation. Ets-2 has been recently characterized as a novel p53-binding protein (50). In our study, co-immunoprecipitation showed that Ets-2 could interact with p53. Silencing of Ets-2 increased p53 expression in A7R5 cells. Our results indicated that Ets-2 may down-regulate VSMC apoptosis through p53 signaling pathway.
Although the majority of previous studies and our study confirmed the anti-apoptotic function of Ets-2, Ets-2 can also mediate the survival and proliferation of normal and cancer cells (51,52), suggesting that Ets-2 may play a double role in regulating cell death and survival. Whether Ets-2 pro-survival pathway is activated in VSMCs and cardiomyocytes in allografts needs further study.
In conclusion, Ets-2 inhibited creased p53 expression and induced Bcl-xl expression in cardiac allograft. The most innovative finding of this study was that Ets-2 down-regulates VSMC apoptosis after rat heart transplantation, indicating that Ets-2 could serve as a possible therapy target to combat CAV and improve the life expectancy of patients after heart transplantation.
Acknowledgements
Funding: This work was supported in part by the National Natural Science Foundation of China (Nos. 81401365, 81373223, 81200918, 81172879), Nantong Science and Technology Project (MS12015056), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Nantong University Graduate Scientific and Technological Innovation Projects (No. YKS14010), Ili Kazak Autonomous Prefecture Science and Technology Planning Project.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Chinese National Committee to the Use of Experimental Animals for Medical Purposes, Jiangsu Branch (No. 20160606-001).
References
- Burch M, Aurora P. Current status of paediatric heart, lung, and heart-lung transplantation. Arch Dis Child 2004;89:386-9. [Crossref] [PubMed]
- Koyanagi T, Noguchi K, Ootani A, et al. Pharmacological inhibition of epsilon pkc suppresses chronic inflammation in murine cardiac transplantation model. J Mol Cell Cardiol 2007;43:517-22. [Crossref] [PubMed]
- Miniati DN, Robbins RC. Heart transplantation: a thirty-year perspective. Annu Rev Med 2002;53:189-205. [Crossref] [PubMed]
- Shi J, Wu K, Yang X, et al. Nur77 is involved in graft infiltrating T lymphocyte apoptosis in rat cardiac transplantation model. Pathol Res Pract 2015;211:633-40. [Crossref] [PubMed]
- Xie A, Wang S, Zhang K, et al. Treatment with interleukin-12/23p40 antibody attenuates acute cardiac allograft rejection. Transplantation 2011;91:27-34. [Crossref] [PubMed]
- Fateh-Moghadam S, Bocksch W, Ruf A, et al. Changes in surface expression of platelet membrane glycoproteins and progression of heart transplant vasculopathy. Circulation 2000;102:890-7. [Crossref] [PubMed]
- Skorić B, Čikeš M, Ljubas Maček J, et al. Cardiac allograft vasculopathy: diagnosis, therapy, and prognosis. Croat Med J 2014;55:562-76. [Crossref] [PubMed]
- Drab M, Verkade P, Elger M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 2001;293:2449-52. [Crossref] [PubMed]
- Pinney SP, Mancini D. Cardiac allograft vasculopathy: advances in understanding its pathophysiology, prevention, and treatment. Curr Opin Cardiol 2004;19:170-6. [Crossref] [PubMed]
- Sedding DG, Braun-Dullaeus RC. Caveolin-1: dual role for proliferation of vascular smooth muscle cells. Trends Cardiovasc Med 2006;16:50-5. [Crossref] [PubMed]
- Albanese C, Johnson J, Watanabe G, et al. Transforming p21ras mutants and c-ets-2 activate the cyclin d1 promoter through distinguishable regions. J Biol Chem 1995;270:23589-97. [Crossref] [PubMed]
- Maroulakou IG, Papas TS, Green JE. Differential expression of ets-1 and ets-2 proto-oncogenes during murine embryogenesis. Oncogene 1994;9:1551-65. [PubMed]
- Yang ZF, Mott S, Rosmarin AG. The Ets transcription factor GABP is required for cell-cycle progression. Nat Cell Biol 2007;9:339-46. [Crossref] [PubMed]
- Deng DX, Jiang J, Garcia B, et al. Endothelin-1, endothelin-3 and their receptors [endothelin (A) and endothelin (B)] in chronic renal transplant rejection in rats. Transpl Int 2000;13:175-82. [PubMed]
- Huang J, Hu XC, Xu J, et al. Construction of the full-length cDNA plasmid library for adult worms of Taenia solium and its ETS sequencing. Chinese Journal of Zoonoses 2008;24:1126-8.
- Zhang L, Eddy A, Teng YT, et al. An immunological renal disease in transgenic mice that overexpress Fli-1, a member of the ets family of transcription factor genes. Mol Cell Biol 1995;15:6961-70. [Crossref] [PubMed]
- Zaldumbide A, Carlotti F, Pognonec P, et al. The role of the ets2 transcription factor in the proliferation, maturation, and survival of mouse thymocytes. J Immunol 2002;169:4873-81. [Crossref] [PubMed]
- Baran CP, Fischer SN, Nuovo GJ, et al. Transcription factor ets-2 plays an important role in the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol 2011;45:999-1006. [Crossref] [PubMed]
- Charreau B. Molecular regulation of endothelial cell activation: Novel mechanisms and emerging targets. Curr Opin Organ Transplant 2011;16:207-13. [Crossref] [PubMed]
- Wu Z, Han M, Chen T, et al. Acute liver failure: mechanisms of immune-mediated liver injury. Liver Int 2010;30:782-94. [Crossref] [PubMed]
- Naito S, Shimizu S, Maeda S, et al. Ets-1 is an early response gene activated by et-1 and pdgf-bb in vascular smooth muscle cells. Am J Physiol 1998;274:C472-80. [PubMed]
- Assoian RK, Marcantonio EE. The extracellular matrix as a cell cycle control element in atherosclerosis and restenosis. J Clin Invest 1997;100:S15-8. [PubMed]
- Crook MF, Olive M, Xue HH, et al. GA-binding protein regulates KIS gene expression, cell migration, and cell cycle progression. FASEB J 2008;22:225-35. [Crossref] [PubMed]
- Hattori T, Shimokawa H, Higashi M, et al. Long-term treatment with a specific rho-kinase inhibitor suppresses cardiac allograft vasculopathy in mice. Circ Res 2004;94:46-52. [Crossref] [PubMed]
- Rahmani M, Cruz RP, Granville DJ, et al. Allograft vasculopathy versus atherosclerosis. Circ Res 2006;99:801-15. [Crossref] [PubMed]
- Shi J, Li Y, Yang X, et al. Upregulation of alpha-enolase in acute rejection of cardiac transplant in rat model: Implications for the secretion of interleukin-17. Pediatr Transplant 2014;18:575-85. [Crossref] [PubMed]
- Soong TR, Pathak AP, Asano H, et al. Lymphatic injury and regeneration in cardiac allografts. Transplantation 2010;89:500-8. [Crossref] [PubMed]
- Tomita Y, Zhang QW, Yoshikawa M, et al. Improved technique of heterotopic cervical heart transplantation in mice. Transplantation 1997;64:1598-601. [Crossref] [PubMed]
- Wang K, Zhang N, Li H. Improved technique of mouse heterotopic heart graft retransplantation. Microsurgery 2006;26:200-2. [Crossref] [PubMed]
- Wang Q, Liu Y, Li XK. Simplified technique for heterotopic vascularized cervical heart transplantation in mice. Microsurgery 2005;25:76-9. [Crossref] [PubMed]
- Li C, Luo L, Lu J, et al. A modified splint tubing technique for heterotopic heart transplantation in mouse. Transplant immunology 2011;25:82-7. [Crossref] [PubMed]
- Liu Y, Chen Y, Lu X, et al. SCYL1BP1 modulates neurite outgrowth and regeneration by regulating the Mdm2/p53 pathway. Mol Biol Cell 2012;23:4506-14. [Crossref] [PubMed]
- Ji L, Li H, Gao P, et al. Nrf2 pathway regulates multidrug-resistance-associated protein 1 in small cell lung cancer. PloS One 2013;8:e63404. [Crossref] [PubMed]
- Baines CP, Kaiser RA, Purcell NH, et al. Loss of cyclophilin d reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005;434:658-62. [Crossref] [PubMed]
- Yao L, Liu YH, Li X, et al. CRMP1 Interacted with Spy1 During the Collapse of Growth Cones Induced by Sema3A and Acted on Regeneration After Sciatic Nerve Crush. Mol Neurobiol 2014;53:879-93. [Crossref] [PubMed]
- Oettgen P. Transcriptional regulation of vascular development. Circ Res 2001;89:380-8. [Crossref] [PubMed]
- Cailhier JF, Laplante P, Hébert MJ. Endothelial apoptosis and chronic transplant vasculopathy: recent results, novel mechanisms. Am J Transplant 2006;6:247-53. [Crossref] [PubMed]
- Papaharalambus CA, Griendling KK. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc Med 2007;17:48-54. [Crossref] [PubMed]
- Wu W, Qiu Q, Wang H, et al. Nrf2 is crucial to graft survival in a rodent model of heart transplantation. Oxid Med Cell Longev 2013;2013:919313.
- Payne GA, Hage FG, Acharya D. Transplant allograft vasculopathy: Role of multimodality imaging in surveillance and diagnosis. J Nucl Cardiol 2016;23:713-27. [Crossref] [PubMed]
- Hiemann NE, Wellnhofer E, Knosalla C, et al. Prognostic impact of microvasculopathy on survival after heart transplantation: evidence from 9713 endomyocardial biopsies. Circulation 2007;116:1274-82. [Crossref] [PubMed]
- Hiemann NE, Wellnhofer E, Lehmkuhl HB, et al. Everolimus prevents endomyocardial remodeling after heart transplantation. Transplantation 2011;92:1165-72. [Crossref] [PubMed]
- Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International society for heart and lung transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant 2010;29:717-27. [Crossref] [PubMed]
- Huibers M, De Jonge N, Van Kuik J, et al. Intimal fibrosis in human cardiac allograft vasculopathy. Transpl Immunol 2011;25:124-32. [Crossref] [PubMed]
- Takahashi H, Chen MC, Pham H, et al. Simultaneous knock-down of Bcl-xL and Mcl-1 induces apoptosis through Bax activation in pancreatic cancer cells. Biochim Biophys Acta 2013;1833:2980-7.
- Danial NN. BCL-2 family proteins: critical checkpoints of apoptotic cell death. Clin Cancer Res 2007;13:7254-63. [Crossref] [PubMed]
- Cao X, Littlejohn J, Rodarte C, et al. Up-regulation of Bcl-xl by hepatocyte growth factor in human mesothelioma cells involves ETS transcription factors. Am J Pathol 2009;175:2207-16. [Crossref] [PubMed]
- Wu W, Zhang S, Li X, et al. Ets-2 regulates cell apoptosis via the Akt pathway, through the regulation of urothelial cancer associated 1, a long non-coding RNA, in bladder cancer cells. PloS One 2013;8:e73920. [Crossref] [PubMed]
- Hoshi T, Sasano H, Kato K, et al. Immunohistochemistry of Caspase3/CPP32 in human stomach and its correlation with cell proliferation and apoptosis. Anticancer Res 1998;18:4347-53. [PubMed]
- Wang Y, Dong Y, Song H, et al. Involvement of gecko SNAP25b in spinal cord regeneration by promoting outgrowth and elongation of neurites. Int J Biochem Cell Biol 2012;44:2288-98. [Crossref] [PubMed]
- Fisher IB, Ostrowski M, Muthusamy N. Role for Ets-2(Thr-72) transcription factor in stage-specific thymocyte development and survival. J Biol Chem 2012;287:5199-210. [Crossref] [PubMed]
- Mathsyaraja H, Thies K, Taffany DA, et al. CSF1-ETS2-induced microRNA in myeloid cells promote metastatic tumor growth. Oncogene 2015;34:3651-61. [Crossref] [PubMed]


