
Riociguat as bridging therapy to pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: a retrospective cohort study
Highlight box
Key findings
• The use of riociguat as bridging therapy demonstrated hemodynamic improvement in patients with high preoperative pulmonary vascular resistance before pulmonary endarterectomy. However, no additional benefits in postoperative mortality or hemodynamics were observed.
What is known and what is new?
• In operable chronic thromboembolic pulmonary hypertension patients, the utilization of bridging therapy with targeted medications prior to pulmonary endarterectomy remains a topic of controversy.
• We aim to assess the impact of riociguat, a soluble guanylate cyclase stimulator, as a bridging therapy on postoperative hemodynamics and outcomes.
What is the implication, and what should change now?
• Active recommendation of short-term riociguat use before pulmonary endarterectomy, particularly in patients with stable hemodynamics, should be approached with caution.
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) results from fibrotic intravascular material in proximal pulmonary arteries and micro vasculopathy of pulmonary arterioles, causing occlusion and stenosis. This increases pulmonary vascular resistance (PVR), leading to progressive right heart failure and eventual mortality (1). Treatment for CTEPH involves a multimodal approach, including pulmonary endarterectomy (PEA), balloon pulmonary angioplasty, and targeted therapy for pulmonary hypertension (PH) (1,2). PEA is presently the most effective and curative therapy for operable patients (3), despite its technical challenges and an observed 2–3% in-hospital mortality rate at experienced centers (4,5).
High preoperative PVR serves as a predictor of perioperative mortality and late survival (6,7). The use of PH targeted medications as a bridging therapy before PEA to optimize hemodynamics may positively impact perioperative outcomes. However, controversy exists regarding the utilization of targeted therapies before PEA (8). Pilot studies with bosentan or prostacyclin demonstrated improved pre-PEA hemodynamics but no significant difference post-PEA compared to controls (9-11). Two retrospective studies suggested better outcomes with the use of PH targeted therapies as a bridge to PEA in patients with severe hemodynamic impairment (12,13). Conversely, a study from the University of California at San Diego (UCSD) indicated that bridging therapy was associated with delayed referral without clear benefits in outcomes (14). Similar findings from a prospective European registry suggested that bridging therapy, although commonly used in patients with severe hemodynamic impairment, was an independent predictor of mortality (15).
Riociguat, a soluble guanylate cyclase (sGC) stimulator, has gained approval for use in patients with inoperable CTEPH and persistent PH based on its outstanding performance in the Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase Stimulator Trial 1 (CHEST-1) (16). Additionally, the utilization of riociguat as a potential bridging therapy in CTEPH patients is becoming increasingly prevalent (17). Unfortunately, the PEA Bridging Study (ClinicalTrials.gov: NCT03273257), designed to explore the efficacy of riociguat in operable CTEPH patients with high PVR, was terminated in 2021 due to slow enrollment. In this study, we aim to assess the clinical impact of riociguat as a bridging therapy on the outcomes of PEA. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-48/rc).
Methods
Study population
This research adopted a retrospective cohort study design. From December 2016 to November 2023, we retrospectively examined all patients undergoing PEA at our institution. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and received approval from the institutional review board of the China-Japan Friendship Hospital (No. 2022-KY-088). Informed consent for data usage was obtained from all patients.
Inclusion criteria comprised: (I) confirmation of chronic pulmonary embolism through computed tomography pulmonary angiography, ventilation/perfusion scan, and pulmonary angiography following adequate anticoagulation for a minimum of 3 months; and (II) resting mean pulmonary artery pressure (PAP) >20 mmHg based on right heart catheterization (RHC).
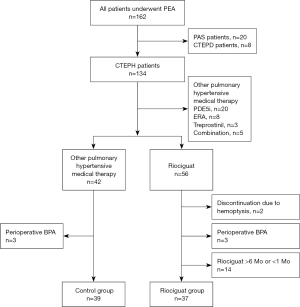
Exclusion criteria included: (I) patients with chronic thromboembolic pulmonary disease or pulmonary artery sarcoma; (II) patients receiving other PH medications before PEA, such as phosphodiesterase type 5 inhibitors, endothelin receptor antagonis, or treprostinil; (III) patients undergoing balloon pulmonary angioplasty before PEA; (IV) discontinuation of riociguat before PEA due to hemoptysis; and (V) patients with a short (<1 month) or long (>6 months) history of riociguat. Patients were categorized into two groups: the riociguat group or the control group, based on the use of riociguat before PEA. Please refer to Figure 1 for the study flow diagram.
Surgical procedure and therapeutic regimen
All patients received anticoagulation and treatment for heart failure. In the riociguat group, riociguat was administered following hemodynamic assessment by RHC. The dose adjustment plan for riociguat, as detailed in a prior study (16), involved initiating a dose of 1 mg three times daily. Subsequent adjustments, either an increase or decrease of 0.5 mg every two weeks, were made based on systolic blood pressure. Riociguat was continued until PEA, with a maximum dose of 2.5 mg three times daily.
RHC was conducted to monitor hemodynamics after anesthesia induction. The PEA procedure, following the UCSD Health Center experiment (18), adhered to fundamental principles including: (I) initiation of deep hypothermia circulatory arrest to ensure optimal visibility and identification of the correct plane when the core temperature reached 20 °C; (II) a maximum circulatory arrest time of 20 minutes, with intermittent re-establishment of cardiopulmonary bypass; and (III) completion of endarterectomy from the main pulmonary artery to the distal ends of the subsegmental vessels.
Data collection and clinical end points
Clinical data were gathered from electronic medical records, encompassing demographic characteristics, laboratory tests, hemodynamic parameters, intraoperative details, perioperative complications, and mortality. For patients in the riociguat group, we assessed transthoracic echocardiography parameters, N-terminal pro-brain natriuretic peptide (NT-proBNP), 6-minute walk distance (6-MWD), and PVR before and after riociguat administration. The PVR after riociguat administration was obtained when RHC was conducted to monitor hemodynamics after anesthesia induction before surgery. The baseline data of general characteristics including laboratory tests, 6-MWD and transthoracic echocardiography were obtained within 1 week before PEA. The baseline hemodynamic characteristics were obtained before riociguat. The follow-up period extended to December 2023, capturing survival status and World Health Organization functional class (WHO-FC). Additionally, the 6-MWD of patients was documented during their last hospital follow-up.
The primary endpoints centered on postoperative outcomes, specifically mortality and complications. Secondary endpoints included hemodynamic measurements post-PEA. Postoperative pulmonary hemodynamic parameters were assessed using a Swan-Ganz catheter in the intensive care unit, defining residual PH as mean PAP ≥25 mmHg. Severe reperfusion pulmonary edema was classified as grade 3 and grade 4, following established criteria (19). Postoperative acute kidney injury was identified as a ≥1.5-fold increase over baseline within 7 days (20).
Propensity score matching analysis
Given the real-world nature of this study, propensity score matching was conducted to facilitate a comparison between the two groups. Propensity scores, based on age, time from diagnosis to PEA, preoperative PVR before riociguat, and WHO-FC, influencing perioperative outcomes, were calculated for each patient. Matching involved pairing each patient in the riociguat group with one patient in the control group using nearest neighbor matching, with a caliper value of 0.1.
Statistical analysis
All statistical analysis were conducted using SPSS Statistics Software 25 (SPSS Inc., Chicago, IL, USA). Continuous variables with normal distribution were described by the mean and standard deviation (mean ± SD), and compared using t-tests. Continuous variables with non-normal distributions were described by the median and interquartile ranges (25th, 75th percentile), and compared using Mann-Whitney U-test. Categorical variables were expressed as numbers with percentages. A Chi-squared test or Fisher’s exact test was used to compare categorical variables. A P value <0.05 (two-sided) was considered statistically significant.
Results
Prevalence of bridging therapy and the effect before PEA
The prevalence of PH targeted therapy before PEA for CTEPH patients was 68.7%. Riociguat emerged as the predominant bridging therapy since 2018, accounting for 41.8% of cases.
Following riociguat administration, with a mean duration of 2.6±1.2 months, 56.8% of patients reached the maximal dose of 2.5 mg three times daily, while 32.4% and 10.4% received riociguat at doses of 2.0 and 1.5 mg, respectively. Notably, the dose of riociguat remained unchanged in 7 patients (18.9%) during the medication period.
Comparative characteristics of patients are presented in Table 1 and Figure 2. Among patients with PVR ≥800 dynes·sec·cm−5, PVR significantly decreased post-riociguat use {1,207 [974–1,698] vs. 1,125 [928–1,486] dynes·sec·cm−5, P<0.01}. Conversely, no significant change was observed in patients with PVR <800 dynes·sec·cm−5 {641 [474–740] vs. 600 [480–768] dynes·sec·cm−5, P=0.46}. The median NT-proBNP decreased after riociguat use {1,162 [231–1,949] vs. 1,479 [432–2,506] pg/mL, P<0.01}. Meanwhile, the median 6-MWD significantly increased from 330 [215–413] to 380 [265–430] m, with a median increase of 18 m. However, transthoracic echocardiography parameters, including systolic PAP and the size of the heart chambers, exhibited only slight improvements with no statistical significance.
Table 1
| Variables | Before riociguat | After riociguat | Change | P value |
|---|---|---|---|---|
| PVR (dynes·sec·cm−5) | ||||
| ≥800, n=22 | 1,207 (974 to 1,698) | 1,125 (928 to 1,486) | −116 (−311 to −21) | <0.01 |
| <800, n=15 | 641 (474 to 740) | 600 (480 to 768) | 26 (−41 to 58) | 0.46 |
| NT-proBNP (pg/mL) | 1,479 (432 to 2,506) | 1,162 (231 to 1,949) | −379 (−784 to −116) | <0.01 |
| 6-MWD (m) | 330 (215 to 413) | 380 (265 to 430) | 18 (6 to 40) | <0.01 |
| Preoperative TTE | ||||
| TDRA (mm) | 55±13.7 | 54±12.1 | −1±5.2 | 0.24 |
| RVTDd (mm) | 49.8±7.4 | 48.5±7.9 | −1.2±4.2 | 0.09 |
| LVEDd (mm) | 41.1±5.3 | 41.1±5.2 | 0.03±2.7 | 0.95 |
| RV/LV | 1.43±0.37 | 1.37±0.33 | −0.06±0.24 | 0.14 |
| TR velocity (cm/sec) | 440 (389 to 488) | 433 (392 to 478) | −5 (−13.5 to 2.5) | 0.22 |
| TAPSE (mm) | 15.35±3.55 | 15.82±3.66 | 0.2 (−0.05 to 0.9) | 0.08 |
| TVRS (cm/sec) | 9.29±2.13 | 9.81±2.54 | 0.08 (−0.1 to 0.85) | 0.07 |
| Systolic PAP (mmHg) | 83.7±21.3 | 81.8±19.6 | −2±7.2 | 0.11 |
Continuous variables are presented as mean ± standard deviation for normally distributed data or median (25th–75th percentile) for non-normally distributed data. PVR, pulmonary vascular resistance; NT-proBNP, N-terminal pro-brain natriuretic peptide; 6-MWD, 6-minute walking distance; TTE, transthoracic echocardiography; TDRA, transverse diameter of the right atrium; RVTDd, diastolic right ventricular basal segment transverse diameter; LVEDd, left ventricular end-diastolic diameter; RV/LV, right ventricular diameter/left ventricular diameter; TR, tricuspid regurgitation; TAPSE, tricuspid annular plane systolic excursion; TVRS, systolic tricuspid velocity of right ventricle; PAP, pulmonary arterial pressure.

Baseline clinical and hemodynamic characteristics
In this study, 76 eligible patients were enrolled, comprising 37 patients in the riociguat group and 39 patients in the control group. Propensity score matching was employed to address selection bias in bridging therapy, resulting in the identification of 26 comparable patients in each group. Table 2 presents a summary of baseline clinical and hemodynamic characteristics.
Table 2
| Variables | Before matching | After matching | |||||
|---|---|---|---|---|---|---|---|
| Riociguat (n=37) | Control (n=39) | P value | Riociguat (n=26) | Control (n=26) | P value | ||
| Gender | 0.37 | 0.77 | |||||
| Male | 23 (62.6) | 28 (71.8) | 17 (65.4) | 18 (69.2) | |||
| Female | 14 (37.8) | 11 (28.2) | 9 (34.6) | 8 (30.8) | |||
| Age (years) | 60 [45–63] | 51 [34–61] | 0.04 | 60 [44–63] | 56 [44–63] | 0.90 | |
| BMI (kg/m2) | 23.53±3.46 | 25.03±3.35 | 0.06 | 23.90±3.05 | 24.94±3.59 | 0.27 | |
| BSA (m2) | 1.7±0.23 | 1.83±0.18 | 0.01 | 1.73±0.16 | 1.81±0.16 | 0.09 | |
| Time from diagnosis to PEA (m) | 14.2 [6.1–23.8] | 7.2 [2.3–15.5] | 0.01 | 6.6 [5.3–15.4] | 9.2 [3.7–18.4] | 0.99 | |
| Smoking history | 13 (35.1) | 16 (41.0) | 0.58 | 8 (30.8) | 11 (42.3) | 0.39 | |
| Acute PE history | 25 (67.6) | 30 (76.9) | 0.36 | 16 (61.5) | 19 (73.1) | 0.38 | |
| Comorbidities | |||||||
| Hypertension | 16 (43.2) | 9 (23.1) | 0.06 | 10 (38.5) | 6 (23.1) | 0.23 | |
| Coronary heart disease | 6 (16.2) | 4 (10.3) | 0.67 | 4 (15.4) | 3 (11.5) | 1 | |
| Hyperlipidemia | 13 (35.1) | 15 (38.5) | 0.76 | 9 (34.6) | 11 (42.3) | 0.57 | |
| COPD | 12 (32.4) | 5 (12.8) | 0.04 | 8 (30.8) | 4 (15.4) | 0.19 | |
| Preoperative laboratory tests | |||||||
| Hemoglobin (g/L) | 142±18.8 | 143.1±20.6 | 0.81 | 140.7±18.2 | 142.7±18.1 | 0.69 | |
| Neutrophil (×109/L) | 3.42 [2.76–4.27] | 3.81 [2.57–4.32] | 0.57 | 3.4 [2.85–4] | 3.57 [2.45–4.22] | 0.96 | |
| Lymphocyte (×109/L) | 1.6±0.68 | 2.05±0.59 | <0.01 | 1.66±0.74 | 1.94±0.47 | 0.12 | |
| Platelet (×109/L) | 204.8±66.6 | 211.8±69.3 | 0.66 | 212.2±70.1 | 210.6±64 | 0.93 | |
| Total bilirubin (μmol×10/L) | 22.68 [14.56–33.59] | 13.69 [10.97–19.48] | <0.01 | 23.83 [14.33–34.11] | 13.53 [11.13–19.44] | <0.01 | |
| Direct bilirubin (μmol/L) | 4.65 [3.05–8.85] | 3.1 [2.09–4.28] | <0.01 | 4.51 [2.86–8.83] | 3.18 [2.43–4.91] | 0.05 | |
| SCR (μmol/L) | 75.3±9.8 | 71.6±12.3 | 0.16 | 76.5±10.9 | 74.8±10.8 | 0.75 | |
| NT-proBNP (pg/mL) | 1,162 [231–1,949] | 677 [179–1,133] | 0.09 | 665 [300–1,901] | 720 [394–1,201] | 0.74 | |
| Preoperative TTE | |||||||
| TDRA (mm) | 54±12.1 | 51±9.9 | 0.24 | 53.5±11.6 | 54±8.8 | 0.84 | |
| RVTDd (mm) | 48.5±7.9 | 48. 8±7.3 | 0.90 | 48.7±7.8 | 50±6.4 | 0.54 | |
| RV/LV | 1.37±0.33 | 1.31±0.32 | 0.39 | 1.35±0.3 | 1.34±0.29 | 0.93 | |
| TR velocity (cm/sec) | 433 [392–478] | 410 [340–453] | 0.06 | 432 [392–477] | 432 [365–465] | 0.41 | |
| TAPSE (mm) | 15.82±3.66 | 17.63±3.32 | 0.03 | 16.61±3.3 | 17.95±3.59 | 0.17 | |
| TVRS (cm/sec) | 9.81±2.54 | 10.8±2.65 | 0.10 | 10.34±2.51 | 10.69±2.63 | 0.63 | |
| Preoperative RHC | |||||||
| Cardiac output (L/min) | 2.93±0.89 | 3.3±0.8 | 0.06 | 3.07±0.88 | 3.08±0.74 | 0.95 | |
| Cardiac index (L/min/m2) | 1.7 [1.33–2.03] | 1.81 [1.43–2.08] | 0.12 | 1.73 [1.4–2.1] | 1.68 [1.4–1.95] | 0.76 | |
| Right atrial pressure (mmHg) | 4 [2–7] | 5 [2–7] | 0.71 | 4 [2–6] | 5 [2–7] | 0.61 | |
| Systolic PAP (mmHg) | 79±18.8 | 75.7±20.6 | 0.46 | 79.3±14 | 79.7±19.6 | 0.94 | |
| Diastolic PAP (mmHg) | 27 [24–32] | 24 [20–29] | 0.12 | 27 [24–32] | 25 [22–30] | 0.44 | |
| Mean PAP (mmHg) | 43.8±9.7 | 41.8±11.1 | 0.42 | 44.5±9.1 | 43.7±10.2 | 0.77 | |
| PVR (dynes·sec·cm−5) | 921 [672–1,400] | 843 [525–1,084] | 0.07 | 874 [646–1,132] | 943 [581–1,164] | 0.71 | |
| 6-MWD (m) | 380 [265–430] | 400 [345–490] | 0.13 | 385 [328–446] | 400 [321–483] | 0.56 | |
| WHO functional class | 0.01 | 0.16 | |||||
| II | 12 (32.4) | 24 (61.5) | 9 (34.6) | 14 (53.8) | |||
| III/IV | 18 (48.6)/7 (18.9) | 11 (28.2)/4 (10.3) | 13 (50.0)/4 (15.4) | 9 (34.6)/3 (11.5) | |||
Continuous variables are presented as mean ± standard deviation for normally distributed data or median [25th–75th percentile] for non-normally distributed data. Categorical variables are reported as frequencies (percentages). BMI, body mass index; BSA, body surface area; PEA, pulmonary endarterectomy; PE, pulmonary embolism; COPD, chronic obstructive pulmonary diseases; SCR, serum creatinine; NT-proBNP, N-terminal pro-brain natriuretic peptide; TTE, transthoracic echocardiography; TDRA, transverse diameter of the right atrium; RVTDd, diastolic right ventricular basal segment transverse diameter; RV/LV, right ventricular diameter/left ventricular diameter; TR, tricuspid regurgitation; TAPSE, tricuspid annular plane systolic excursion; TVRS, systolic tricuspid velocity of right ventricle; RHC, right heart catheterization; PAP, pulmonary arterial pressure; PVR, pulmonary vascular resistance; 6-MWD, 6-minute walking distance; WHO, World Health Organization.
Before matching, the riociguat group exhibited a higher proportion of patients with WHO-FC III/IV (67.6% vs. 38.5%, P=0.01) and chronic obstructive pulmonary diseases (32.4% vs. 12.8%, P=0.04). However, significant differences were observed in the median age {60 [45–63] vs. 51 [34–61] years, P=0.04}, body surface area (1.7±0.23 vs. 1.83±0.18 m2, P=0.01), and time from diagnosis to PEA {14.2 [6.1–23.8] vs. 7.2 [2.3–15.5] m, P=0.01} between the two groups. Additionally, the riociguat group presented higher serum total bilirubin {22.68 [14.56–33.59] vs. 13.69 [10.97–19.48] µmol/L, P<0.01} and serum direct bilirubin {4.65 [3.05–8.85] vs. 3.1 [2.09–4.28] µmol/L, P<0.01}, along with a lower tricuspid annular plane systolic excursion (15.82±3.66 vs. 17.63±3.32 mm, P=0.03), indicating worse right heart function in the riociguat group. Following matching, all preoperative variables were similar between the groups, except for serum total bilirubin (Figure 3). Moreover, the effect of using riociguat among 26 patients in riociguat group after matching are presented in Table S1, which were similar to the data in Table 1.

Intraoperative variables and early outcomes of PEA
After matching, no differences were observed in intraoperative variables between the two groups (Table 3), including total operative time (587±83 vs. 602±87 min, P=0.54), cardiopulmonary bypass time (339±53 vs. 346±52 min, P=0.65), aortic blocking time (158±31 vs. 166±41 min, P=0.41), and circulatory arrest time (63±17 vs. 62±16 min, P=0.78). The distribution of surgical classifications was similar in both groups, with the majority of patients exhibiting type I disease (57.7% vs. 61.5%, P=0.68).
Table 3
| Variables | Before matching | After matching | |||||
|---|---|---|---|---|---|---|---|
| Riociguat (n=37) | Control (n=39) | P value | Riociguat (n=26) | Control (n=26) | P value | ||
| Intraoperative data | |||||||
| CPB time (min) | 338±49 | 336±48 | 0.81 | 339±53 | 346±52 | 0.65 | |
| Aortic blocking time (min) | 158±27 | 162±40 | 0.59 | 158±31 | 166±41 | 0.41 | |
| Circulatory arrest time (min) | 63±16 | 57±17 | 0.09 | 63±17 | 62±16 | 0.78 | |
| Total operation time (min) | 582±74 | 583±91 | 0.95 | 587±83 | 602±87 | 0.54 | |
| Blood loss volume (mL) | 400 [300–800] | 500 [200–800] | 0.76 | 400 [300–1,000] | 400 [200–800] | 0.07 | |
| Surgical classification | 0.16 | 0.68 | |||||
| I | 25 (67.6) | 21 (53.8) | 15 (57.7) | 16 (61.5) | |||
| II | 8 (21.6) | 16 (41) | 7 (26.9) | 8 (30.8) | |||
| III | 4 (10.8) | 2 (5.1) | 4 (15.4) | 2 (7.7) | |||
| Postoperative cardiac function | |||||||
| Systolic PAP (mmHg) | 34.8±13.4 | 35.2±14.1 | 0.89 | 34.1±13.4 | 38.9±14.9 | 0.23 | |
| Mean PAP (mmHg) | 21.9±7.5 | 21.5±6.6 | 0.80 | 21.7±7.7 | 22.9±6.7 | 0.54 | |
| Mean PAP ≥25 mmHg | 11 (29.7) | 10 (25.6) | 0.69 | 6 (23.1) | 8 (30.8) | 0.53 | |
| PVR (dynes·sec·cm−5) | 349 [291–456] | 313 [225–406] | 0.15 | 326 [254–398] | 361 [290–445] | 0.35 | |
| Decrease in systolic PAP (mmHg) | 44.8±18.6 | 40.5±22.1 | 0.36 | 46±14.4 | 40.8±23.1 | 0.34 | |
| Decrease in mean PAP (mmHg) | 22.2±10.2 | 20.4±11.1 | 0.45 | 23.2±10.3 | 20.7±10.8 | 0.41 | |
| Decrease in PVR (dynes·sec·cm−5) | 588 [344–907] | 439 [218–670] | 0.20 | 606 [348–899] | 483 [338–835] | 0.46 | |
| TR velocity (cm/sec) | 282 [246–325] | 256 [241–291] | 0.12 | 283 [249–321] | 263 [245–306] | 0.76 | |
| RV/LV | 1.02±0.03 | 0.93±0.02 | <0.01 | 1±0.13 | 0.97±0.12 | 0.30 | |
| Mortality | 2 (5.4) | 0 | 0.23 | 1 (3.8) | 0 | >0.99 | |
| Mechanical ventilation (h) | 65.6 [41.5–92.6] | 45.4 [38.1–67.5] | 0.03 | 64 [40.6–84.7] | 61.2 [41.1–67.9] | 0.87 | |
| Length of ICU stays (h) | 116 [92–174] | 91 [70–164] | 0.10 | 115 [90–139] | 115 [84–164] | 0.92 | |
| Length of hospital stays (d) | 20 [15–26.5] | 20 [16–27] | 0.71 | 19.5 [14–25] | 20.5 [16–24] | 0.24 | |
| Complication | |||||||
| PMV | 14 (37.8) | 8 (20.5) | 0.10 | 5 (19.2) | 4 (15.4) | >0.99 | |
| Atrial fibrillation | 10 (27.0) | 9 (23.1) | 0.69 | 7 (26.9) | 6 (23.1) | 0.75 | |
| Severe infection | 5 (13.5) | 3 (7.7) | 0.65 | 2 (7.7) | 1 (3.8) | >0.99 | |
| Pericardial effusion | 4 (10.8) | 7 (17.9) | 0.38 | 2 (7.7) | 3 (11.5) | >0.99 | |
| Severe RPE | 9 (24.3) | 6 (15.4) | 0.33 | 5 (19.2) | 4 (15.4) | >0.99 | |
| Acute kidney injury | 23 (62.2) | 20 (51.3) | 0.34 | 16 (61.5) | 14 (53.8) | 0.56 | |
| Delirium | 3 (8.1) | 2 (5.1) | 0.95 | 2 (7.7) | 1 (3.8) | >0.99 | |
Continuous variables are presented as mean ± standard deviation for normally distributed data or median [25th–75th percentile] for non-normally distributed data. Categorical variables are reported as frequencies (percentages). CPB, cardiopulmonary bypass; PAP, pulmonary arterial pressure; PVR, pulmonary vascular resistance; TR, tricuspid regurgitation; RV/LV, right ventricular diameter/left ventricular diameter; ICU, intensive care unit; PMV, prolonged mechanical ventilation; RPE, reperfusion pulmonary edema.
Postoperative hemodynamic features were comparable between the two groups (Table 3). Postoperative mean PAP (21.7±7.7 vs. 22.9±6.7 mmHg, P=0.54) and PVR {326 [254–398] vs. 361 [290–445] dynes·sec·cm−5, P=0.35} were slightly lower in the riociguat group than in the control group, although the differences were not statistically significant. Moreover, the percentage decrease in PVR {62.3% [52.1–75.8%] vs. 58.2% [42.4–69.6%], P=0.17} was comparable in patients with preoperative PVR ≥800 {66% [61.3–76.4%] vs. 62.2% [53.8–76.5%], P=0.23} and <800 dynes·sec·cm−5 {51.4% [43.9–68.9%] vs. 42.4% [34.9–61.2%], P=0.19} (Figure 4). The incidence of residual PH (23.1% vs. 30.8%, P=0.53) was also similar between the two groups.

In 76 patients, the overall perioperative mortality rate stood at 2.6%. Following matching, the riociguat group experienced a sole in-hospital death. Examination of Table 3 revealed no discernible disparities in major postoperative complications between the riociguat and control groups. Notably, only one patient in the riociguat group necessitated extracorporeal membrane oxygenation support post-PEA, with no occurrences of stroke or mediastinal hemorrhage requiring surgical intervention. Furthermore, there were no statistically significant variances in the duration of intensive care unit stays {115 [90–139] vs. 115 [84–164] hours, P=0.92} or hospital stays {19.5 [14–25] vs. 20.5 [16–24] days, P=0.24}.
Subgroup analysis in patients with high preoperative PVR
Among the 42 patients (55.3%) with preoperative PVR ≥800 dynes·sec·cm−5, the riociguat and control groups comprised 22 and 20 patients, respectively. Baseline characteristics and postoperative outcomes are detailed in Tables S2,S3. Generally, these patients exhibited exacerbated hemodynamic impairments. Moreover, the control group had a higher proportion of males (80% vs. 45.5%, P=0.02). Although not statistically significant, the time from diagnosis to PEA was longer in the riociguat group {15.5 [6.5–29.2] vs. 7.5 [2.5–17.9] months, P=0.06}. Riociguat group displayed higher preoperative total bilirubin {27 [14.58–41.61] vs. 14.36 [11.92–22.16] µmol/L, P=0.01} and lower cardiac output (2.46±0.37 vs. 2.89±0.64 L/min, P=0.04). Postoperatively, both groups demonstrated comparable hemodynamic improvement and clinical outcomes, but mechanical ventilation duration was significantly longer in the riociguat group {66.9 [64–98] vs. 53.3 [40.1–68.6] hours, P=0.01}.
Long-term outcomes
Of the 51 patients surviving to hospital discharge (25 in the riociguat group, 26 in the control group), follow-up occurred over a median period of 31 and 42 months, respectively. Late death transpired in one patient due to cerebral hemorrhage, and another patient was re-hospitalized for right heart failure. Among the surviving patients, WHO-FC was classified as I, II, and III in 44, 5, and 1 patient, respectively. Mean 6-MWD (500±60 vs. 512±52 m, P=0.44) and median increase in 6-MWD {109 [57–172] vs. 87 [58–183] m, P=0.86} demonstrated similarity between the two groups.
Discussion
PEA remains the gold standard for operable CTEPH patients. Nevertheless, the inoperable patient cohort spans from 20% to 50% (21,22). There exists an imbalance between elevated PVR and the availability of surgically accessible thrombotic material, contributing significantly to the inoperability of CTEPH (22). Moreover, a heightened preoperative PVR correlates with an increased postoperative mortality rate (6,7). Consequently, the adoption of bridging therapy utilizing PH targeted medications has become common. The rationale behind administering bridging therapy before PEA is to optimize hemodynamics, potentially enhancing postoperative outcomes. Nevertheless, the impact of bridging therapy remains a topic of controversy.
While previous investigations predominantly centered on phosphodiesterase type 5 inhibitors, endothelin receptor antagonists, or prostacyclin analogues, riociguat, functioning as a sGC stimulator, exhibits a dual mode of action. It elevates cyclic guanosine monophosphate production by directly stimulating sGC and synergizing with endogenous nitric oxide to augment sGC activation (23,24). Over the last decade, riociguat has been recommended for patients with inoperable CTEPH and persistent PH (1). However, the sole clinical study (ClinicalTrials.gov: NCT03273257) evaluating riociguat as a bridge to PEA was terminated in 2021.
In this retrospective cohort study involving operable CTEPH patients, bridging therapy with riociguat demonstrated hemodynamic improvements in individuals with PVR ≥800 dynes·sec·cm−5. Despite this, no discernible positive impact on postoperative outcomes or hemodynamics was observed.
A pre-PEA reassessment revealed a slight hemodynamic and clinical improvement in the riociguat group. Post-riociguat administration of 2.6 months, there was a reduction in postoperative PVR and NT-proBNP, accompanied by an increase in the 6-MWD, akin to findings in the CHEST-1 study and RACE study (16,25). However, the reduction in PVR after riociguat among patients with PVR ≥800 dynes·sec·cm−5 was lower compared with previous studies, which was 200 dynes·sec·cm−5 after 26 weeks in RACE study and 226 dynes·sec·cm−5 after 16 weeks in CHEST-1 study. We suppose that two possible reasons contribute to the difference. Firstly, the duration of riociguat administration in our study was shorter than that in RACE and CHEST-1 study, which might result in suboptimal effect. Secondly, the objects in RACE and CHEST-1 were inoperable CTEPH and persistent PH patients, while the objects in our study were operable CTEPH patients with proximal lesions, which could lead to poor efficacy of riociguat. Furthermore, among patients with PVR <800 dynes·sec·cm−5, no differences in PVR were noted. This could be attributed to milder micro-vasculopathy, the target for riociguat, and the shorter duration of medication in these patients.
After meticulous exclusion and propensity score matching analyses, patients receiving riociguat before PEA were compared with those receiving supportive therapy. Overall, hemodynamic parameters and clinical status were comparable between the two groups, except for serum total bilirubin, indicating greater severity of right heart failure in the riociguat group. Consistent with prior studies (14), our investigation demonstrated significant hemodynamic improvements after PEA, with no notable differences between the riociguat group and the control group. However, a German study reported a significantly larger decrease in PVR among patients receiving bosentan as bridging therapy, particularly in cases with extremely high PVR (13). While PH was alleviated in the majority, a noteworthy proportion in both the riociguat group (23.1%) and the control group (30.8%) exhibited mean pulmonary artery pressure over 25 mmHg. These findings align with a reported meta-analysis in which persistent PH was observed in up to 25% of patients post-PEA (26).
In this study, no differences were observed in postoperative mortality and major complications between the two groups. The overall in-hospital mortality in this series was 2.6% (2/76 patients), a rate lower than 5% and consistent with previously reported rates of approximately 2.5% (4,5). Additionally, there were no discernible differences in the length of intensive care unit stays or hospital stays in this investigation. These findings collectively indicate that bridging therapy utilizing riociguat confers no additional positive impact on short-term outcomes after PEA. However, the RACE study showed riociguat administered prior to balloon pulmonary angioplasty significantly decreased complications, possibly due to decreasing PVR. Although PEA and balloon pulmonary angioplasty are not identical, the less effects in PVR decrease may account for no difference in surgical outcomes in this study. Whether a greater decrease in PVR will improve the postoperative outcomes needs to be confirmed by further studies.
Notably, a recent Brazilian study suggested that employing medical therapies before PEA in patients with low cardiac output was linked to improved short-term outcomes (12). However, the overall 1-year survival rate of 82.5% was relatively low, rendering their conclusion less generalizable. In our study, a subgroup analysis was conducted on patients with high preoperative PVR. Despite more severe hemodynamic impairments in the riociguat group, postoperative hemodynamics and clinical profiles were similar between the two groups. Moreover, the riociguat group exhibited a longer duration of mechanical ventilation, potentially associated with compromised preoperative cardiopulmonary function.
Several inherent limitations are found in this study. Firstly, it is a retrospective cohort study with a small sample size. The possibility of selection bias exists for patients receiving riociguat as a bridge to PEA, as this bridging therapy might be administered to patients with more severe hemodynamic impairment. To mitigate this bias, propensity score matching analysis was conducted to align the hemodynamic profiles between the two groups. Secondly, some patients with preoperative PVR <800 dynes·sec·cm−5 were included. In clinical practice, the decision to use riociguat before PEA for these patients was influenced by patients’ consideration of whether to pursue surgical treatment. Finally, the duration of riociguat use before PEA lacked a standardized protocol. Although patients with an excessively short period of bridging therapy were excluded from our study, the mean duration of riociguat use was still shorter than that in the CHEST-1 study (16). Consequently, the optimal regimen for bridging therapy using riociguat remains unclear.
Conclusions
In conclusion, the utilization of riociguat as bridging therapy to PEA demonstrated a notable association with hemodynamic improvement before PEA among patients exhibiting elevated preoperative PVR. However, our analysis did not reveal any additional advantages in terms of postoperative mortality or hemodynamics. We assert that the active recommendation of short-term riociguat use before PEA, particularly in patients with stable hemodynamics, should be approached with caution.
Furthermore, we advocate for more extensive cohort studies and randomized controlled trials involving riociguat to comprehensively assess whether bridging therapy confers benefits to postoperative outcomes.
Acknowledgments
Funding: This work was supported by grants from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-48/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-48/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-48/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-48/coif). All authors report that this study was supported by the National Natural Science Foundation of China, National High Level Hospital Clinical Research Funding, the International S&T Cooperation Program and Major New Drug Creation Special Project. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of China-Japan Friendship Hospital (No. 2022-KY-088). Informed consent for data usage was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022;43:3618-731. [Crossref] [PubMed]
- Poch D, Mandel J. Pulmonary Hypertension. Ann Intern Med 2021;174:ITC49-64. [Crossref] [PubMed]
- Kim NH, Delcroix M, Jais X, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019;53:1801915. [Crossref] [PubMed]
- Lankeit M, Krieg V, Hobohm L, et al. Pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2017;S1053-2498(17)31877-6.
- Madani MM, Auger WR, Pretorius V, et al. Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg 2012;94:97-103; discussion 103. [Crossref] [PubMed]
- Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011;141:702-10. [Crossref] [PubMed]
- Cannon JE, Su L, Kiely DG, et al. Dynamic Risk Stratification of Patient Long-Term Outcome After Pulmonary Endarterectomy: Results From the United Kingdom National Cohort. Circulation 2016;133:1761-71. [Crossref] [PubMed]
- Faccioli E, Verzeletti V, Perazzolo Marra M, et al. Pulmonary Endarterectomy for Chronic Thromboembolic Pulmonary Hypertension: A Systematic Review of the Most Updated Literature. J Clin Med 2022;11:6976. [Crossref] [PubMed]
- Nagaya N, Sasaki N, Ando M, et al. Prostacyclin therapy before pulmonary thromboendarterectomy in patients with chronic thromboembolic pulmonary hypertension. Chest 2003;123:338-43. [Crossref] [PubMed]
- Bresser P, Fedullo PF, Auger WR, et al. Continuous intravenous epoprostenol for chronic thromboembolic pulmonary hypertension. Eur Respir J 2004;23:595-600. [Crossref] [PubMed]
- Reesink HJ, Surie S, Kloek JJ, et al. Bosentan as a bridge to pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg 2010;139:85-91. [Crossref] [PubMed]
- Castro MA, Piloto B, Dos Santos Fernandes CJC, et al. Use of medical therapies before pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension patients with severe hemodynamic impairment. PLoS One 2020;15:e0233063. [Crossref] [PubMed]
- Kunihara T, Wilkens H, Halank M, et al. Haemodynamic benefit of bridging use of bosentan prior to pulmonary endarterectomy. Eur J Cardiothorac Surg 2021;60:840-7. [Crossref] [PubMed]
- Jensen KW, Kerr KM, Fedullo PF, et al. Pulmonary hypertensive medical therapy in chronic thromboembolic pulmonary hypertension before pulmonary thromboendarterectomy. Circulation 2009;120:1248-54. [Crossref] [PubMed]
- Delcroix M, Lang I, Pepke-Zaba J, et al. Long-Term Outcome of Patients With Chronic Thromboembolic Pulmonary Hypertension: Results From an International Prospective Registry. Circulation 2016;133:859-71. [Crossref] [PubMed]
- Ghofrani HA, D'Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013;369:319-29. [Crossref] [PubMed]
- Balasubramanian VP, Beutner M, Gill K, et al. Real-world experience with riociguat as potential bridging therapy in patients with chronic thromboembolic pulmonary hypertension: a case series. Pulm Circ 2020;10:2045894019898377. [Crossref] [PubMed]
- Madani MM. Surgical Treatment of Chronic Thromboembolic Pulmonary Hypertension: Pulmonary Thromboendarterectomy. Methodist Debakey Cardiovasc J 2016;12:213-8. [Crossref] [PubMed]
- Ma J, Li C, Zhai Z, et al. Distribution of thrombus predicts severe reperfusion pulmonary edema after pulmonary endarterectomy. Asian J Surg 2023;46:3766-72. [Crossref] [PubMed]
- Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158:825-30. [Crossref] [PubMed]
- Quadery SR, Swift AJ, Billings CG, et al. The impact of patient choice on survival in chronic thromboembolic pulmonary hypertension. Eur Respir J 2018;52:1800589. [Crossref] [PubMed]
- Madani M, Ogo T, Simonneau G. The changing landscape of chronic thromboembolic pulmonary hypertension management. Eur Respir Rev 2017;26:170105. [Crossref] [PubMed]
- Klinger JR, Chakinala MM, Langleben D, et al. Riociguat: Clinical research and evolving role in therapy. Br J Clin Pharmacol 2021;87:2645-62. [Crossref] [PubMed]
- Kenny M, Clarke MM, Pogue KT. Overview of Riociguat and Its Role in the Treatment of Pulmonary Hypertension. J Pharm Pract 2022;35:437-44. [Crossref] [PubMed]
- Jaïs X, Brenot P, Bouvaist H, et al. Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension (RACE): a multicentre, phase 3, open-label, randomised controlled trial and ancillary follow-up study. Lancet Respir Med 2022;10:961-71. [Crossref] [PubMed]
- Hsieh WC, Jansa P, Huang WC, et al. Residual pulmonary hypertension after pulmonary endarterectomy: A meta-analysis. J Thorac Cardiovasc Surg 2018;156:1275-87. [Crossref] [PubMed]


