Modified bronchial anastomosis in video-assisted thoracoscopic sleeve lobectomy: a report of 32 cases
Introduction
In selected patients with central type non-small cell lung cancer (NSCLC), sleeve lobectomy can achieve clinical outcomes comparable with those of pneumonectomy and offers better preservation of lung function, a better quality of life, and better long-term survival than pneumonectomy (1,2). Although sleeve lobectomy has been considered a contraindication to thoracoscopic surgery during the past few years (3,4), a growing number of institutions are now capable of conducting video-assisted thoracoscopic sleeve lobectomy and double sleeve lobectomy using a multiple-port or even single-port approach with improvements in thoracoscopic instrumentation and surgical skill (5-8). Therefore, sleeve lobectomy by video-assisted thoracoscopic surgery (VATS) is technically feasible. However, development of this technique has been slow since Santambrogio et al. (9) first reported VATS sleeve lobectomy in 2002. Rapid development has been prevented by strict operative indications and surgical difficulties in key techniques, including bronchial anastomosis. The need for a high level of surgical skill has also contributed to the small number of cases reported in the literature (5,10).
A safe, quick, convenient bronchial anastomosis technique is crucial for promotion of VATS sleeve lobectomy. Between October 2010 and October 2015, 32 VATS sleeve lobectomies were conducted by a single group of surgeons at Fujian Medical University Fujian Union Hospital, Fuzhou, Fujian, China. A modified bronchial anastomosis technique developed by the authors was applied in all patients.
Patients and methods
Of 32 patients, 28 were male and 4 were female, with a mean age of 55.8 years (range, 18–76 years). Recurrent cough and bloody sputum were the main clinical manifestations in 23 patients, while 9 were asymptomatic. Electronic bronchoscopy was performed to confirm the location and pathological type of tumor. All patients were diagnosed with NSCLC. Enhanced thoracic computed tomography, brain magnetic resonance imaging, whole-body bone emission computed tomography, and abdominal and cervical lymph node ultrasonography was performed to exclude metastases. Laboratory blood tests, electrocardiographic examination, and lung function tests were also performed to evaluate the feasibility for VATS sleeve lobectomy. Systematic lymphadenectomy was conducted in all cases.
Operative procedure
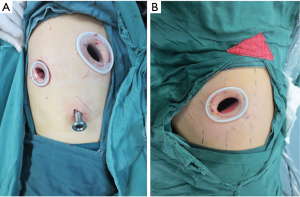
The operation was performed under intravenous–inhalational anesthesia and double-lumen tube intubation with unilateral pulmonary ventilation. The patient was placed in the lateral decubitus position. For three-port VATS sleeve lobectomy, a 1.5-cm observation port was made at the seventh intercostal space on the median axillary line, a 4-cm main operating port at the fourth intercostal space on the anterior axillary line, and a 1.5-cm auxiliary operating port at the seventh intercostal space on the posterior axillary line.
For the single-port approach, a 4- to 5-cm single incision was made at the fourth or fifth intercostal space on the anterior axillary line (Figure 1). All incisions were made with no rib spreading and were kept open by a silicon lap protector. The surgeon and camera holder stood on the ventral side of the patient for three-port VATS sleeve lobectomy, while the camera holder stood opposite the surgeon for the single-port approach (Figure 2).
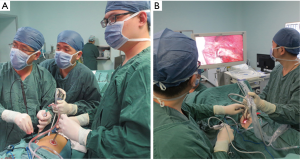
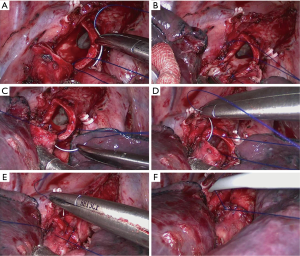
First, we released the inferior pulmonary ligament and divided the mediastinal pleura around the hilum from posterior to anterior. The pulmonary vein, artery, and their branches were divided and exposed superficially to radially. The vessel branches were then dissected by an ultrasonic scalpel or endoscopic stapler according to the specific situation. The interlobar fissures were also divided and stapled. A long scissors and scalpel were used to transect the target bronchus. After the lobe had been completely dissected, the specimen was pulled out of the chest cavity using sterile gloves. The cut ends of the bronchus were clipped to make them smoother and easier to anastomose. The cut ends were sent out for intraoperative frozen pathology to exclude tumor invasion. Before the bronchial anastomosis, we dissected the mediastinal lymph nodes in the order of station 2, 3, 4R, 7, 8, and 9 for right pulmonary operations and station 4L, 5, 6, 7, 8, and 9 for left pulmonary operations. End-to-end anastomosis was performed using a continuous 3–0 prolene suture.
Bronchial anastomosis was begun by placing the first suture in the inner side of the deepest point of the posterior bronchial wall and knotted outside the bronchus. A continuous suture was made on the interior quarter of the posterior lumen circumference. The superficial quarter of the posterior lumen circumference was then sutured. After completing posterior wall anastomosis, the sutures were tightened to regulate the tension around the anastomosis. The remaining two bilateral quarters of the anterior wall were sutured in the same manner. Finally, the last knot was made on the anterior portion with careful regulation of the suture tightness (Figures 3-5). All knots were placed outside the bronchial lumen to prevent postoperative persistent cough. A 3.2-mm fiber bronchoscope was inserted to observe the anastomotic stoma and ensure that the bronchial lumen was unobstructed. The anastomosis was checked for air leakage underwater at 30 cm of H2O airway pressure, and the lung was fully inflated with the same pressure. We placed a 28-F chest drainage tube in the main operating port and a 32-F chest drainage tube in the observation port. In single-port VATS sleeve lobectomy, a 28-F chest drainage tube was placed in the operating port, and an extra central venous catheter was placed in the seventh or eighth intercostal space on the posterior axillary line when conducting upper lobe dissection.


Follow-up
All patients were followed up by telephone or clinic consultation every 3 months and chest CT scan and bronchoscopic examination every 6 months to observe the anastomosis. All data were collected from the hospital’s database, from the medical records, or by telephone. The last investigation was finished in March 2016.
Statistical analysis
Clinical data were input into Microsoft Excel for further processing. Enumeration data are presented as frequencies and percentages. Measurement data are presented as mean ± standard deviation.
Results
Postoperative characteristics
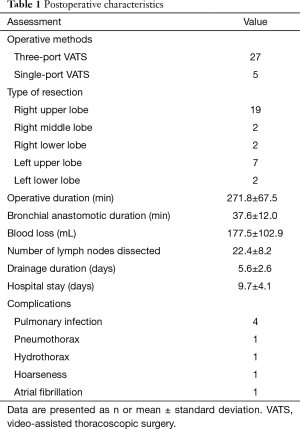
The postoperative characteristics of the patients are summarized in Table 1. No conversion to thoracotomy or reoperation occurred. Twenty-seven cases were conducted by the three-port approach, while five were conducted by the single-port approach. The mean tumor size was 2.8±1.6 cm. There were no perioperative deaths. The mean operative time was 271.8±67.5 with 37.6±12.0 min for bronchial anastomosis. The mean blood loss volume was 177.5±102.9 mL, and the mean number of dissected lymph nodes was 22.4±8.2. The mean duration of chest tube drainage and hospital stay were 5.6±2.6 and 9.7±4.1 days, respectively. The postoperative complication rate was 25.0% (8/32). The specific operative complications are also listed in Table 1. All patients recovered and were discharged uneventfully with active management, and none were transferred to the intensive care unit for further treatment.
Full table
Pathological type and staging
Pathologic examination revealed squamous cell carcinoma in 22 patients, adenocarcinoma in 4, adenosquamous carcinoma in 3, large cell neuroendocrine carcinoma in 1, mucoepidermoid carcinoma in 1, and adenoid cystic carcinoma in 1. The postoperative stage was IB in 11 patients, IIA in 7, IIB in 4, IIIA in 9, and IIIB in 1.
Follow-up
All patients were followed up for a mean duration of 21.0±11.7 months with the exception of one who was lost to follow-up; the follow-up rate was 96.9% (31/32). Electronic bronchoscopy reexamination was performed 6 months postoperatively and detected an unobstructed bronchus and fine healing stoma in all patients. No bronchial stenosis, bronchopleural fistula, or other anastomotic complications were detected at the time of this writing. During the follow-up, no recurrence was reported, while the metastasis rate was 19.4% (6/31, bone metastasis in 3, brain metastasis in 1, bilateral pulmonary in 1, and adrenal metastasis in 1). And the overall 1-year survival rate was 75.9% (22/29). The median survival was 21.9 months.
Discussion
The main difference in the conduct of the operation between VATS lobectomy and VATS sleeve lobectomy is the need for a bronchial anastomosis (5). Bronchial anastomosis is technically challenging in VATS and anastomotic complications are difficult to address, restricting the promotion of VATS sleeve lobectomy. Therefore, only a small number of case series have been reported (5,10,13).
With respect to the anastomosis method, most authors (7,13) have described end-to-end anastomosis by interrupted sutures. Although interrupted sutures are widely applied, twisting of the suture threads frequently occurs; this often breaks the continuity of the operation and prolongs the operative time for unskilled surgeons. Continuous sutures are considered easy to perform, and the clinical results are comparable with those of reported series using interrupted sutures for tracheal and bronchial anastomosis (14). However, Li et al. (10) believed that tightening the sutures may lead to anastomotic leakage or, in the worst-case scenario, anastomotic dehiscence. To overcome the disadvantage of simple interrupted sutures and simple continue sutures, some authors have developed modified methods. Yu et al. (15) reported the use of continuous sutures combined with discontinuous consolidation in bronchial anastomosis. Li et al. (10) performed end-to-end bronchial anastomosis by simple continuous and simple interrupted suturing of the membranous and cartilaginous portions of the bronchus. However, we believe that the use of mixed suture styles is a relatively complex process, and inexperienced surgeons may be slow to adopt it. Modified bronchial anastomosis technique in this report is based on continuous suturing, and the whole process is completed using a single prolene suture thread. We believe that this management technique has the following advantages. First, the working process is simple and convenient because it is a substantially modified continuous suture technique. The advantage of continuous suturing over interrupted suturing in bronchial anastomosis is its technical simplicity and reduced operative time; little tying of knots is required. This is particularly important in thoracoscopic surgery, in which surgeons have a limited operative view and narrow working space (16). Second, good exposure of the operative view is maintained. We follow a “from difficult to easy” principle during the bronchial anastomosis. The posterior wall of the bronchus is positioned deeply with poor exposure, making it the most challenging portion to address during the bronchial anastomosis. Therefore, placing the first stitch at this location can help to approximate the bronchial ends and reduce the tension in the anastomotic stoma. Additionally, the bilateral quarters of the lumen circumference were continuously sutured to finish the posterior wall suture. The remainder of the bronchial lumen is then sutured with the posterior wall visible in the monitor to prevent accidental injury. During left upper sleeve lobectomy, we encountered limited exposure of the left main bronchus and paratracheal and subcarinal lymph nodes, which are located beneath the aortic arch in the lateral decubitus position. We changed the patient to a semiprone position by tilting the operative bed 45 degrees to the patient’s ventral side. In this way, excellent exposure was attained through downward positioning of the lung by gravity. For better exposure and accessibility, we sometimes raised the left main bronchus by passing two 1–0 silk sutures, respectively ligated with both sides of the wall of the left main bronchus, through the anterior and posterior chest wall using a crochet needle and lifted the trunk of the left pulmonary artery by threading a 1–0 silk suture through the posterior chest wall with a crochet needle (17). Third, anastomotic tension is controllable with the modified bronchial anastomosis technique. We chose a 3–0 prolene suture because it is smooth and induces less tissue injury, allowing us to easily control the anastomotic tension. During this process, we gradually tightened the anastomosis from posterior to anterior, and the anastomotic tension was adjusted again according to our experience before knotting the last stitch. We believe that by using this method, the anastomotic tension can be efficiently controlled and anastomotic complications can thus be reduced.
Postoperative characteristics such as the anastomosis time, blood loss volume, drainage duration, and hospital stay in this study were comparable with those reported by other authors (5,10,13,15). Yildizeli et al. (18) reported an anastomotic complication rate of 6.4%, including bronchopleural fistulas, bronchial stenosis, local necrosis, bronchovascular fistulas, and bronchial rupture. No anastomotic complications were observed in this study. Besides the advantages of the modified bronchial anastomosis technique, equally important to successful bronchial anastomosis are the improvement in the operative process, clipping of the bronchial cut ends, and prevention of suture thread twisting.
A surgically sound bronchial anastomosis consists of tension-free anastomosis with accurate mucosa-to-mucosa approximation (19). Therefore, we clipped the cut ends of the bronchus for size matching to reduce the anastomotic tension. The following methods were applied when encountering size differences between the main bronchus and bronchi. First, a V-notch was made or the smaller end was obliquely cut off. Second, the larger end was contracted by constricted the suture on the membrane part. Third, the stitch length of both ends was adjusted (the stitch length of the larger end was extended and that of the smaller end was decreased). We also made some modifications to the operative process. First, we released the hilar structure and surrounding tissues of the anastomotic stoma. Agasthian reported that in sleeve lobectomy, release procedures such as division of the inferior ligament are routinely performed to relieve tension prior to anastomosis (13). For hilar structure division, Li and Wang (10) recommended dividing the bronchus first because of anatomical tumor and lymph node considerations for the left upper lobe. However, we believe that release of tension at the bronchial anastomosis is the priority; thus, we divide and dissect the bronchus after the pulmonary vein and artery. We recommend then dissecting the mediastinal lymph nodes prior to performing the bronchial anastomosis. Performing the procedures in this order helps to prevent tractive injury to the anastomotic stoma when performing lymph node dissection. Agasthian (13) reported that when possible, the anastomosis should be performed last to prevent injury or disruption to the anastomosis. Additionally, Lu et al. (20) believed that preferential dissection of the mediastinal lymph nodes could ensure a good field of view under VATS during the bronchus anastomosis.
Twisting of the suture thread occurred in our early stages of practice. With progression in VATS technical skill and additional experience, we developed a method to overcome this difficulty. First, because most of the thread twisting occurs in the chest cavity, we pull the needle out of the incision after finishing each seam to straighten the suture thread and prevent twisting. The assistant pulls the other end of the suture to prevent suture loosening. Second, the surgeon should keep the end of suture at the inferior aspect of the utility incision and the assistant keeps the other end at the superior aspect. In this way, crossing of the suture threads can be prevented and the operative space between the suture threads eases the subsequent manipulation. Third, the surgeon’s manipulation should rely on physiological wrist movement. A rough operating style is forbidden, especially during needle insertion and withdrawal. By following the above methods, twisting of the suture threads seldom occurred in the later operations, and the anastomosis time was significantly reduced.
In this study, five cases of VATS sleeve lobectomy were performed by the single-port approach. Wang et al. (21) reported that single-incision thoracoscopic lobectomy and segmentectomy are technically feasible methods and that their perioperative outcomes are similar to those of the multiple-incision approach. Unfortunately, no study has compared single-port VATS sleeve lobectomy with the multiple approaches. In our practice, single-port VATS sleeve lobectomy is technically feasible and safe. However, the limited operative space and interference with the view caused by instrumentation are the main disadvantages and require accumulation of experience for unskilled surgeons to overcome. Although all single-port VATS procedures in this study were performed with traditional thoracoscopic instruments, we believe that prolonged or two-joint devices may help to reduce the difficulty of the operation. Modified bronchial anastomosis technique could also be used in single-port VATS bronchial anastomosis, which will broaden its application range.
Conclusions
In conclusion, modified bronchial anastomosis technique is safe and feasible in three-port or single-port VATS sleeve lobectomy with satisfactory short-term and long-term outcomes. Adequate management of the operative process and bronchial anastomosis technique could help to reduce the difficulty of VATS sleeve lobectomy. However, considerable experience in VATS is still needed for the widespread application of this technique.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by ethics committee of Fujian Medical University Fujian Union Hospital (No. 20101008) and written informed consent was obtained from all patients.
References
- Ma Z, Dong A, Fan J, et al. Does sleeve lobectomy concomitant with or without pulmonary artery reconstruction (double sleeve) have favorable results for non-small cell lung cancer compared with pneumonectomy? A meta-analysis. Eur J Cardiothorac Surg 2007;32:20-8. [Crossref] [PubMed]
- Shi W, Zhang W, Sun H, et al. Sleeve lobectomy versus pneumonectomy for non-small cell lung cancer: a meta-analysis. World J Surg Oncol 2012;10:265. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Hanna JM, Berry MF, D'Amico TA. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J Thorac Dis 2013;5 Suppl 3:S182-9. [PubMed]
- Mahtabifard A, Fuller CB, McKenna RJ Jr. Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg 2008;85:S729-32. [Crossref] [PubMed]
- Huang J, Li J, Qiu Y, Xu X, et al. Thoracoscopic double sleeve lobectomy in 13 patients: a series report from multi-centers. J Thorac Dis 2015;7:834-42. [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: first report. J Thorac Cardiovasc Surg 2013;145:1676-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Double sleeve uniportal video-assisted thoracoscopic lobectomy for non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:E2. [PubMed]
- Santambrogio L, Cioffi U, De Simone M, et al. Video-assisted sleeve lobectomy for mucoepidermoid carcinoma of the left lower lobar bronchus: a case report. Chest 2002;121:635-6. [Crossref] [PubMed]
- Li Y, Wang J. Video-assisted thoracoscopic surgery sleeve lobectomy with bronchoplasty: an improved operative technique. Eur J Cardiothorac Surg 2013;44:1108-12. [Crossref] [PubMed]
- Chen H, Huang L, Chen C, et al. Modified bronchial anastomosis technique in three-port video-assisted thoracoscopic surgery (VATS) right upper sleeve lobectomy. Asvide 2016;3:334. Available online: http://www.asvide.com/articles/1103
- Chen H, Huang L, Chen C, et al. Modified bronchial anastomosis technique in single-port video-assisted thoracoscopic surgery (VATS) right middle and lower sleeve lobectomy. Asvide 2016;3:335. Available online: http://www.asvide.com/articles/1104
- Agasthian T. Initial experience with video-assisted thoracoscopic bronchoplasty. Eur J Cardiothorac Surg 2013;44:616-23. [Crossref] [PubMed]
- Kutlu CA, Goldstraw P. Tracheobronchial sleeve resection with the use of a continuous anastomosis: results of one hundred consecutive cases. J Thorac Cardiovasc Surg 1999;117:1112-7. [Crossref] [PubMed]
- Yu D, Han Y, Zhou S, et al. Video-assisted thoracic bronchial sleeve lobectomy with bronchoplasty for treatment of lung cancer confined to a single lung lobe: a case series of Chinese patients. J Cardiothorac Surg 2014;9:67. [Crossref] [PubMed]
- Nakagawa T, Chiba N, Ueda Y, et al. Clinical experience of sleeve lobectomy with bronchoplasty using a continuous absorbable barbed suture. Gen Thorac Cardiovasc Surg 2015;63:640-3. [Crossref] [PubMed]
- Xu G, Zheng W, Guo Z, et al. Complete video-assisted thoracoscopic surgery upper left bronchial sleeve lobectomy. J Thorac Dis 2013;5 Suppl 3:S298-300. [PubMed]
- Yildizeli B, Fadel E, Mussot S, et al. Morbidity, mortality, and long-term survival after sleeve lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;31:95-102. [Crossref] [PubMed]
- Chakaramakkil MJ, Jim LY, Soon JL, et al. Continuous absorbable suture technique for tracheobronchial sleeve resections. Asian Cardiovasc Thorac Ann 2011;19:44-7. [Crossref] [PubMed]
- Lu H, Zhang Z, Li W, et al. Video-assisted thoracic surgery right sleeve lobectomy. J Thorac Dis 2013;5 Suppl 3:S323-4. [PubMed]
- Wang BY, Liu CY, Hsu PK, et al. Single-incision versus multiple-incision thoracoscopic lobectomy and segmentectomy: a propensity-matched analysis. Ann Surg 2015;261:793-9. [Crossref] [PubMed]


