
Different treatment modalities on the prognosis of patients with stage I–IIIa small cell lung cancer: a population based study
Highlight box
Key findings
• For patients with stage I–IIIa small cell lung cancer (SCLC), lobectomy may have similar or even superior efficacy to chemoradiotherapy, and postoperative radiotherapy does not improve the prognosis of patients.
What is known and what is new?
• Previous studies have shown that surgery can improve the prognosis of patients with limited-stage SCLC (LS-SCLC), and postoperative radiotherapy does not improve the prognosis of patients.
• In this study, we comprehensively compared the effects of seven treatment modalities on the prognosis of LS-SCLC patients using large-sample data from the SEER database, especially comparing the role of lobectomy and chemoradiotherapy in improving the prognosis of patients with stage I–IIIa SCLC. The results showed that compared with chemotherapy after lobectomy, chemoradiation after lobectomy may not significantly improve the prognosis of patients with stage I–IIIa SCLC. For patients with stage II–IIIa SCLC, there may be no significant difference in prognosis between chemoradiation and lobectomy combined with chemotherapy ± radiotherapy. This gives us insight into how to avoid overtreatment.
What is the implication, and what should change now?
• This study provides support for reconsidering the positive role of surgery in patients with stage I–IIIa SCLC, but further large-sample prospective cohort studies are needed for validation.
Introduction
Around the world, lung cancer-related deaths account for a majority of all cancer-related deaths (1-3). Based on histopathological classification, lung cancer is classified into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). Based on the Veterans Affairs Lung Cancer Study Group classification, SCLC is frequently divided into limited-stage (LS) and extensive stage (ES). The LS is defined as the stage I–III (any T-stage, any N-stage, M0) and can be treated with radiotherapy and chemotherapy, and the 5-year overall survival (OS) rate of LS-SCLC patients is only about 25% (4).
With recent advances in targeted therapy for lung cancer, the standard treatment for LS-SCLC is radiotherapy combined with chemotherapy (5,6). Although surgery has been largely abandoned as a treatment for patients with LS-SCLC, it can improve the prognosis of patients with stage I–IIA SCLC and stage IIB, and there is no significant difference in the 5-year survival rate between patients with stage I and stage II SCLC who receive surgery (63.8% vs. 65.5%) (7-14).
Therefore, the aim of this study is to compare the effects of each treatment modality (no treatment, lobectomy, radiotherapy, chemotherapy, chemoradiotherapy, lobectomy plus postoperative chemotherapy, lobectomy plus postoperative chemoradiotherapy) on lung cancer specific survival (LCSS) and OS in LS-SCLC patients based on the information from Surveillance, Epidemiology and End Results (SEER) database. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1899/rc).
Methods
SEER database
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The ICD-O-3 histology codes 8041, 8043, 8044, 8045, and 8073 were used to identify SCLC patients. The SEER program version 8.4.1 (National Cancer Institute, Bethesda, MA, USA) was used to identify clinical, treatment, and survival information from the SEER 2000 registry for patients diagnosed with LS-SCLC between 2004 and 2015.
Specific information mainly included age, gender, race, year of diagnosis, laterality, treatment-related conditions (surgery, radiotherapy, chemotherapy), order of radiotherapy and surgery, order of chemotherapy and surgery, tumor size, patient survival month, SEER cause-specific death classification, SEER other cause of death classification, and tumor-node-metastasis (TNM) staging data from the 6th edition of American Joint Committee on Cancer (AJCC). The clinical staging results of the 6th edition of the AJCC were subsequently converted to the 8th edition. All patients in this study were analyzed according to the TNM staging system of the 8th edition of the AJCC.
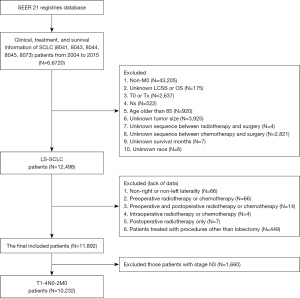
Exclusion criteria were as follows: (I) non-M0 patients; (II) patients with unclear T or N stage and T0 patients; (III) patients older than 85 years; (IV) patients with a non-left or non-right laterality; (V) patients with unknown tumor size or results showing less than 4 or 5 cm, of which the staging cannot be converted from the 6th edition TNM to the 8th edition; (VI) patients with a non-0 or non-lobectomy procedure, with the aim to preserve the patients who had a lobectomy and those who had not a surgery; (VII) patients receiving lobectomy treated with non-postoperative radiotherapy or non-postoperative chemotherapy, with the aim to exclude the patients who had preoperative or intraoperative radiotherapy; (VIII) patients with unknown LCSS or OS results; (IX) patients with unknown survival months; (X) patients treated with radiotherapy alone after lobectomy (Figure 1).
Statistical analysis
Descriptive statistics were calculated to characterize the study cohort associated with demographic, prognostic, and treatment factors of interest. The primary endpoints were LCSS and OS for LS-SCLC. LCSS was determined by selecting lung cancer-related causes of death in the SEER database, and OS was determined by selecting lung cancer-related and other causes of death in the SEER database. LCSS was defined as the time from diagnosis to death due to lung cancer, and OS was defined as the time from diagnosis to death.
Kaplan-Meier (K-M) survival analysis was performed to assess LCSS and OS, and the log-rank test was used to compare the relationship between LCSS and OS among different treatment groups of interest. Multivariate analysis (MVA) using Cox proportional hazard regression model was performed to assess the independent effect on LCSS or OS of factors significantly associated with patient prognosis by K-M survival analysis. The adjusted hazard ratio (HR) was calculated based on the MVA and reflected an increased or decreased risk of death from lung cancer. All P values were two-sided and statistical significance was assessed at P<0.05. All analyses were performed using SPSS version 26.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
A total of 11,892 patients with LS-SCLC were included in this study (Table S1). Of the patients who underwent lobectomy, only one patient was at stage N3. Therefore, corresponding to the eighth AJCC stage, these patients had stage IIIB or IIIC. Surgery is not the primary treatment option for patients with this subset of SCLC, so we included this subset of patients (N3) in the exclusion criteria. A total of 10,232 patients with T1-4N0-2M0 SCLC were included in this study, and they were divided into stage I, II, IIIa, and IIIb (T3-4N2M0) (Table S2).
A total of 1,362 stage I patients were enrolled, including 61, 137, 139, 555, 105, 162 and 203 patients who underwent lobectomy plus postoperative chemoradiotherapy, lobectomy plus postoperative chemotherapy, lobectomy, chemoradiotherapy, radiotherapy, chemotherapy and no treatment, respectively. The details of their characteristics were shown in Table 1.
Table 1
| Patient characteristics | Lobectomy + pCt + pRt (n=61) | Lobectomy + pCt (n=137) | Lobectomy alone (n=139) | Radiotherapy + chemotherapy (n=555) | Radiotherapy alone (n=105) | Chemotherapy alone (n=162) | None (n=203) |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| ≥70 (n=721) | 13 | 50 | 65 | 296 | 82 | 92 | 123 |
| <70 (n=641) | 48 | 87 | 74 | 259 | 23 | 70 | 80 |
| Gender | |||||||
| Male (n=621) | 20 | 58 | 61 | 270 | 42 | 73 | 97 |
| Female (n=741) | 41 | 79 | 78 | 285 | 63 | 89 | 106 |
| Race | |||||||
| White (n=1,198) | 59 | 130 | 121 | 485 | 84 | 140 | 179 |
| Others (n=164) | 2 | 7 | 18 | 70 | 21 | 22 | 24 |
| Year of diagnosis | |||||||
| 2006–2010 (n=623) | 30 | 63 | 77 | 247 | 44 | 77 | 85 |
| 2011–2015 (n=739) | 31 | 74 | 62 | 308 | 61 | 85 | 118 |
| Laterality | |||||||
| Left (n=593) | 25 | 57 | 61 | 236 | 48 | 81 | 85 |
| Right (n=769) | 36 | 80 | 78 | 319 | 57 | 81 | 118 |
| T_stage | |||||||
| T1 (n=909) | 46 | 91 | 95 | 349 | 87 | 103 | 138 |
| T2 (n=453) | 15 | 46 | 44 | 206 | 18 | 59 | 65 |
| T3 (n=0) | – | – | – | – | – | – | – |
| T4 (n=0) | – | – | – | – | – | – | – |
| N_stage | |||||||
| N0 (n=1,362) | 61 | 137 | 139 | 555 | 105 | 162 | 203 |
| N1 (n=0) | – | – | – | – | – | – | – |
| N2 (n=0) | – | – | – | – | – | – | – |
| Follow-up time (months) | 80 (8–155) | 56 (2–167) | 55 (0–165) | 27 (0–164) | 21 (1–119) | 12 (0–160) | 7 (0–159) |
Data are presented as n or range scope. SCLC, small cell lung cancer; pCt, postoperative chemotherapy; pRt, postoperative radiotherapy.
A total of 1,078 stage II patients were enrolled, including 59, 53, 42, 610, 39, 126 and 149 patients who received lobectomy plus postoperative chemoradiotherapy, lobectomy plus postoperative chemotherapy, lobectomy, chemoradiotherapy, radiotherapy, chemotherapy and no treatment, respectively. The details of their characteristics were shown in Table 2.
Table 2
| Patient characteristics | Lobectomy + pCt + pRt (n=59) | Lobectomy + pCt (n=53) | Lobectomy alone (n=42) | Radiotherapy + chemotherapy (n=610) | Radiotherapy alone (n=39) | Chemotherapy alone (n=126) | None (n=149) |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| ≥70 (n=519) | 16 | 25 | 21 | 253 | 27 | 80 | 97 |
| <70 (n=559) | 43 | 28 | 21 | 357 | 12 | 46 | 52 |
| Gender | |||||||
| Male (n=500) | 28 | 26 | 19 | 279 | 17 | 61 | 70 |
| Female (n=578) | 31 | 27 | 23 | 331 | 22 | 65 | 79 |
| Race | |||||||
| White (n=938) | 53 | 49 | 38 | 531 | 27 | 111 | 129 |
| Others (n=140) | 6 | 4 | 4 | 79 | 12 | 15 | 20 |
| Year of diagnosis | |||||||
| 2006–2010 (n=499) | 27 | 28 | 20 | 268 | 23 | 59 | 74 |
| 2011–2015 (n=579) | 32 | 25 | 22 | 342 | 16 | 67 | 75 |
| Laterality | |||||||
| Left (n=499) | 27 | 29 | 19 | 278 | 13 | 67 | 66 |
| Right (n=579) | 32 | 24 | 23 | 332 | 26 | 59 | 83 |
| T_stage | |||||||
| T1 (n=360) | 28 | 25 | 17 | 213 | 8 | 36 | 33 |
| T2 (n=472) | 25 | 22 | 16 | 263 | 17 | 59 | 70 |
| T3 (n=246) | 6 | 6 | 9 | 134 | 14 | 31 | 46 |
| T4 (n=0) | – | – | – | – | – | – | – |
| N_stage | |||||||
| N0 (n=446) | 10 | 13 | 19 | 235 | 23 | 62 | 84 |
| N1 (n=632) | 49 | 40 | 23 | 375 | 16 | 64 | 65 |
| N2 (n=0) | – | – | – | – | – | – | – |
| Follow-up time (months) | 30 (6–164) | 24 (2–124) | 16 (0–144) | 28 (0–155) | 12 (1–141) | 12 (0–101) | 4 (0–105) |
Data are presented as n or range scope. SCLC, small cell lung cancer; pCt, postoperative chemotherapy; pRt, postoperative radiotherapy.
A total of 3,847 patients with stage IIIa were enrolled, including 61, 34, 25, 2,380, 135, 658 and 554 patients who received lobectomy plus postoperative chemoradiotherapy, lobectomy plus postoperative chemotherapy, lobectomy, chemoradiotherapy, radiotherapy, chemotherapy and no treatment, respectively. The details of their characteristics were shown in Table 3.
Table 3
| Patient characteristics | Lobectomy + pCt + pRt (n=61) | Lobectomy + pCt (n=34) | Lobectomy alone (n=25) | Radiotherapy + chemotherapy (n=2,380) | Radiotherapy alone (n=135) | Chemotherapy alone (n=658) | None (n=554) |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| ≥70 (n=1,562) | 23 | 16 | 13 | 804 | 73 | 313 | 320 |
| <70 (n=2,285) | 38 | 18 | 12 | 1,576 | 62 | 345 | 234 |
| Gender | |||||||
| Male (n=1,710) | 28 | 21 | 13 | 1,076 | 54 | 273 | 245 |
| Female (n=2,137) | 33 | 13 | 12 | 1,304 | 81 | 385 | 309 |
| Race | |||||||
| White (n=3,344) | 55 | 31 | 24 | 2,068 | 106 | 574 | 486 |
| Others (n=503) | 6 | 3 | 1 | 312 | 29 | 84 | 68 |
| Year of diagnosis | |||||||
| 2006–2010 (n=1,891) | 33 | 14 | 17 | 1,166 | 67 | 333 | 261 |
| 2011–2015 (n=1,956) | 28 | 20 | 8 | 1,214 | 68 | 325 | 293 |
| Laterality | |||||||
| Left (n=1,596) | 24 | 13 | 9 | 990 | 53 | 260 | 247 |
| Right (n=2,251) | 37 | 21 | 16 | 1,390 | 82 | 398 | 307 |
| T_stage | |||||||
| T1 (n=1,170) | 24 | 9 | 4 | 793 | 34 | 180 | 126 |
| T2 (n=1,193) | 16 | 12 | 6 | 752 | 39 | 202 | 166 |
| T3 (n=129) | 2 | 0 | 1 | 81 | 6 | 18 | 21 |
| T4 (n=1,355) | 19 | 13 | 14 | 754 | 56 | 258 | 241 |
| N_stage | |||||||
| N0 (n=937) | 8 | 9 | 6 | 507 | 38 | 195 | 174 |
| N1 (n=547) | 13 | 4 | 9 | 328 | 24 | 81 | 88 |
| N2 (n=2,363) | 40 | 21 | 10 | 1,545 | 73 | 382 | 292 |
| Follow-up time (months) | 28 (3–137) | 22 (3–142) | 11 (0–125) | 19 (0–165) | 10 (0–131) | 10 (0–161) | 3 (0–164) |
Data are presented as n or range scope. SCLC, small cell lung cancer; pCt, postoperative chemotherapy; pRt, postoperative radiotherapy.
A total of 3,945 patients with T3-4N2M0 (IIIB) were enrolled (Table S3). Among them, only 21 received lobectomy, only 14 received lobectomy plus postoperative chemoradiotherapy, only two received lobectomy plus postoperative chemotherapy, and only five received lobectomy. Therefore, the prognostic differences of these patients with different treatment modalities were not analyzed in this study.
K-M survival analysis
Univariate analysis was performed using the K-M survival curve and variables were assigned as follows: age, 1 for ≥70 years and 2 for <70 years; gender, 1 for male and 2 for female; year of diagnosis, 1 for 2006–2010 and 2 for 2011–2015; race, 1 for white, 2 for others; laterality, 1 for tumor located in the left lung and 2 for in the right lung; clinical T-stage, 1–4 for T1–4, respectively; clinical N-stage, 1–3 for N0–2, respectively; LCSS, 1 for survival or death from other causes, 2 for death from lung cancer; OS, 1 for survival, 2 for death; treatment modality, 1 for lobectomy combined with postoperative radiotherapy and chemotherapy, 2 for lobectomy plus postoperative chemotherapy, 3 for lobectomy alone, 4 for radiotherapy combined with chemotherapy, 5 for radiotherapy alone, 6 for chemotherapy alone, and 7 for no relevant treatment.
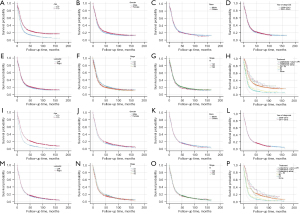
The results showed that for stage I patients, age (P<0.001), gender (P=0.008), T-stage (P=0.03), and treatment modality (P<0.001) might be the influencing factors of LCSS, while age, gender, and treatment modality might be the influencing factors of OS (all P<0.001). For stage II patients, age (P<0.001), year of diagnosis (P=0.04), T-stage (P=0.02), N-stage (P=0.01), and treatment modality (P<0.001) might be the influencing factors of LCSS, while age (P<0.001), N-stage (P=0.04), and treatment modality (P<0.001) might be the influencing factors of OS. For stage IIIa patients, age, gender, T-stage, and treatment modality might be influential factors of LCSS and OS (all P<0.01). The details were shown in Table 4, and the corresponding K-M curves were shown in Figures 2-4.
Table 4
| Variables | Stage I | Stage II | Stage IIIa | |||||
|---|---|---|---|---|---|---|---|---|
| P (LCSS) | P (OS) | P (LCSS) | P (OS) | P (LCSS) | P (OS) | |||
| Age | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Gender | 0.008 | <0.001 | 0.054 | 0.10 | 0.005 | 0.001 | ||
| Race | 0.91 | 0.63 | 0.85 | 0.37 | 0.95 | 0.86 | ||
| Year of diagnosis | 0.73 | 0.27 | 0.04 | 0.11 | 0.38 | 0.65 | ||
| Laterality | 0.10 | 0.23 | 0.58 | 0.76 | 0.25 | 0.22 | ||
| T_stage | 0.03 | 0.10 | 0.02 | 0.07 | 0.001 | 0.001 | ||
| N_stage | – | – | 0.01 | 0.042 | 0.07 | 0.32 | ||
| Therapy | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
LCSS, lung cancer specific survival; OS, overall survival; SCLC, small cell lung cancer.


Multi-factor analysis
Variables with P<0.05 in univariate analysis were analyzed using Cox proportional hazard regression model. The results showed that for stage I patients, age, gender, T stage, and treatment modality were influencing factors of LCSS (all P<0.05), while age, gender, and treatment modality were influencing factors of OS (all P<0.05). Lobectomy plus postoperative chemoradiotherapy was significantly better than chemoradiotherapy and lobectomy in improving LCSS and OS of the patients (all P<0.05). For stage II patients, age and treatment modality were influential factors of LCSS and OS (both P<0.05). For stage IIIa patients, age, gender, T stage, and treatment modality were influential factors of LCSS and OS (all P<0.05). For both stage II and IIIa patients, lobectomy plus postoperative chemotherapy ± radiotherapy had similar efficacy to chemoradiotherapy in improving LCSS and OS of the patients (all P>0.05), and lobectomy plus postoperative chemoradiotherapy did not significantly improve LCSS or OS compared with lobectomy plus postoperative chemotherapy or lobectomy (all P>0.05). When age, gender, and T stage had effect on the prognosis of SCLC patients, the prognosis of male older than 70 years was much worse, and the prognosis of T2–T4 might be worse than that of T1 (all HR >1). The details were shown in Table 5, and the K-M curves of different treatment modalities on LCSS and OS for MVA were shown in Figure 5.
Table 5
| Variables | Stage I | Stage II | Stage IIIa | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LCSS | OS | LCSS | OS | LCSS | OS | ||||||||||||
| Hazard ratio | P | Hazard ratio | P | Hazard ratio | P | Hazard ratio | P | Hazard ratio | P | Hazard ratio | P | ||||||
| Age (years) | |||||||||||||||||
| ≥70 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||||||
| <70 | 0.745 (0.645–0.860) | <0.001 | 0.670 (0.593–0.758) | <0.001 | 0.698 (0.601–0.810) | <0.001 | 0.642 (0.561–0.735) | <0.001 | 0.752 (0.698–0.810) | <0.001 | 0.706 (0.659–0.757) | <0.001 | |||||
| Gender | |||||||||||||||||
| Male | 1 (ref) | 1 (ref) | – | – | – | 1 (ref) | 1 (ref) | ||||||||||
| Female | 0.849 (0.738–0.978) | 0.02 | 0.797 (0.708–0.898) | <0.001 | – | – | – | – | 0.902 (0.839–0.969) | 0.005 | 0.892 (0.834–0.954) | 0.001 | |||||
| Year of diagnosis | |||||||||||||||||
| 2006–2010 | – | – | – | 1 (ref) | – | – | – | ||||||||||
| 2011–2015 | – | – | – | – | 0.893 (0.772–1.034) | 0.13 | – | – | – | – | – | – | |||||
| T_stage | |||||||||||||||||
| T1 | 1 (ref) | – | – | 1 (ref) | – | – | 1 (ref) | 1 (ref) | |||||||||
| T2 | 1.222 (1.054–1.418) | 0.008 | – | – | 1.138 (0.939–1.379) | 0.19 | – | – | 1.154 (1.052–1.265) | 0.002 | 1.158 (1.063–1.261) | 0.001 | |||||
| T3 | – | – | – | – | 1.190 (0.888–1.595) | 0.24 | – | – | 1.099 (0.898–1.346) | 0.36 | 1.049 (0.865–1.271) | 0.63 | |||||
| T4 | – | – | – | – | – | – | – | – | 1.130 (1.033–1.235) | 0.007 | 1.097 (1.010–1.192) | 0.03 | |||||
| N_stage | |||||||||||||||||
| N0 | – | – | – | – | 1 (ref) | 1 (ref) | – | – | – | – | |||||||
| N1 | – | – | – | – | 1.073 (0.861–1.337) | 0.53 | 1.037 (0.906–1.186) | 0.60 | – | – | – | – | |||||
| N2 | – | – | – | – | – | – | – | – | – | – | – | – | |||||
| Therapy | |||||||||||||||||
| Lobectomy+ pCt + pRt | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||||||
| Lobectomy + pCt | 1.914 (1.063–3.445) | 0.03 | 1.382 (0.908–2.102) | 0.13 | 1.026 (0.646–1.630) | 0.91 | 0.937 (0.606–1.447) | 0.77 | 0.912 (0.554–1.501) | 0.72 | 0.920 (0.579–1.462) | 0.73 | |||||
| Lobectomy alone | 2.557 (1.434–4.560) | 0.001 | 1.816 (1.205–2.737) | 0.004 | 1.124 (0.668–1.892) | 0.66 | 1.378 (0.878–2.161) | 0.16 | 1.291 (0.737–2.262) | 0.37 | 1.420 (0.852–2.366) | 0.18 | |||||
| Rt + Ct | 3.837 (2.241–6.572) | <0.001 | 2.609 (1.796–3.789) | <0.001 | 1.076 (0.772–1.499) | 0.67 | 1.134 (0.836–1.537) | 0.42 | 1.206 (0.894–1.626) | 0.22 | 1.270 (0.959–1.681) | 0.10 | |||||
| Rt alone | 4.400 (2.440–7.932) | <0.001 | 3.363 (2.213–5.110) | <0.001 | 1.592 (0.968–2.618) | 0.07 | 1.865 (1.199–2.900) | 0.006 | 1.987 (1.399–2.822) | <0.001 | 2.092 (1.507–2.904) | <0.001 | |||||
| Ct alone | 8.714 (4.988–15.23) | <0.001 | 5.719 (3.846–8.505) | <0.001 | 2.255 (1.550–3.282) | <0.001 | 2.330 (1.648–3.294) | <0.001 | 2.380 (1.750–3.237) | <0.001 | 2.426 (1.818–3.238) | <0.001 | |||||
| None | 9.620 (5.530–16.74) | <0.001 | 6.264 (4.236–9.262) | <0.001 | 4.240 (2.922–6.153) | <0.001 | 4.486 (3.183–6.323) | <0.001 | 4.707 (3.452–6.418) | <0.001 | 4.629 (3.460–6.194) | <0.001 | |||||
LCSS, lung cancer specific survival; OS, overall survival; SCLC, small cell lung cancer; pCt, postoperative chemotherapy; pRt, postoperative radiotherapy; Rt, radiotherapy; Ct, chemotherapy.

Discussion
Historically, the standard treatment for LS-SCLC has been chemotherapy and radiotherapy, but surgery in the treatment of SCLC has been controversial. Lobectomy is the most common procedure in thoracic surgery and previous studies have suggested that it may be superior to other types of procedures other than pneumonectomy in improving survival in patients with LS-SCLC. For instance, a retrospective study of 54 patients with SCLC from 1985 to 2012 showed the 5-year OS rate of 37% after surgical resection, confirming a higher survival rate with lobectomy and pneumonectomy compared to wedge resection and segmental resection (15). Analysis of 3,566 patients with stage I or II SCLC showed a median survival of 39.0 months after lobectomy or pneumonectomy, significantly longer than that after wedge resection (28.0 months) (16).
In terms of radiotherapy, a meta-analysis of 11 randomized trials showed that chemotherapy combined with radiotherapy could increase the 2-year survival by 5.4% and intrathoracic tumor control by 25.3% (17). Another meta-analysis including 13 trials of LS-SCLC showed an OS of 5.4% and a relative risk of death of 0.86 in the combination group compared to patients receiving chemotherapy alone (18). Although previous study showed that patients who received chemoradiotherapy had a higher survival rate compared to those who received chemotherapy alone, surgery combined with radiotherapy could not significantly improve disease-specific survival (DSS) or OS compared with surgery alone (19). In this study, we found that seven patients received postoperative radiotherapy after restricting the type of surgery to lobectomy. The results showed that lobectomy plus postoperative chemoradiotherapy was superior to chemoradiotherapy in improving LCSS and OS in patients with stage I SCLC, and lobectomy and/or postoperative chemotherapy had similar or even superior efficacy to chemoradiotherapy. However, for stage II–IIIa patients, either lobectomy alone or lobectomy plus postoperative chemotherapy ± radiotherapy had similar efficacy to chemoradiotherapy in improving LCSS and OS, and there is no need for postoperative chemotherapy and/or radiotherapy. In addition, the role of surgery in patients with stage IIIb–IIIc SCLC may be overstated.
This study has several limitations. First, the outcomes of I–IIIa SCLC patients can only be analyzed with retrospective evaluation, rather than randomized controlled trials. Second, some details are absent from the SEER database, such as the specific timing and protocol associated with radiotherapy and chemotherapy. In addition, information on chemotherapy regimens for all patients is not collected in the SEER database. Third, due to the small number of patients, the impact of lobectomy plus pre- or post-operative radiotherapy, lobectomy plus preoperative chemotherapy, and the corresponding treatment modalities for patients receiving both pre- and post-operative adjuvant therapy on the prognosis of I–IIIa SCLC patients was not analyzed. With the same reason, we did not analyze other surgical methods, such as wedge resection or total pneumonectomy. Fourth, if patients were older than 85 years, they are recorded as 85+ in the SEER database and their exact age would not be clarified, so these patients were excluded from our study.
Conclusions
Lobectomy plus postoperative chemoradiotherapy is superior to chemoradiotherapy in improving LCSS and OS in patients with stage I SCLC, and lobectomy ± postoperative chemotherapy has similar or even superior efficacy to chemoradiotherapy. However, for stage II–IIIa patients, either lobectomy alone or lobectomy plus postoperative chemotherapy and/or radiotherapy has similar efficacy to chemoradiotherapy in improving LCSS and OS, and there is no need for postoperative chemotherapy and/or radiotherapy.
Acknowledgments
Thanks to the relevant staff and data from the Surveillance, Epidemiology and End Results (SEER) database.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1899/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1899/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1899/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Hernandez D, Cheng CY, Hernandez-Villafuerte K, et al. Survival and comorbidities in lung cancer patients: Evidence from administrative claims data in Germany. Oncol Res 2022;30:173-85. [Crossref] [PubMed]
- Zou K, Sun P, Huang H, et al. Etiology of lung cancer: Evidence from epidemiologic studies. Journal of the National Cancer Center 2022;2:216-25. [Crossref]
- Luo J, Song J, Xiao L, et al. Simultaneous integrated dose reduction intensity-modulated radiotherapy effectively reduces cardiac toxicity in limited-stage small cell lung cancer. Cancer Biol Med 2023;20:452-64. [Crossref] [PubMed]
- Rosell R, Jain A, Codony-Servat J, et al. Biological insights in non-small cell lung cancer. Cancer Biol Med 2023;20:500-18. [Crossref] [PubMed]
- Shi J, Chen Y, Peng C, et al. Advances in Targeted Therapy Against Driver Mutations and Epigenetic Alterations in Non-Small Cell Lung Cancer. Oncologie 2022;24:613-48. [Crossref]
- Combs SE, Hancock JG, Boffa DJ, et al. Bolstering the case for lobectomy in stages I, II, and IIIA small-cell lung cancer using the National Cancer Data Base. J Thorac Oncol 2015;10:316-23. [Crossref] [PubMed]
- Liu T, Chen Z, Dang J, et al. The role of surgery in stage I to III small cell lung cancer: A systematic review and meta-analysis. PLoS One 2018;13:e0210001. [Crossref] [PubMed]
- Gaspar LE, McNamara EJ, Gay EG, et al. Small-cell lung cancer: prognostic factors and changing treatment over 15 years. Clin Lung Cancer 2012;13:115-22. [Crossref] [PubMed]
- Yao Y, Zhou Y, Yang Z, et al. Adjuvant Chemotherapy Following Surgical Resection Improves Survival in Patients With Early Stage Small Cell Lung Cancer. Oncol Res 2019;27:203-10. [Crossref] [PubMed]
- Xu L, Zhang G, Song S, et al. Surgery for small cell lung cancer: A Surveillance, Epidemiology, and End Results (SEER) Survey from 2010 to 2015. Medicine (Baltimore) 2019;98:e17214. [Crossref] [PubMed]
- Yang Y, Yuan G, Zhan C, et al. Benefits of surgery in the multimodality treatment of stage IIB-IIIC small cell lung cancer. J Cancer 2019;10:5404-12. [Crossref] [PubMed]
- Du X, Tian D, Liu L, et al. Surgery in patients with small cell lung cancer: A period propensity score matching analysis of the Seer database, 2010-2015. Oncol Lett 2019;18:4865-81. [Crossref] [PubMed]
- Bi N, Xu K, Ge H, et al. Real-world treatment patterns and clinical outcomes in EGFR-mutant locally advanced lung adenocarcinoma: A multi-center cohort study. Journal of the National Cancer Center 2023;3:65-71. [Crossref]
- Stish BJ, Hallemeier CL, Olivier KR, et al. Long-Term Outcomes and Patterns of Failure After Surgical Resection of Small-Cell Lung Cancer. Clin Lung Cancer 2015;16:e67-73. [Crossref] [PubMed]
- Weksler B, Nason KS, Shende M, et al. Surgical resection should be considered for stage I and II small cell carcinoma of the lung. Ann Thorac Surg 2012;94:889-93. [Crossref] [PubMed]
- Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 1992;10:890-5. [Crossref] [PubMed]
- Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618-24. [Crossref] [PubMed]
- Kim SK, Manzerova J, Christos P, et al. Impact of Radiation Therapy in Surgically Resected Limited-Stage Small Cell Lung Carcinoma. Lung 2017;195:341-6. [Crossref] [PubMed]


