
Analysis of the resistance profile of real-world alectinib first-line therapy in patients with ALK rearrangement-positive advanced non-small cell lung cancer using organoid technology in one case of lung cancer
Highlight box
Key findings
• Patient-derived organoid drug sensitivity testing for lung cancer determines subsequent drug therapy in anaplastic lymphoma kinase (ALK) rearrangement-positive patients.
What is known and what is new?
• Blind sequencing of other second-generation tyrosine kinase inhibitors (TKIs) or third-generation lorlatinib after progression of first-line to second-generation ALK-TKI therapy does not always lead to satisfactory tumor suppression. It is crucial to propose a novel approach to bridge this gap.
• In this study, a new strategy for patient-derived drug sensitivity testing of lung cancer-like organs is proposed, which is anticipated to be an effective tool for predicting drug sensitivity and guiding individualized dosing.
What is the implication, and what should change now?
• Patient-derived organoid drug susceptibility testing for lung cancer improves the effectiveness of clinical treatment in ALK-resistant patients. However, extending the use of organoid drug sensitivity testing to all lung cancer clinical situations requires randomized clinical trial validation.
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide, including in China. Non-small cell lung cancer (NSCLC) constitutes 80–85% of primary lung malignancies (1,2). Due to atypical early clinical manifestations, most lung cancer patients are detected in the middle to late stages of the disease, with a 5-year survival rate of 10% for stage IVA patients and almost 0 for stage IVB (3). With continuous advancements in lung cancer treatment, an expanding array of therapeutic approaches is being implemented. In 2007, Soda et al. (4) first identified the echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK) fusion gene in NSCLC tissues. This discovery introduced a novel therapeutic concept for NSCLC and ALK rearrangement (ALK fusion), constituting 3–7% of NSCLC cases (5,6). Alectinib is a highly selective oral ALK inhibitor. In the ALEX trial, the median progression-free survival (mPFS) in the alectinib group was 34.8 months, which is a substantial improvement from 10.9 months in the crizotinib group [hazard ratio (HR) =0.43; P<0.001] and the objective response rate (ORR) (7). In China, alectinib is the preferred recommendation for first-line treatment of patients with ALK-positive advanced NSCLC. However, most patients inevitably develop drug resistance after treatment, causing disease progression. The mechanisms of drug resistance in patients with ALK fusion gene-positive NSCLC include ALK signaling pathway-dependent (ALK gene amplification and kinase domain mutation), ALK signaling pathway-independent (bypass activation and tissue-type transformation), and concomitant gene (EGFR, KRAS, and TP53) alterations. Clarifying the resistance mechanism in patients and selecting targeted agents for subsequent treatment is crucial. The preferred therapeutic strategy for resistance development is the selection of mutation-targeted next-generation ALK inhibitors. In this study, we retrospectively analyzed the efficacy of the first-line use of alectinib in ALK rearrangement-positive patients with advanced NSCLC and the use of drugs after resistance. We evaluated patients’ mutation profiles at baseline and after first-line resistance based on next-generation sequencing (NGS) technology and explored the therapeutic options after alectinib resistance by applying the drug sensitivity test for lung cancer-like organs originating from patients. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1964/rc).
Methods
General information
We selected data from 35 patients with advanced ALK gene-positive NSCLC treated with first-line alectinib between November 2018 and April 2022 at the First Affiliated Hospital of Guangzhou Medical University. The inclusion criteria were as follows: (I) stage III–IV NSCLC with eighth-edition the tumour, nodes, and metastasis (TNM) staging system staging confirmed by histopathology; and (II) tissue samples or blood samples were taken for genetic testing and were positive for the ALK fusion gene positivity indicated by NGS. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the First Affiliated Hospital of Guangzhou Medical University (Medical Research and Lunar Review 2020, No. 79), and individual consent for this retrospective analysis was waived.
Patients’ baseline data were collected, including age, sex, smoking status, Eastern Cooperative Oncology Group performance status (ECOG PS) score, pathologic type, clinical stage, metastatic site, genetic testing status, progression characteristics, post-progression genetic characteristics, and progression follow-up treatment plan.
Treatment process
All patients underwent relevant examinations before drug administration, including routine blood tests to assess liver and kidney function, coagulation function, tumor markers, cardiopulmonary function, and imaging examinations for systemic assessment. After the exclusion of contraindications to targeted therapy, oral alectinib 600 mg was administered twice daily for four weeks until disease progression or intolerable adverse effects were observed.
Efficacy methods and follow-up methods
Throughout the medication period, regular blood routine, liver function, renal function, coagulation function, electrolytes, tumor markers, urine and feces routine, and periodic blood pressure monitoring were performed. Imaging examinations were conducted every 8 weeks. Alectinib efficacy was assessed according to the criteria for evaluating the effectiveness of solid tumors (RECIST version 1.1) (8). The imaging methods included computed tomography (CT), magnetic resonance imaging (MRI) and chest frontal and lateral imaging, and the assessment findings were divided into four grades: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD).
Survival analysis
Outpatients and inpatients were used for follow-up until March 01, 2023, and the progression-free survival (PFS) of all patients was recorded.
Culture of patient-derived organoid (PDO)
A tissue sample is procured from a patient’s tumor, samples were washed with 10 mL of Hank’s balanced salt solution (HBSS) containing 5% antibiotics 8–10 times and minced into 1 mm3 in size. The tissue are then immersed in a mixture containing 0.001% DNase from Sigma-Aldrich (Louis, Missouri, USA), 1 mg/mL collagenase/dispase from Roche (Indianapolis, Indiana, USA) and a combination of 200 U/mL penicillin, 200 mg/mL streptomycin, and 0.5 mg/mL amphotericin B, which accounts for 2% of the total antibiotics, all obtained from Sigma. This mixture is prepared in a DMEM/F12 medium from Lonza (Gampel, Switzerland) and incubated at a temperature of 37 °C for 3 hours with periodic gentle stirring and resuspension. Post-incubation, the tissue is pipetted repeatedly and strained through a 70-micron mesh, followed by centrifugation at 112 ×g for 3 minutes to form a cell pellet. The pellet is then treated with a lysis buffer (product number: 00443357 from Invitrogen eBioscience, Carlsbad, California, USA) for 5 minutes to lyse red blood cells. The resulting cell pellet is resuspended in 100 µL of a serum-free DMEM/F12 medium known as minimum basal medium (MBM), also supplied by Lonza, and is further supplemented with additives: 20 ng/mL of basic fibroblast growth factor (bFGF) and 50 ng/mL of human epidermal growth factor (EGF), both sourced from Invitrogen (Carlsbad, California, USA), N2 and B27 supplements, a 10 µM concentration of ROCK-inhibitor from Enzo Life Sciences (Farmingdale, New York, USA), and 1% penicillin-streptomycin from Gibco (Oklahoma City, Oklahoma, USA). Following this, 200 µL of Matrigel, a product of Corning (Corning, New York, USA) is combined with the 100 µL cell suspension to initiate organoid formation. The mixture is then set to solidify on pre-warmed 6-well culture plates from Corning at 37 °C for 30 minutes. Once the Matrigel has set, an additional 3 mL of MEM is added to the well, with the culture medium being replaced every 4 days. The organoids are passaged every two weeks at a ratio of either 1:2 or 1:3.
Organoid drug response assay
The organoids are harvested and dissociated into clusters using 1× TrypLE Express Enzyme from Gibco, with clusters numbering between 100 to 500 being plated in 40-µL of a 5% Matrigel/organoid culture medium mixture within a 384-well plate from Corning, in triplicate. The plates, which have a clear bottom, are pre-coated with 10-µL of collagen from Thermo Fisher Scientific (Waltham, Massachusetts, USA), prior to cell seeding. Following a 48-hour post-seeding period, a range of concentrations (10, 2.5, 0.625, 0.156, 0.039, 0.0098 µM) of each compound is introduced using automated liquid handling. After a 4-day incubation with the drugs, cell viability is evaluated using the CellTiter-Glo assay from Promega (Madison, Wisconsin, USA), with the results normalized to vehicle-only controls. Data analysis is performed using GraphPad Prism 6.0 software, where the half-maximal inhibitory concentration (IC50) values are calculated using a non-linear regression model.
Statistical analysis
Statistical Package for the Social Sciences (SPSS, version 25.0) was used for data processing. Measurement data were expressed as , and count data were expressed as percentages. Survival analysis was performed using the Kaplan-Meier curve method.
Results
The baseline characteristics of the patients
Out of 35 eligible patients, seven (20%) developed baseline brain metastases before the initiation of alectinib therapy. The patient’s median age was 53 years (range, 20–89 years), 63% (22/35) were younger than 60 years, 57% (20/35) were male, and 60% (21/35) had never smoked. All patients were diagnosed with adenocarcinoma, and 80% (28/35) had stage IV lung cancer at baseline. All patients had an ECOG PS score of 0–2 at baseline. Moreover, 20% (7/35) had brain metastases at baseline, 51% (18/35) had classical EML4-ALK, and 11% (4/35) had combined TP53 mutations. The most common sites of metastasis were bones (37%), brain (20%), adrenal glands (11%), and liver (9%). The other demographic and clinical characteristics are displayed in Table 1.
Table 1
| Characteristics | Number [%] |
|---|---|
| Age (years) | |
| <60 | 22 [63] |
| ≥60 | 13 [37] |
| Sex | |
| Male | 20 [57] |
| Female | 15 [43] |
| Smoking | |
| Yes | 14 [40] |
| No | 21 [60] |
| TNM stage | |
| Stage III | 7 [20] |
| Stage IV | 28 [80] |
| Central nervous system metastasis | |
| Yes | 7 [20] |
| No | 28 [80] |
| ECOG PS score | |
| 0 | 2 [6] |
| 1 | 30 [86] |
| 2 | 3 [8] |
| Genotype | |
| EML4-ALK | 18 [51] |
| Complex ALK fusion | 17 [49] |
| Combined TP53 mutation | 4 [11] |
TNM, tumour, nodes, and metastasis; ECOG PS, Eastern Cooperative Oncology Group performance status; EML4-ALK, echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase.
Efficacy of alectinib treatment
At follow-up until March 01, 2023, 35 patients were enrolled, and 31 remained in PFS, with a PFS of 4.3–35.0 months. Only 4 cases showed disease progression, and the median PFS of first-line alectinib treatment was not reached (Figure 1). Almost all patients with primary lung tumors started responding within one month after the initiation of ALK-tyrosine kinase inhibitors (TKIs) treatment, and among the 35 patients, there were 0 CR (0%), 22 PR (62.86%), 9 SD (25.71%), and 4 PD (11.43%). The ORR was 62.86%, and the DCR was 88.57%. Most patients (ORR, 62.86%) responded to treatment, with 22 (62.86%) achieving PR and 9 (25.71%) achieving SD. Seven intracranial metastases showed varying degrees of remission, and the ORR of patients with measurable central nervous system lesions was 100%. Two patients with brain metastases achieved CR in their brain lesions after dosing. As of March 01, 2023, four patients experienced disease progression (any organ; Figure 2).

Genetic profiling of patients
Baseline gene mutations in patients
The NGS results indicated substantial interindividual differences in the ALK gene mutation profiles. The ALK fusion variants detected were EML4 exon 6a-ALK exon 20 (n=6; 17%), EML4 exon 20-ALK exon 20 (n=4; 11%), EML4 exon 13-ALK exon 20 (n=3; 9%), EML4 exon 18-ALK exon 20 (n=2; 6%), EML4 exon 19-ALK exon 20 (n=1; 3%), EML4 exon 14-ALK exon 21 (n=1; 3%), EML4 exon 21-ALK exon 20 (n=1; 3%), ALK-UBE2E3 (n=1; 3%), PIGF-ALK (n=1; 3%), and FRZB-ALK (n=1; 3%). Eighteen (51%) patients exhibited EML4-ALK fusions. Four (11%) patients had combined TP53 mutations, including three classical EML4-ALK fusions.
Gene mutations after first-line alectinib resistance
Four patients exhibited treatment resistance progression. After progression, all patients underwent a second biopsy, and the pathology remained lung adenocarcinoma. NGS was performed on the tissues after progression, and in three cases, the results implied the presence of the original baseline EML4-ALK fusion gene without new mutants. The other patient had a structural rearrangement of ALK and UBE2E3 genes, with concomitant mutations in the ARID1A and TP53. This patient developed de novo mutants (ALK V1180L and ALK E1161D) during first-line ALK-TKI resistance.
Treatment after first-line alectinib resistance
During follow-up until March 01, 2023, all four drug-resistant patients received sequential ceritinib (450 mg) once daily. This approach was taken because the third-generation drug lorlatinib was not covered by health insurance reimbursement policies at the time. The tissue NGS results of three patients proposed that the original baseline EML4-ALK fusion gene was retained, and the PFS of ceritinib treatment was 0.5–1.3 months. One patient developed acquired resistance mutations in the structural domains of ALK protein kinase (V1180L and E1161D) and progressed again after 6.7 months of sequential ceritinib treatment with a de novo resistance mutant ALK I1174L.
In one patient who retained the original baseline ALK fusion without resistance mutation sites after the progression of first-line alectinib resistance, we conducted a puncture biopsy of the liver metastasis site after 1.3 months of sequential ceritinib treatment for re-progression of resistance. The punctured tissue was taken for lung cancer organoid culture and drug susceptibility testing (Figures 3,4).
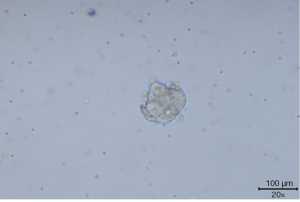
The lung cancer organoid culture of the patient detected seven drugs eligible for drug sensitivity testing, and following the clinical drug protocol, buxtitinib, lorlatinib, gemcitabine, paclitaxel, vincristine, carboplatin, and anilotinib were selected. The inhibition rate method (a parameter describing the inhibitory effect of a drug on organoid growth at the tested concentration) was applied to detect the inhibition of organoid growth by drug concentration. The cell viability value [adenosine triphosphate (ATP) chemiluminescence assay] of the no-drug addition control group was used as 100% to calculate the cell viability value after drug intervention. Pemetrexed was not selected for the drug sensitivity test because the patient was proposed to be treated with pemetrexed combined with carboplatin chemotherapy. The organoid drug sensitivity results are depicted in Figure 5.
The patient was a 34-year-old woman with pathology indicative of primary lung adenocarcinoma at the time of sample collection, diagnosed with T4N3M1c stage IVB lung adenocarcinoma and had metastases to the liver, bones, and adrenal glands. Tissue genetic testing revealed an EML4-ALK rearrangement (E20:A20). First-line alectinib treatment was initiated on July 16, 2021, with a PFS of 4.9 months. Tissue genetic testing on post-progression biopsy on December 11, 2021, continued to show an EML4-ALK (E20:A20) mutation. Sequential ceritinib (450 mg) was administered once daily with a meal and orally with alectinib. On January 18, 2022, the patient received pemetrexed combined with carboplatin chemotherapy after the ceritinib resistance progression. On January 30, 2022, the drug sensitivity report indicated the patient’s highest sensitivity to vincristine and that the second-generation buxtitinib was more sensitive to third-generation lorlatinib the ALK-targeted drugs. On September 29, 2022, the patient’s intrahepatic metastases were enlarged compared with the previous one; however, the patient declined further chemotherapy and started taking second-generation ALK-TKI brigatinib 180 mg orally once daily on September 30, 2022, with a PFS of 3.2 months. On January 3, 2023, CT indicated that metastases had progressed in the liver, and third-generation ALK-TKI lorlatinib was administered orally once daily at a sequential dose of 100 mg, with a PFS of 1.9 months. On February 28, 2023, CT revealed that the metastases continued progressing. On March 14, 2023, 12 mg once daily vascular targeted therapy with amlotinib was administered, and three courses of ramucirumab (1,200 mg) were administered outside the hospital from March 21 to May 23, 2023, with a follow-up CT suggesting metastases in the liver region. A repeat CT demonstrated that the metastases in the liver region continued to enlarge. Fortunately, ablation of the liver metastases was performed at an outside hospital.
Discussion
The most common resistance mechanism of ALK inhibitors is secondary resistance mutations in ALK. For example, the common resistance mutations in the first-generation ALK inhibitor crizotinib are ALK G1269A, L1196M, and C1156Y mutations. The common resistance mutations in the second-generation ALK inhibitor ceritinib are G1202R and F1174C mutations, and treatment with lectinic causes resistance mutations I1171T/N/S, V1180L, and G1202R (9,10). Mutations in ALK L1198, V1180L, I1171N, and L1196 affect conformational changes in the ATP-binding portal and hinge regions, inducing changes in the “gating” between alectinib and ALK. Two “gating” mutations, V1180L, ALK L1196M/Q, and I1171T/N/S, are resistant to both alectinib and crizotinib (11).
The preferred next-generation ALK inhibitors for ALK-TKI resistance after treatment include first-generation TKI resistance sequential second-generation TKI, or second-generation TKI first-line resistance directly sequential third-generation TKI. The third-generation ALK inhibitor lorlatinib is the only inhibitor that effectively inhibits all ALK secondary mutations. Moreover, it is a replacement for second-generation TKIs. Some patients exhibiting secondary mutation resistance with a second-generation ALK inhibitor are sensitive to another drug. For example, the F1174C mutation is among the most common ceritinib resistance mutations; however, it is sensitive to alectinib, whereas V1180L and I1171T mutations cause alectinib resistance but are sensitive to ceritinib (12). The aforementioned successful switching therapy was certainly due to NGS testing. Hida et al. (13) conducted a phase 2 study in patients with ALK-rearranged NSCLC previously treated with alectinib. The ORR of ceritinib was only 25%, with an mPFS of 3.7 months. All four patients in this study who received ceritinib after progression on first-line alectinib had a recurrence of resistant progression at 0.5–6.7 months, which is consistent with the outcomes of that study.
In lung adenocarcinomas with ALK gene rearrangements, the proportion of coexisting mutations in EML4-ALK and TP53 was approximately 5.5% (14), which is comparable to the proportion of four patients (11%) with combined TP53 mutations in this study. Christopoulos et al. (15) reported that in patients with ALK-positive lung cancer, TP53 mutations were detected in tissue biopsies or fluids at the time of disease progression. Acquisition of a TP53 mutation at progression was associated with more aggressive disease, shorter TKI response, and poorer OS than those cases with primary TP53 mutations. Among 90 alectinib-treated patients with NSCLC, TP53 co-mutant patients exhibited significantly worse PFS than TP53 wild-type patients. PFS, 11.7 months [95% confidence interval (CI): 6.3–not reached] vs. not reached (95% CI: 23.6–not reached); P<0.001; HR =0.33 (95% CI: 0.17–0.65) (16). In this study, two of the four patients with combined TP53 mutations developed first-line alectinib resistance, with a PFS of 4.3 and 7.4 months, respectively. The incidence of resistance mutations in patients with TP53 co-mutations was higher than that in patients with wild-type TP53, indicating that TP53 mutations may cause tumor suppressor function impairment, impact genomic stability, promote gene evolution, and ultimately induce drug-resistant mutations (17).
With the ongoing advancements in targeted therapy and immunotherapy for lung cancer, precision therapy assumes a progressively significant function in the comprehensive management of patients with lung cancer. The determination of personalized treatment plans largely depends on the results of molecular pathology, particularly NGS. NGS can identify tumor driver genes with the corresponding range of targeted drug choices; however, it cannot provide therapeutic insights regarding the drug to which the patient is sensitive. Because not all patients carrying drug target genes can benefit from targeted therapy and some patients have primary resistance to individual drugs, selecting sensitive drugs is a current clinical challenge (18).
In this investigation, only one of the four patients with first-line alectinib-resistant progression had an acquired resistance mutation site that could be selected as a corresponding sensitive drug. Identification of suitable and effective medications for patients who remain ALK rearrangement-positive at baseline after resistance is a pressing concern in the current precision treatment of lung cancer. The in vitro organoid model as a functional model can effectively fill this void.
Organoids are three-dimensional in vitro structures generated from autologous tissues and differentiated stem cells. They can summarize the original tissue’s in vivo cellular heterogeneity, genetic characteristics, structure, and function. The drug response of PDOs is consistent with that of patients and correlates with genetic alterations. Tumor-like organs can condense the original tumor’s multi-omics features, imitating its drug-sensitive properties. PDOs in colorectal, gastrointestinal, and rectal cancers have been demonstrated to predict the clinical response to radiotherapy and targeted therapies, providing new insights into the clinical utility of organoids (19-21). In this study, a patient who remained original baseline ALK rearrangement-positive without acquired mutation underwent lung cancer organoid culture with sequential brigatinib and lorlatinib after progression of ceritinib resistance, with a PFS of 3.2 and 1.9 months, respectively, consistent with the corresponding drug sensitivity test results of this patient.
PDO in lung cancer can be established by surgical resection of tissue, puncture biopsy, and pleural fluid and has become an emerging technology to assist in drug selection in refractory lung cancer. It can condense the original tumor’s main features on the genome, transcriptome, and translation products. Presently, lung adenocarcinoma, squamous carcinoma, adenosquamous carcinoma, large cell carcinoma, and small cell carcinoma can be used to effectively culture PDO (22). Surgical resection of tissue, puncture samples, or pleural effusions has been used to successfully establish organoid cultures and has been validated for driver mutations and drug efficacy in concordance with the original tumor (23,24). Wang and colleagues (25) used lung cancer organoid models for sensitivity testing of targeted and chemotherapeutic drugs to predict clinical response. The outcomes indicated that the overall alignment between drug sensitivity and clinical response reached 83.33%, confirming the ability of organoids to predict drug response in precision diagnosis and treatment.
Conclusions
For ALK rearrangement-positive patients, blind sequencing of other second-generation TKIs or third-generation lorlatinib may not achieve satisfactory tumor suppression after first-line administration of second-generation ALK-TKI alectinib for treatment progression. Organoid-individualized drug sensitivity assays can be used to understand the sensitivity of tumor cells to various medications in an in vitro culture environment. The higher the sensitivity of tumor cells to a certain drug in vitro, the better the efficacy of the patient treated with this drug; conversely, the lower the sensitivity of tumor cells to a certain drug or even drug resistance, the lower the likelihood that the patient will have a successful efficacy after being treated with this drug. PDO can serve as an in vitro replacement for the original tumor and may eventually contribute to the development of real customized anticancer therapy for lung cancer in precision medicine. However, the results of drug testing by PDOs also need to be further verified by more clinical evidence.
Acknowledgments
We thank all patients who participated in this study and their families. Besides, we thank Burning Rock Biotech for their valuable assistance in NGS data analysis and interpretation. Finally, the authors thank Accurate International Biotechnology Co. (Guangzhou, China) for their assistance with organoid culturing and drug testing.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1964/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1964/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1964/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1964/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the First Affiliated Hospital of Guangzhou Medical University (Medical Research and Lunar Review 2020, No. 79), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hirsch FR, Suda K, Wiens J, et al. New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet 2016;388:1012-24. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Tan DS, Thomas M, Kim DW, et al. Genetic landscape of patients with ALK-rearranged non-small-cell lung cancer (NSCLC) and response to ceritinib in ASCEND-1 study. Lung Cancer 2022;163:7-13. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [Crossref] [PubMed]
- Gadgeel S, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol 2018;29:2214-22. [Crossref] [PubMed]
- Shah MA, Bodoky G, Starodub A, et al. Phase III Study to Evaluate Efficacy and Safety of Andecaliximab With mFOLFOX6 as First-Line Treatment in Patients With Advanced Gastric or GEJ Adenocarcinoma (GAMMA-1). J Clin Oncol 2021;39:990-1000. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Noé J, Lovejoy A, Ou SI, et al. ALK Mutation Status Before and After Alectinib Treatment in Locally Advanced or Metastatic ALK-Positive NSCLC: Pooled Analysis of Two Prospective Trials. J Thorac Oncol 2020;15:601-8. [Crossref] [PubMed]
- He M, Li W, Zheng Q, et al. A molecular dynamics investigation into the mechanisms of alectinib resistance of three ALK mutants. J Cell Biochem 2018;119:5332-42. [Crossref] [PubMed]
- Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov 2014;4:662-73. [Crossref] [PubMed]
- Hida T, Seto T, Horinouchi H, et al. Phase II study of ceritinib in alectinib-pretreated patients with anaplastic lymphoma kinase-rearranged metastatic non-small-cell lung cancer in Japan: ASCEND-9. Cancer Sci 2018;109:2863-72. [Crossref] [PubMed]
- Tao H, Shi L, Zhou A, et al. Distribution of EML4-ALK fusion variants and clinical outcomes in patients with resected non-small cell lung cancer. Lung Cancer 2020;149:154-61. [Crossref] [PubMed]
- Christopoulos P, Dietz S, Kirchner M, et al. Detection of TP53 Mutations in Tissue or Liquid Rebiopsies at Progression Identifies ALK+ Lung Cancer Patients with Poor Survival. Cancers (Basel) 2019;11:124. [Crossref] [PubMed]
- Tanimoto A, Matsumoto S, Takeuchi S, et al. Proteasome Inhibition Overcomes ALK-TKI Resistance in ALK-Rearranged/TP53-Mutant NSCLC via Noxa Expression. Clin Cancer Res 2021;27:1410-20. [Crossref] [PubMed]
- Yang Y, Huang J, Wang T, et al. Decoding the Evolutionary Response to Ensartinib in Patients With ALK-Positive NSCLC by Dynamic Circulating Tumor DNA Sequencing. J Thorac Oncol 2021;16:827-39. [Crossref] [PubMed]
- Lin A, Giuliano CJ, Palladino A, et al. Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials. Sci Transl Med 2019;11:eaaw8412. [Crossref] [PubMed]
- Ooft SN, Weeber F, Dijkstra KK, et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci Transl Med 2019;11:eaay2574. [Crossref] [PubMed]
- Vlachogiannis G, Hedayat S, Vatsiou A, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018;359:920-6. [Crossref] [PubMed]
- Yao Y, Xu X, Yang L, et al. Patient-Derived Organoids Predict Chemoradiation Responses of Locally Advanced Rectal Cancer. Cell Stem Cell 2020;26:17-26.e6. [Crossref] [PubMed]
- Jia Z, Liang N, Li S. Application of Organoids in Lung Cancer Precision Medicine. Zhongguo Fei Ai Za Zhi 2020;23:615-20. [PubMed]
- Shi R, Radulovich N, Ng C, et al. Organoid Cultures as Preclinical Models of Non-Small Cell Lung Cancer. Clin Cancer Res 2020;26:1162-74. [Crossref] [PubMed]
- Endo H, Okami J, Okuyama H, et al. Spheroid culture of primary lung cancer cells with neuregulin 1/HER3 pathway activation. J Thorac Oncol 2013;8:131-9. [Crossref] [PubMed]
- Wang HM, Zhang CY, Peng KC, et al. Using patient-derived organoids to predict locally advanced or metastatic lung cancer tumor response: A real-world study. Cell Rep Med 2023;4:100911. [Crossref] [PubMed]





