Characteristics of patients with pseudochylothorax—a systematic review
Introduction
Pseudochylothorax (PCT) is a rare form of pleural effusion (PE), also called chyliform or cholesterol PE, and is characterized by its high cholesterol content and milky pleural fluid (PF). PCT and chylothorax both classically have a turbid or milky appearance due to their high lipid content. Apart from that, their etiologies, pathogenesis, and clinical implications differ, making it important to distinguish between them (1).
The most frequent causes of PCT include tuberculosis (TB), rheumatoid arthritis (RA), paragonimiasis, echinococcosis, neoplasia, or trauma (2). The pathogenesis of PCT has never been investigated in depth. However, the presence of an effusion for at least 5 years in the setting of a fibrous, thickened pleura due to chronic pleuritis is characteristic (2,3). It is believed that, as a result of the pleural thickening that blocks the drainage of fluids to the pleural wall lymphatic system, the cholesterol and lecithin-globulin complexes liberated after red cell and neutrophil lysis in the PF become trapped in the pleural cavity. In fact, some authors consider the PCT as a form of “lung entrapment” in the context of chronic inflammation (1). The diagnosis of PCT is established by the evaluation of the PF: milky PE, cholesterol level greater than 200 mg/dL, triglyceride level typically below 110 mg/dL [always with a cholesterol/triglycerides ratio (CHOL/TG ratio) >1], and often with cholesterol crystals seen on microscopy (1,4,5). The initial step in the management of PCT is treatment of the underlying disease process, which is usually benign. Additional components of the management approach, such as therapeutic thoracentesis and decortication, depend on the severity of the patient’s respiratory symptoms, degree and pattern of impairment in pulmonary function tests, and the ability of the lung to re-expand.
Despite these data, there are no sufficiently large series describing its features, and our aim was to document its etiology and behavior in these patients, as well as evaluate the PF analysis and the outcomes by presenting a systematic review.
Methods
This systematic review employed a methodology based on the principles of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) study (6). As there were not enough large series that responded to the needs of the study, the cases described in the literature were added using this methodology.
Selection criteria
All cases of any age published in any format were deemed eligible for inclusion, except abstracts of papers presented at conferences, and editorials, reviews, or letters to the editor that did not document new cases.
Sources of information
The search strategy included several sources of free databases available by the year of publication, although the full text of the study had to be in English, French, or Spanish. The literature search included the following electronic databases (online): Medline (through PubMed interface), and Scopus. Searches were conducted between 1 November and 31 November 2015. The following search terms were adopted for each database: pseudochylothorax OR cholesterol pleural effusion OR chyliform pleural effusion.
In addition to the electronic data bases consulted, a manual search was performed on the reference lists of the included articles. Any studies fulfilling the above criteria were included. Each article was then independently screened and assessed, and potentially relevant ones identified. Studies were reviewed in three stages, based on the title, abstract, and then full text, with consensus sought at each stage of review.
Data collection process
Data from selected studies were extracted electronically (Microsoft Excel 2010, Microsoft Corp, USA). The information extracted included the following: authors, date of publication, number of cases in the series, age, gender, personal and family history, presence of dyspnea and chest pain, side and size of PE, thoracic CT findings, PF appearance, characteristics of transudate or exudate, total and percentage nucleated cell count, levels of total protein in PF, lactate dehydrogenase (LDH), albumin, cholesterol, triglycerides, glucose, pH, adenosine deaminase (ADA), cholesterol crystals, culture results, cytology and pleural biopsy (PB), causes of PCT, treatments received and their responses, complications and deaths.
Methodological quality of individual studies
Because the articles reviewed were mostly case descriptions, their quality was not assessed in relation to the assessment of study type, internal validity, generalizability, heterogeneity, or precision.
Outcomes of interest
Outcomes of interest were to know the patient demographic data, associated diseases, causes of PCT, biochemical and cytological characteristics of the PE, and the response to various treatments.
Statistical analysis
Due to the wide heterogeneity and descriptive nature of the studies, a simple descriptive statistic (proportion, median and range) of each outcome of interest was calculated.
Results
Sixty-two studies (2,3,7-25) involving 104 patients (26-50) were selected for review (51-66). Figure 1 presents a flowchart for the complete breakdown in the identification of appropriate studies corresponding to isolated case reports and a retrospective series (35).
Demographic and clinical characteristics
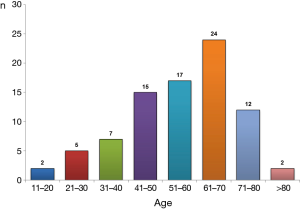
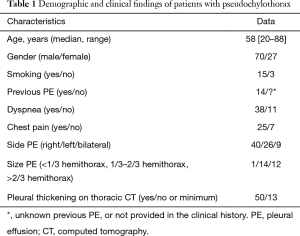
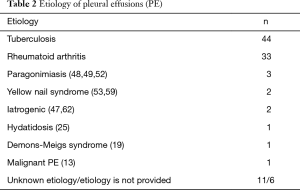
Clinical and demographic characteristics of the 104 patients included in the study are shown in Table 1, and their age-group distribution appears in Figure 2. The median age was 58 years (range, 20–88 years). The number of cases increased with age until the seventh decade of life, and in 77.1% (64/83) it was from 40–75 years (Figure 2). Age was not available for 21 patients. Of the final study population, 69/96 (71.9%) were men (ratio 2.6/1). Gender was not available for eight patients. In 77/87 cases (88.5%), the origin of PCT was TB (44 patients) or RA (33). The cause was unknown in 11 patients, and the origin was not stated in another 6 cases (Table 2). A previous PE was recorded in 14 patients (time interval: 2–40 years (2,8,9,17,23,24,39,53,56,57,59,65). In 10 (71.4%) of these (2,8,9,17,23,24,39,59,65), the previous PE was on the same side as the subsequent PCT; in two (14.3%) (56,57) the previous unilateral PE was found to be bilateral when the PCT was diagnosed; in one case (7.1%) (17) the previous bilateral PE was unilateral when the PCT was diagnosed, and in another, the side of the previous PE was not specified (53).
Full table
Full table
PE
PE was unilateral in 66/75 patients (88%), and right-sided in 40 (60.6%). The PE was bilateral in nine (12%) patients. The authors did not specify whether it was unilateral or bilateral in 29 cases. Pleural thickening was evaluated in 63 (60.6%) patients. In 13 (20.6%) cases, the thickening was minimum [five patients (49,55,58,63,64)], or non-existent [eight patients (50,53,55,66)].
In the 83 cases of patients with PE and descriptions of its appearance, 38 were milky, 33 cloudy, 7 opaque, 2 oily yellow (9,59), 1 bloody (33), 1 bloody-chylous (62), and 1 serous (23). Biochemical characteristics and cellular differentiation of PF are described in Table 3. The question of whether the PE was transudate or exudate was only addressed in 14 cases, and all corresponded to the latter. Cholesterol levels were available in 67.3% (70/104) of patients. The median was 332 mg/dL (range, 80–8,983 mg/dL), 18 (25.7%) patients had cholesterol levels <200 mg/dL (2,8-10,13,20,35,37,39,44-46,55,57,61,66), and 25% of patients (10/40) had triglycerides levels >110 mg/dL (2,18,41,43,46,52,55,57,62) (range, 4–716 mg/dL). The CHOL/TG ratio was >1 in 37/38 (97.4%). The only case with a CHOL/TG ratio <1 corresponded to a patient with RA (57). No cholesterol crystals were detected in the 10.3% (8/78) of patients (16,17,22,46,55). No chylomicrons were detected in the 18 cases in which they were determined (2,34,37-39,44-46,51,55,57,65).
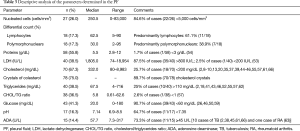
Full table
PF culture was available in 62 (59.6%) patients, and was positive in 16 (34.1%) (15 Mycobacterium tuberculosis (2,13,15,36,40,43,46,47,59,62,65) and 1 Clostridium (46). PF cytology was performed in 29 (27.9%) patients, and was negative in all cases.
PB
PB was performed on 26 patients (Figure 3). In the six cases in which the final diagnosis was TB (2,18,26,29,50,58), the biopsy culture was positive in only one patient (29).
Treatment of PE
Table 4 summarizes the most frequently used treatments (medical treatment, therapeutic thoracentesis, decortication/pleurectomy, pleurodesis, chest drainage, and thoracoscopic drainage, among others), as well as the outcomes (favorable: total or partial control of PE or its symptoms; unfavorable: no control of PE, recurrence of symptoms). Other treatments were ovarian tumor surgery in a patient with Demons-Meigs syndrome (19), and pneumonectomy in another patient (46), both with a favorable response. Although nine patients did not receive treatment, seven (77.8%) had a favorable outcome (2,20,34,35,37). The reported complications following diagnostic procedures were empyema in eight patients (2,18,38,46,57,64), and bronchopleural fistula in two (35,46).
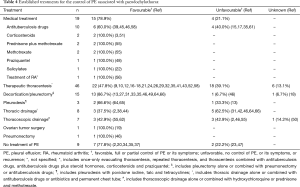
Full table
Discussion
Publications on PCT involve isolated case reports and a small series (35), so key issues such as clinical course, characteristics of PE or the most effective treatments are not well known. These questions were addressed using a systematic review of the literature relating to this disease.
Although PCT can occur at any age from the second decade of life, the typical patient is male (71.9%; ratio 2.6/1), aged between 40–75 years (77.1%), with dyspnea (77.6%), chest pain (78.1%), unilateral PE (88%), and size greater than one-third of the hemithorax (96.3%). The pleural thickening is not a requirement for the diagnosis (absent in 20.6%) and it is not unusual to find a history of a previous PE (9,17). Its origin is usually TB or RA (88.5%).
It was classically considered that the pleural thickening “retained” the cholesterol and the lecithin-globulin complexes liberated after red cell and neutrophil lysis in the PF, on blocking the drainage of fluid to the pleural wall lymphatic system. It is possible that this may be the cause in those patients that had presented with a previous PE, but the finding that 20% of the PCT do not involve pleural thickening, implies that other pathogenic mechanisms must be involved.
The terms “milky”, “cloudy”, “opaque”, etc., used to describe the characteristic appearance of PCT, can be interpreted as similar. However, serous (23), or bloody (33) fluids have been described in which the absence of the typical appearance of PCT does not exclude the diagnosis.
PCT are exudates, with a low count of nucleated cells (84.6%, <5,000 cells/mm3) and usually lymphocyte-predominant (61.1%) (Table 3). The characteristics of PF analysis are representative of a pleural space inflammation (87.5% of cases have an LDH >800 IU/L), as well as of increased capillary permeability or lymphatic dysregulation (98.3% of cases with proteins >3 g/dL). Moreover, glucose and pH values are generally less than 60 mg/dL (90.7%) and 7.35 mg/dL (64.7%) respectively. Cholesterol values >200 mg/dL, triglycerides <110 mg/dL, CHOL/TG ratio >1 and the presence of cholesterol crystals in PF were classically considered as useful tests for the diagnosis of PCT. However, 25.7% of the cases have cholesterol levels <200 mg/dL, and another 25% with triglycerides values >110 mg/dL, thus these determinations do not appear to be conclusive by themselves. A CHOL/TG ratio >1 (97.4%) and the presence of cholesterol crystals (89.7%) is more sensitive. This systematic review is not designed to establish the specificity of any test but, as far as the authors know, the specificity of the CHOL/TG ratio and cholesterol crystals is currently reported as being 100%. The PF culture may be useful in the diagnosis of PFs of tuberculous origin (positive in the 34.1% of cases), unlike PB, which was only positive in one patient (29). In general, the findings of PB have little relevance, except in the specific cases shown in Figure 3. Therefore, its role seems limited to a subset of patients that is not suspected RA or TB, but the diagnosis of a PCT is not yet clear. In the case that showed a pleural metastasis due to a lung adenocarcinoma, cholesterol crystals were also observed in the PF (13).
The management of PCT is not well defined thus far. Experts recommend a sequential approach by treating the underlying cause first (4). If the patient has symptoms or the PE increases, invasive techniques may be resorted to (depending on the complexity) in order to control the symptoms produced by a recurrent symptomatic PE (67). The most frequently used treatments are therapeutic thoracentesis (46 patients; alone or combined with medical treatment or another type of intervention), medical treatment on its own (19), and decortication/pleurectomy (15) with a favorable response from 47.8%, 78.9%, and 86.7%, respectively (Table 4). It is worth mentioning the high number of unfavorable outcomes with TB treatment (40%), although in at least two patients the treatment prescribed was not that currently recommended (15,17). Although it is classically said that PCT usually have a favorable outcome, the therapeutic thoracentesis, the pleurodesis, the thoracic drainage and the thoracoscopic drainage (all of them using their different methods) showed a high percentage of unfavorable outcomes (39.1%, 33.3%, 62.5% and 42.9%, respectively). Decortication/pleurectomy only had a poor outcome in one patient (6.7%) and it was necessary to perform a pneumonectomy (46). On the other hand, favorable outcomes have been reported (stabilization of symptoms and the size of the PE, and even a spontaneous improvement of the PCT) without having received any type of treatment (2,20,34,35,37). However, the favorable results, possibly very optimistic, should be interpreted with caution, since in the majority of these studies they were associated with various treatments and, sometimes it is difficult to know to which one the favorable outcome was due. Furthermore, the follow-up period was generally short in the majority of cases. Moreover, interventional procedures are not exempt from complications, such as empyema and bronchopleural fistulas (2,18,35,38,46,57,64).
Our review has some limitations. The most important was the reliance on descriptions of case reports, instead of case series or comparative trials. Therefore, the evaluation of the quality of the reported literature could limit internal validity, generalizability, and accuracy. In addition, some articles highlight the clinical characteristics of the patients, while others stress diagnostic and therapeutic aspects. Due to the heterogeneity of the reported information, it is not possible to provide the details required, making it difficult to correctly classify some effusions or evaluate the response to a particular treatment. As in all rare disease, the publication bias would be another limitation to consider since the successfully treated cases or atypical cases are more likely to get published.
In summary, the mechanism by which a PCT is produced is not sufficiently clear. They are effusions that are more common in males, and its origin is usually TB or AR. It is not unusual to find a history of a previous PE recorded in the clinical notes, and the lack of pleural thickening does not rule out the diagnosis. PE is usually unilateral, and the PF is an exudate of milky appearance in which the CHOL/TG ratio is almost always >1, and cholesterol crystals are usually observed. The culture may be useful for the TB diagnosis and the PB does not usually provide useful information except the observation of cholesterol crystals in some cases. Finally, the treatment must be sequential, treating the underlying cause in the first place and, afterwards, assessing the need to resort to interventionist techniques (starting with the least invasive) in order to control the symptoms of the patient, and also taking into account that these are not exempt from complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Agrawal V, Sahn SA. Lipid pleural effusions. Am J Med Sci 2008;335:16-20. [Crossref] [PubMed]
- Garcia-Zamalloa A, Ruiz-Irastorza G, Aguayo FJ, et al. Pseudochylothorax. Report of 2 cases and review of the literature. Medicine (Baltimore) 1999;78:200-7. [Crossref] [PubMed]
- Ferguson GC. Cholesterol pleural effusion in rheumatoid lung disease. Thorax 1966;21:577-82. [Crossref] [PubMed]
- Sassoon CS, Light RW. Chylothorax and pseudochylothorax. Clin Chest Med 1985;6:163-71. [PubMed]
- Hillerdal G. Chylothorax and pseudochylothorax. Eur Respir J 1997;10:1157-62. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [Crossref] [PubMed]
- Schulman M. Pleural effusion, largely cholesterol. JAMA 1917;LXVIII:1256. [Crossref]
- Weems BF. Cholesterohydrothorax. Am J Med Sci 1918;156:20. [Crossref]
- Evander LC. Cholesterol pleural effusion. Am Rev Tuberc 1946;54:504-8. [PubMed]
- Stein HF. Cholesterol pleural effusion: report of a case. Am Rev Tuberc 1947;56:305-7. [PubMed]
- Curran TM. Cholesterol pleural effusion. Edinb Med J 1948;55:252-5. [PubMed]
- Lyons HA. Cholesterol pleural effusion. Dis Chest 1949;16:495-500. [Crossref] [PubMed]
- Goldman A, Burford TH. Cholesterol pleural effusion: A report of 3 cases with a cure by decortication. Dis Chest 1950;18:586-94. [Crossref] [PubMed]
- Cuddihey KP. Crystalline cholesterol effusion of the pleural space. Ir J Med Sci 1950.389-91. [Crossref] [PubMed]
- Frew HW, Campbell-fowler C. Cholesterol pleural effusion. Practitioner 1956;176:416-9. [PubMed]
- Lopez sendon JL. Pleuritis colesterinica. Rev Clin Esp 1958;70:106-9. [PubMed]
- Coe JE, Aikawa JK. Cholesterol pleural effusion. Report of 2 cases studied with isotopic techniques and review of the world literature. Arch Intern Med 1961;108:763-74. [Crossref] [PubMed]
- Mannerberg FD. Brandt En Jr, Shook MB. Cholesterol pleural effusion. J Okla State Med Assoc 1962;55:378-83. [PubMed]
- Ledoux A, Gille P, Pageaut G, et al. Demons-meigs syndrome with chyliform effusions and ovarian goiter. Ann Med Nancy 1964;150:1150-8. [PubMed]
- Dodson WH, Hollingsworth JW. Pleural effusion in rheumatoid arthritis. Impaired transport of glucose. N Engl J Med 1966;275:1337-42. [Crossref] [PubMed]
- Stengel BF, Watson RR, Darling RJ. Pulmonary rheumatoid nodule with cavitation and chronic lipid effusion. JAMA 1966;198:1263-6. [Crossref] [PubMed]
- Bower GC. Chyliform pleural effusion in rheumatoid arthritis. Am Rev Respir Dis 1968;97:455-9. [PubMed]
- Laguarda R, Benner MH, Snider GL. Calcified cholesterol-containing pleural effusion. Chest 1971;60:597-8. [Crossref] [PubMed]
- Matsuura C, Murakami T, Kajiyama G, et al. A case of cholesterol pleurisy with special reference to lipid analysis of the serum and pleuiral fluid. Hiroshima J Med Sci 1971;20:195-206. [PubMed]
- Carel RS, Schey G, Bruderman I. Chyliform pleural effusion. An unusual manifestation of hepatothoracic echinococcus cysts. Chest 1975;68:598-9. [Crossref] [PubMed]
- Purohit SD, Sharma GS, Banerjee K. Cholesterol pleural effusion. Ind J Tub 1978;25:43-4.
- Wagoner J Jr, Doherty JE, Straub KD. Radiocarbon cholesterol turnover in cholesterol thorax. J Ark Med Soc 1979;75:321-7. [PubMed]
- Riou R, Mauduit G, Touraine R. Rheumatoid pleurisy with cholesterol pleural effusion. A single case. Literature review (author's transl). Poumon Coeur 1980;36:213-4. [PubMed]
- Ayensa Dean C, Muñoz Fernández J, Pérez Martí M, et al. Cholesterol pleurisy. Med Clin (Barc) 1982;78:352. [PubMed]
- Marsac J, Frija G, Bismuth V. Chylothorax and the pathology of the lymphatic pleura. Rev Fr Mal Respir 1982;10:227-41. [PubMed]
- Ayzenberg O, Reiff DB, Levin L. Bilateral pneumothoraces and pleural effusions complicating rheumatoid lung disease. Thorax 1983;38:159-60. [Crossref] [PubMed]
- Shiel WC Jr, Prete PE. Pleuropulmonary manifestations of rheumatoid arthritis. Semin Arthritis Rheum 1984;13:235-43. [Crossref] [PubMed]
- Lee SS, Trimble RB. Rheumatoid arthritis with bloody and cholesterol pleural effusion. Arch Pathol Lab Med 1985;109:769-71. [PubMed]
- Barberá Mir JA, Medina Fernández-Aceytuno C, Vallejo Galbete J, et al. Cholesterol pleural effusion. Rev Clin Esp 1985;176:372-3. [PubMed]
- Hillerdal G. Chyliform (cholesterol) pleural effusion. Chest 1985;88:426-8. [Crossref] [PubMed]
- Engel U, Aru A, Francis D. Rheumatoid pleurisy. Specificity of cytological findings. Acta Pathol Microbiol Immunol Scand A 1986;94:53-6. [PubMed]
- Van Meerhaeghe A, Ilunga E. Chyliform pleurisy. Rev Med Brux 1987;8:494-6. [PubMed]
- Rodriguez Glez-Moro JM, Izquierdo-Alonso JL, Gomez-Nebreda MJ, et al. Derrames pleurales colesterínicos. An Med Intern (Madrid) 1987;4:602-4.
- Bargay J, Tarrasa J, Salva F, et al. Tuberculous pseudochylothorax with thoracic wall involvement. Med Clin (Barc) 1990;94:76-7. [PubMed]
- Teira Cobo R, Fariña Sarasqueta S, Merino Múgica J. Pseudoquilotórax: Presentación de un caso. Arch Bronconeumol 1991;27:85. [Crossref]
- Hamm H, Pfalzer B, Fabel H. Lipoprotein analysis in a chyliform pleural effusion: implications for pathogenesis and diagnosis. Respiration 1991;58:294-300. [Crossref] [PubMed]
- Debieuvre D, Gury JP, Ory JP, et al. Association of pseudochylothorax and pleural tuberculosis. Apropos of a case Rev Pneumol Clin 1994;50:175-7. [PubMed]
- González R, Ramírez-Rivera J. Chyliform (pseudochylous) pleural effusion. Bol Asoc Med P R 1994;86:50-2. [PubMed]
- Caballero N, Viejo Menéndez MC, Arévalo González M, et al. Pseudochylothorax: apropos a case. An Med Interna 1995;12:202-3. [PubMed]
- Ribas Sola J, Ruiz Manzano J, Gallego Díaz M, et al. Tuberculous pseudochylothorax. Presentation of a case with positive Mycobacterium tuberculosis culture and review of the literature. Arch Bronconeumol 1995;31:80-2. [Crossref] [PubMed]
- Campos Rodríguez F, Alfageme Michavila I, Hernández Borge J, et al. Pseudochylothorax. Review of 5 cases. Arch Bronconeumol 1997;33:422-5. [Crossref] [PubMed]
- Garcia-Pachon E, Fernandez LC, Lopez-Azorin F, et al. Pseudochylothorax in pleural effusion due to coronary artery bypass surgery. Eur Respir J 1999;13:1487-8. [Crossref] [PubMed]
- Inoue Y, Kawaguchi T, Yoshida A, et al. Paragonimiasis miyazakii associated with bilateral pseudochylothorax. Intern Med 2000;39:579-82. [Crossref] [PubMed]
- Song JW, Im JG, Goo JM, et al. Pseudochylous pleural effusion with fat-fluid levels: report of six cases. Radiology 2000;216:478-80. [Crossref] [PubMed]
- Nogueras C, Monteagudo M, Vila M, et al. Recent-onset tuberculous pleurisy presenting as pseudochylothorax. Am J Med 2002;113:166-8. [Crossref] [PubMed]
- Canalejo Castrillero E, Carratalá Blasco C, Matanza Rodríguez I, et al. Pleural effusion in patient with long course rheumatoid arthritis. Rev Clin Esp 2005;205:627-9. [Crossref] [PubMed]
- Thewjitcharoen Y, Poopitaya S. Paragonimiasis presenting with unilateral pseudochylothorax: case report and literature review. Scand J Infect Dis 2006;38:386-8. [Crossref] [PubMed]
- Koegelenberg CF, Theron J, Brundyn K, et al. A patient with a persistent pleural effusion. Respiration 2006;73:120-3. [Crossref] [PubMed]
- Shen PU, Blair JL. Cholesterol crystals causing falsely elevated automated cell count. Am J Clin Pathol 2006;125:358-63. [Crossref] [PubMed]
- Wrightson JM, Stanton AE, Maskell NA, et al. Pseudochylothorax without pleural thickening: time to reconsider pathogenesis? Chest 2009;136:1144-7. [Crossref] [PubMed]
- Inonu H, Yeginsu A, Duran Yücesoy F, et al. Pseudochylothorax Secondary to Rheumatoid Arthritis: Case Report. Turkiye Klinikleri J Med Sci 2011;31:1274-7. [Crossref]
- Chong SG, Chauhan Z, Di Nino E, et al. Effusion under the microscope. Monaldi Arch Chest Dis 2012;77:32-4. [PubMed]
- Malhotra P, Watson JP, Plant PK, et al. A cloudy pleural effusion. Thorax 2012;67:658-9. [Crossref] [PubMed]
- Raljevic S, Pesut DP, Stjepanovic MI, et al. Yellow nail syndrome: Recurrent pleural effusion in a patient with severe chronic obstructive pulmonary disease. Healthmed 2012;6:640-2.
- Genzen JR, Motin A. Cholesterol crystals in pleural fluid. Am J Respir Crit Care Med 2012;185:586. [Crossref] [PubMed]
- Hibino M, Hikino K, Oe M, et al. Cholesterol crystals in pleural effusion. Intern Med 2013;52:2685. [Crossref] [PubMed]
- Puttagunta HK, Seneviratne C, Kupfer Y, et al. Pseudochylothorax and diaphragmatic weakness secondary to a misplaced central venous catheter. BMJ Case Rep 2013;2013.
- Yokosuka T, Suda A, Sugisaki M, et al. Rheumatoid pleural effusion presenting as pseudochylothorax in a patient without previous diagnosis of rheumatoid arthritis. Respir Med Case Rep 2013;10:37-9. [PubMed]
- Ergin M, Yeginsu A, Gurlek K, et al. Pseudochylothorax due to Rheumatoid Arthritis; A Very Rare Entity. J Clin Anal Med 2014;5:62-4. [Crossref]
- Berg J, Guiot J, Heinen V, et al. Comparison between chylothorax and pseudochylothorax. Rev Med Liege 2015;70:73-7. [PubMed]
- Priya MP, Dharmic S, Kar A, et al. An unusual case of pseudochylothorax. J Pharm Bioallied Sci 2015;7:S80-2. [Crossref] [PubMed]
- Ryu JH, Tomassetti S, Maldonado F. Update on uncommon pleural effusions. Respirology 2011;16:238-43. [Crossref] [PubMed]