
An analysis of residual lung volume changes after segmentectomy based on three-dimensional computed tomography
Highlight box
Key findings
• Extensive compensatory volume changes occur not only in the ipsilateral lung but also in the contralateral lung, with no significant volume decrease in preserved segments.
What is known and what is new?
• Previous studies investigating lobectomy cases showed an increased compensatory lung growth rate with larger resection volumes.
• In segmentectomy cases, the compensated group included a greater number of cases with a larger volume of resected segments, indicating a more extensive resection volume. The volume of the lobe including the resected segment, is maintained. In addition, postoperative compensatory volume changes occur not only in the ipsilateral lung but also in the contralateral lung.
What is the implication, and what should change now?
• Conservation of the segment is crucial for postoperative outcomes. The lung volume of after segmentectomy provides greater residual capacity for compensatory changes in comparison to lobectomy, potentially broadening treatment options for secondary cancers and other diseases. Further study is needed to determine the significance of segment preservation.
Introduction
Lobectomy is recognized as a conventional therapeutic approach for the treatment of primary lung cancer. The detection of small-sized peripheral lung cancers has recently increased, due to the increased use of computed tomography (CT) and advancements in imaging modalities. Small peripheral lung cancers are known to exhibit favorable prognoses following surgical intervention alone. The JCOG0802 study, a phase III multicenter randomized controlled trial in Japanese patients, and the U.S.-based CALGB/Alliance 140503 trial, both of which compared sublobar resection and lobectomy for early-stage lung cancer, established segmentectomy as a standard procedure for early-stage lung cancer (1,2). Consequently, the implementation of segmentectomy is anticipated to escalate. The efficacy of segmentectomy in preserving postoperative pulmonary function remains debatable. While segmentectomy tends to be associated with an improved postoperative pulmonary function, the extent of preservation observed has not been as substantial as anticipated. Within the context of the JCOG0802 study, segmentectomy was associated with more favorable postoperative forced expiratory volume in one second (FEV1) outcomes; however, the observed difference between the two groups was only 3.5%, which is below the pre-established threshold of 10% for clinical significance (1). Similarly, in the CALGB study, the disparity in postoperative FEV1 was only 2%, with the sublobar resection cohort demonstrating marginally superior outcomes (2). However, the clinical relevance of this difference remains unclear.
Approximately 6 months to 1 year following pulmonary resection, the resultant cavity typically undergoes compensation through deviation of the mediastinum and diaphragm, alongside compensatory expansion of the remaining lung tissue. Notably, in numerous instances, the actual postoperative lung function surpassed the preoperatively predicted residual lung function. This observation is attributed to postoperative compensatory expansion of the residual lung (3-6). Additionally, several studies have proposed the concept of compensatory lung growth, wherein new lung tissue regeneration occurs in the remaining lung tissue after resection. Functional and histological examinations have indicated that compensatory alterations in the residual lung are characterized not only by hyperexpansion of alveolar tissue but also by augmentation of functional lung tissue (4,7-11).
Three-dimensional computed tomography (3D-CT) is useful for preoperative planning of segmentectomy. 3D-CT is useful not only for defining the anatomy of the pulmonary vasculature and bronchi but also for calculating lung volumes. Several studies have reported that the estimated postoperative pulmonary function based on preoperative 3D-CT is well correlated with pulmonary function test values (12,13).
This study aimed to analyze the characteristics of compensatory lung expansion after segmentectomy using 3D-CT volumetry. Based on the results obtained, the reasons of pulmonary function after segmentectomy were not preserved as well as expected will be discussed. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-83/rc).
Methods
Patient selection
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committees of the Faculty of Medicine, Oita University (approval No. 2023-2642) and individual consent for this retrospective analysis was waived. A total of 77 cases underwent segmentectomy as the initial surgery at the Department of Thoracic and Breast Surgery, Oita University Hospital, from April 2015 to March 2022. The study excluded patients who underwent additional lung resections within one year, those without available postoperative thin-slice CT imaging at one year, and those for whom lung field delineation was not possible using SYNAPSE VINCENT (Fujifilm Holdings Corporation, Tokyo, Japan). Based on these criteria, some cases were excluded from the analysis. The following information was collected retrospectively using medical records. age, sex, smoking history, number of resected segments, presence or absence of emphysematous or interstitial disease pattern, surgical approach, method of intersegmental plane division, presence of intrathoracic adhesions, occurrence of postoperative air leaks, and pleurodesis procedure.
Segmentectomy
In the context of this study, segmentectomy is defined as a surgical procedure involving the dissection of the pulmonary arteries, veins, and bronchi corresponding to each segment. Intersegmental planes were identified using two distinct methodologies: the inflation-deflation technique (14) and indocyanine green (ICG) imaging (15). To establish intersegmental planes, the surgical team employed either an electrocautery device or surgical stapler, depending on the specific requirements of each case.
Evaluation of lung volume
Lung volume (mL) was calculated by 3D-CT volumetry, specifically employing SYNAPSE VINCENT (Fujifilm Holdings Corporation, Tokyo, Japan), both preoperatively and at the one-year postoperative mark. The predicted residual volume (mL) was determined by subtracting the volume of the resected segment from the total lung volume measured preoperatively. Utilizing the predicted residual volume (mL) and postoperative total lung volume (mL), the rate of postoperative lung volume increase was calculated as [(postoperative total lung volume/predicted residual volume) × 100] (%). Additionally, cases were categorized based on this rate: those with a postoperative lung volume increase of ≥100% were classified into the compensatory group, while those with an increase rate of <100% were assigned to the non-compensatory group.
This study entailed a comparative analysis of the detailed volumetric changes between the compensatory and non-compensatory groups. Additionally, we examined the clinical background factors, postoperative complications, and pulmonary function alterations between the two groups. Among the 31 cases for which pulmonary function test data at one year postoperatively were available, we further analyzed the actual measured values of pulmonary function. Predicted postoperative vital capacity (VC) and FEV1 were estimated using the anatomical segment counting method. This method bases calculations on the number of resected segments, following the formula [preoperative VC (or FEV1) × (19 − n)/19] (mL), where ‘n’ represents the number of segments resected, and 19 denotes the total number of lung segments (16).
Statistical analysis
All statistical analyses were conducted using the RcmdrPlugin.EZR (Easy R, version 1.51) software package (17). Clinical variables were compared between the compensatory and non-compensatory groups using Fisher’s exact test. Based on a receiver operating characteristic (ROC) curve analysis, the cutoff value for the pack-year index was 27.5. Comparisons of the rate of postoperative lung volume increase, as observed on computed tomography (CT), and the results of pulmonary function tests were performed using the Wilcoxon signed-rank test. For all statistical tests, P values of <0.05 were considered to indicate statistical significance.
Results
Patients
The cohort in this study consisted of 77 patients. The average age was 68.8 years (15–86 years). Thirty-seven of the participants were male and 41 were identified as current or ex-smokers. The average pack year index (PYI) was 23.8 (0–153), and 30 patients had over 27.5 PYI. Most cases involved primary lung cancer (65 cases), including adenocarcinoma (53 cases), squamous cell carcinoma (8 cases), and other types (4 cases). Metastatic lung tumors were present in nine cases, three of which were categorized as other types. Regarding the side of surgery, 38 cases involved right-sided operations, while 39 involved left-sided operations. The distribution of resected segments varied: in the right lung, there were 15 segmentectomies in segment 6 (S6), 12 in the basal segment, and 11 in other segments (three in the upper lobe and eight in the lower lobe). In the left lung, segmentectomies included five in segment 1+2 (S1+2), eight in the upper segment (S1+2+3), 15 in S6, six in the basal segment, and five in other segments (three in the upper lobe and two in the lower lobe). CT findings revealed emphysematous disease pattern in 14 cases, with two cases exhibiting both emphysematous and interstitial disease pattern, and one case displaying interstitial disease pattern. The intersegmental plane division employed two primary techniques: 52 cases used a stapler exclusively, while 25 cases predominantly used an electrocautery device (Table 1).
Table 1
| Variables | All patients, n (%) | Compensated group, n (%) | Non-compensated group, n (%) | P value |
|---|---|---|---|---|
| Age | 0.01 | |||
| Under 65 years old | 20 (26.0) | 18 (34.6) | 2 (8.0) | |
| 65 years old or older | 57 (74.0) | 34 (65.4) | 23 (92.0) | |
| Sex | 0.81 | |||
| Male | 37 (48.1) | 24 (46.2) | 13 (52.0) | |
| Female | 40 (51.9) | 28 (53.8) | 12 (48.0) | |
| Thoracotomy | 0.23 | |||
| Open | 5 (6.5) | 2 (3.8) | 3 (12.0) | |
| Small incision | 64 (83.1) | 43 (82.7) | 21 (84.0) | |
| Complete VATS | 8 (10.4) | 7 (13.5) | 1 (4.0) | |
| Smoking history | 0.09 | |||
| Current/ex-smoker | 41 (53.2) | 24 (46.2) | 17 (68.0) | |
| Never | 36 (46.8) | 28 (53.8) | 8 (32.0) | |
| Pack year index | 0.046 | |||
| <27.5 | 47 (61.0) | 36 (69.2) | 11 (44.0) | |
| ≥27.5 | 30 (39.0) | 16 (30.8) | 14 (56.0) | |
| Background lung | 0.38 | |||
| Normal | 60 (77.9) | 42 (80.8) | 18 (72.0) | |
| COPD | 14 (18.2) | 7 (13.5) | 7 (28.0) | |
| IP | 1 (1.3) | 1 (1.9) | 0 (0.0) | |
| COPD + IP | 2 (2.6) | 2 (3.8) | 0 (0.0) | |
| Pulmonary function test | 0.50 | |||
| Normal | 51 (66.2) | 35 (67.3) | 16 (64.0) | |
| Obstructive disease | 23 (29.9) | 16 (30.8) | 7 (28.0) | |
| Restrictive disease | 3 (3.9) | 1 (1.9) | 2 (8.0) | |
| Resected segment number | 0.04 | |||
| 1 or 2 | 53 (68.8) | 31 (59.6) | 22 (88.0) | |
| 3 or 4 | 24 (31.2) | 21 (40.4) | 3 (12.0) | |
| Formation of intersegmental planes | 0.009 | |||
| Stapler | 52 (67.5) | 30 (57.7) | 22 (88.0) | |
| Electrocautery | 25 (32.5) | 22 (42.3) | 3 (12.0) | |
| Pleurodesis | 0.49 | |||
| Present | 10 (13.0) | 8 (15.4) | 2 (8.0) | |
| Absent | 67 (87.0) | 44 (84.6) | 23 (92.0) |
VATS, video-assisted thoracoscopic surgery; COPD, chronic obstructive pulmonary disease (emphysematous disease pattern observed on CT); IP, interstitial pneumonia (interstitial disease pattern observed on CT); CT, computed tomography.
Postoperative lung volume increase and patient characteristics
In this cohort of 77 patients, the average rate of postoperative lung volume increase was 104.6% (range, 79.5–149.8%). The compensatory group included 52 patients, while the non-compensatory group included 25 patients. A comparative analysis was conducted to examine factors such as age, sex, smoking history, number of resected segments, presence or absence of emphysematous or interstitial disease pattern CT appearance, preoperative pulmonary function test (obstructive disease or restrictive disease), surgical approach, method of intersegmental plane division, presence of intrathoracic adhesions, occurrence of postoperative air leaks, and pleurodesis procedure. The results indicated that the compensatory group had significantly higher proportions of individuals of under 65 years old of age (P=0.01), those with less than 27.5 PYI (P=0.046), those with ≥3 resected segments (P=0.04), and cases in which intersegmental plane division was performed using an electrocautery device (P=0.009) (Table 1).
Among the 31 cases for which preoperative and one-year postoperative pulmonary function test data were available, we observed a notable trend within the compensatory group (20 patients). In this group, the postoperative measured values of VC and FEV1 exceeded the preoperatively predicted value for the remaining lung volume (VC, P=0.005; FEV1, P=0.01). On the other hand, there was no significant difference between the postoperative measured VC and FEV1 values and the preoperatively predicted value in the non-compensatory group (VC, P=0.41; FEV1, P=0.58).
Details of the change in remaining lung volume
To investigate the location of the postoperative compensatory lung volume increase phenomenon and the differences between the compensated and non-compensated groups, the residual lungs were divided into three parts for a detailed comparison: (I) the volume of the lobe including the resected segment (preservation segment); (II) the total volume of the ipsilateral lobes excluding the resected segment; and (III) the total volume of the contralateral lung (Figure 1, Table 2).
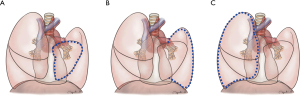
Table 2
| Volume of the lobe including the resected segment | Total volumes of the ipsilateral lobes excluding the resected segment | Total volume of the contralateral lung | ||||||
|---|---|---|---|---|---|---|---|---|
| Rates (%), range | P value | Rates (%), range | P value | Rates (%), range | P value | |||
| Compensated group (n=52) | 53.6–176.8 | 0.63 | 86.9–173.5 | <0.001 | 93.2–157.7 | <0.001 | ||
| Non-compensated group (n=25) | 33.3–115.9 (decrease) | 0.003 | 66.9–126.2 | 0.25 | 67.8–112.9 | 0.09 | ||
In the compensated group, there was no significant change in (I) the volume of the lobe, including the resected segment (range of the rate of postoperative lung volume increase: 53.6–176.8%, P=0.63). In addition, the postoperative lung volume was significantly increased in comparison to the preoperative and postoperative volumes of (II) the total volume of the ipsilateral lobes excluding the lobe with resected segments (range: 86.9–173.5%, P<0.001), and (III) the total volume of the contralateral lung (range: 93.2–157.7%, P<0.001) (Table 2). In the contralateral lung, both the upper and lower lobes exhibited significant expansion (right side, 20 cases; left upper lobe, P=0.002; left lower lobe, P<0.001; left side, 26 cases: right upper lobe, P<0.001; right lower lobe, P<0.001). However, no significant differences were observed in the right middle lobe (P=0.056).
In contrast, the non-compensatory group showed a significant volume decrease in (I) the lobe including the resected segment (range: 33.3–115.9%, P=0.003), but no significant change was found in (II) the total volume of the ipsilateral lobes excluding the lobe with resected segments (range: 66.9–126.2%, P=0.25), and (III) the total volume of the contralateral lung (range: 67.8–112.9%, P=0.09) (Table 2).
Discussion
The results of the JCOG0802 trial revealed no statistically significant difference in the postoperative FEV1 values between lobectomy and segmentectomy (1). This finding was unexpected for thoracic surgeons and necessitates further exploration of the benefits of segment preservation in sublobar resection for early-stage lung cancer.
The findings of this study may be contextualized within the broader scope of existing research, including several previous studies on lobectomy cases that have demonstrated a correlation between increased compensatory lung growth rate and larger resection volumes (4,5,13,16,18). Ueda et al. conducted a comparative investigation of the rate of postoperative lung volume increase between lobectomy and segmentectomy, revealing that lobectomy cases experienced more pronounced expansion of the remaining lung (5). Similarly, in our study evaluating segmentectomy, the compensatory group included a higher number of cases with more extensive resection of the lung segments, indicating a greater volume of resection. This observation is consistent with prior findings from lobectomy cases and suggests distinctive characteristics of compensatory changes following anatomical lung resection. Notably, dynamic movements within the thoracic cavity and extensive expansion and shift of the residual lung were observed in patients classified within the compensatory group after segmentectomy (Figure 2). These findings align with alterations in chest X-ray observations that are routinely noted by thoracic surgeons in clinical practice. Particularly in the compensated group, the volume of the lobe encompassing the resected segments was preserved and closely aligned with the preoperative volume predictions. This underlines the importance of preserving lung segments during surgery. Additionally, increases in the total volume of the contralateral and ipsilateral lobes, excluding the resected segments, were noted. Such changes effectively counterbalance any reduction in postoperative lung volume. These volumetric observations were corroborated by both preoperative and postoperative pulmonary function test results and three-dimensional computed tomography (3D-CT) volume measurements. The previous study has reported associations between preoperative predictive volumes obtained from 3D-CT and postoperative the results of postoperative pulmonary function tests (12). In addition, the increase in lung volume measured before and after surgery was significantly correlated with improvement in pulmonary function test results (5). Nomori et al. reported the outcomes of respiratory function tests and lung perfusion single photon emission computed tomography (SPECT)/CT conducted before and 6 months following lobectomy and segmentectomy. They observed that lung function in the contralateral lung improved following both surgical interventions, whereas the function of the ipsilateral lobes excluding the resected segment exhibited an increase only in cases of segmentectomy (19). It was suggested that the absence of improvement in lung function of the ipsilateral lobes within the lobectomy cases could be attributed to bronchial and vascular distortions caused by the excursion of the residual lung (19). Although the scope of this study was confined to segmentectomy, the excursion of the residual lung, bronchial and vascular distortions caused by the excursion of the residual lung might explain the observed lack of compensatory lung expansion in the ipsilateral other lobes among the non-compensated group.

Previous studies have presented mixed findings concerning the preservation of the postoperative pulmonary function. Some studies favor segmentectomy over lobectomy, yet many thoracic surgeons have questioned the clinical significance of these differences (1,2,18-23). Additionally, the JCOG0802 study reported a higher overall survival rate in the segmentectomy group than in the lobectomy group, supporting the potential benefits of lung preservation through segmentectomy. Patients who undergo segmentectomy may be better positioned to withstand various treatments for subsequent secondary cancers than those who undergo lobectomy. In this study, there was no significant volume loss in the remaining area in the compensated group, which underlines the importance of segment preservation. Furthermore, both this study and previous research have indicated that postoperative compensatory lung expansion is more commonly observed in patients who have undergone resection of a larger number of segments. These findings imply that in cases where only a small number of segments are resected and postoperative lung function does not significantly exceed preoperative projections, there may still be potential for further lung capacity expansion following secondary anatomic lung resection. Consequently, the advantages of lung preservation afforded by segmentectomy may not be immediately apparent following initial surgery.
Previous reports have suggested that an increase in functional lung parenchyma in the residual lung after surgery may be more likely to occur in younger patients (7-10). Ohata et al. reported observations of compensatory neo-alveolarization after lung resection in large animals, both through imaging and histological examination (11). In general, the number of alveoli in humans continues to increase after birth, but reaches an upper limit during school age. In contrast, Butler et al. (9) reported that an increase in functional lung parenchyma may occur after pneumonectomy in young adults with lung cancer. Mizobuchi et al. (10) reported on living lung transplant donors. This study showed more cases with small PYI and younger age in the compensated group. These results suggest that the postoperative compensatory lung expansion in the compensated group may have included not only hyperinflation of the remaining lung, but also an increase in the functional lung parenchyma.
Furthermore, we observed a predominance of intersegmental plane formation using electrocautery in the compensated group. With respect to the techniques employed for this procedure, previous reports have indicated no significant difference in postoperative pulmonary function when comparing different devices, such as surgical staplers or electrocautery (24-26). In this study, electrocautery was used in a limited number of patients. Additionally, in cases where the stapler was employed, there might have been incursions into the areas intended for preservation during the actual surgical procedure.
The present study was associated with some limitations. First, there was a small number of patients available for postoperative pulmonary function test results. This included a relatively small sample size and the absence of pulmonary function tests in all patients. Second, unevaluable background factors may have affected the condition of the contralateral lung or the mediastinum. For example, full adhesions of the contralateral lung and poor mediastinal mobility may inhibit the expansion of the contralateral lung, which is associated with postoperative compensatory lung volume gain. Third, only 3D-CT and pulmonary function tests, which mainly focus on lung air volume, were performed. In other words, indices such as pulmonary blood flow, oxygen uptake, and symptoms of patients, such as percutaneous oxygen saturation (SpO2), partial pressure of arterial oxygen (PaO2), diffusing capacity of lung for carbon monoxide (DLCO), and the 6-minute walking test (22,27,28). Furthermore, in the analysis of volumetric changes before and after surgery, as conducted in this study, where the residual lungs divided into several parts, investigations utilizing imaging techniques for a more detailed local volume analysis that reflects more functional lung volume assessments—such as these reported by previous studies with lung perfusion SPECT/CT, radiologic lung weight, and functional lung volume measurement for example excluding low attenuation area—were not undertaken in our research (10,11,19,29,30).
Conclusions
In conclusion, postoperative compensatory volume changes occur in an extensive area of the thoracic cavity, not only in the ipsilateral lung but also in the contralateral lung. In addition, there was no significant decrease in volume in any of the residual segments compared to the preoperative volume, suggesting the importance of segment preservation.
Acknowledgments
This original article was proofread by Dr. Brian Quinn (Japan Medical Communication).
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-83/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-83/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-83/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-83/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committees of the Faculty of Medicine, Oita University (approval No. 2023-2642) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Hoshino H, Koba H, Sekine K, et al. Compensatory increases in residual lobar volume following lung resection. Nihon Kokyuki Gakkai Zasshi. 1999;37:783-9. [PubMed]
- Mizobuchi T, Wada H, Sakairi Y, et al. Spirometric and radiological evaluation of the remnant lung long after major pulmonary resection: can compensatory phenomena be recognized in clinical cases? Surg Today 2014;44:1735-43. [Crossref] [PubMed]
- Ueda K, Tanaka T, Hayashi M, et al. Computed tomography-defined functional lung volume after segmentectomy versus lobectomy. Eur J Cardiothorac Surg 2010;37:1433-7. [Crossref] [PubMed]
- Yokoba M, Ichikawa T, Harada S, et al. Postoperative pulmonary function changes according to the resected lobe: a 1-year follow-up study of lobectomized patients. J Thorac Dis 2018;10:6891-902. [Crossref] [PubMed]
- Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med 2004;170:319-43. [Crossref] [PubMed]
- Fernandez LG, Mehta CK, Kron IL, et al. Reinitiation of compensatory lung growth after subsequent lung resection. J Thorac Cardiovasc Surg 2007;134:1300-5. [Crossref] [PubMed]
- Butler JP, Loring SH, Patz S, et al. Evidence for adult lung growth in humans. N Engl J Med 2012;367:244-7. [Crossref] [PubMed]
- Mizobuchi T, Chen F, Yoshino I, et al. Radiologic evaluation for volume and weight of remnant lung in living lung donors. J Thorac Cardiovasc Surg 2013;146:1253-8. [Crossref] [PubMed]
- Ohata K, Chen-Yoshikawa TF, Hamaji M, et al. Radiologic evaluation of compensatory lung growth using computed tomography by comparison with histological data from a large animal model. Sci Rep 2022;12:2520. [Crossref] [PubMed]
- Fernández-Rodríguez L, Torres I, Romera D, et al. Prediction of postoperative lung function after major lung resection for lung cancer using volumetric computed tomography. J Thorac Cardiovasc Surg 2018;156:2297-2308.e5. [Crossref] [PubMed]
- Wakamatsu I, Matsuguma H, Nakahara R, et al. Factors associated with compensatory lung growth after pulmonary lobectomy for lung malignancy: an analysis of lung weight and lung volume changes based on computed tomography findings. Surg Today 2020;50:144-52. [Crossref] [PubMed]
- Oizumi H, Kato H, Endoh M, et al. Techniques to define segmental anatomy during segmentectomy. Ann Cardiothorac Surg 2014;3:170-5. [PubMed]
- Yotsukura M, Okubo Y, Yoshida Y, et al. Indocyanine green imaging for pulmonary segmentectomy. JTCVS Tech 2021;6:151-8. [Crossref] [PubMed]
- Yoshiyasu N, Kojima F, Takahara H, et al. Efficacy of the Segment-Counting Method in Predicting Lung Function and Volume Following Stapler-Based Thoracoscopic Segmentectomy. Ann Thorac Cardiovasc Surg 2022;28:121-8. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Lee SG, Lee SH, Cho SH, et al. Changes in Forced Expiratory Volume in 1 Second after Anatomical Lung Resection according to the Number of Segments. J Chest Surg 2021;54:480-6. [Crossref] [PubMed]
- Nomori H, Shiraishi A, Cong Y, et al. Differences in postoperative changes in pulmonary functions following segmentectomy compared with lobectomy. Eur J Cardiothorac Surg 2018;53:640-7. [Crossref] [PubMed]
- Charloux A, Quoix E. Lung segmentectomy: does it offer a real functional benefit over lobectomy? Eur Respir Rev 2017;26:170079. [Crossref] [PubMed]
- Suzuki H, Morimoto J, Mizobuchi T, et al. Does segmentectomy really preserve the pulmonary function better than lobectomy for patients with early-stage lung cancer? Surg Today 2017;47:463-9. [Crossref] [PubMed]
- Xu Y, Qin Y, Ma D, et al. The impact of segmentectomy versus lobectomy on pulmonary function in patients with non-small-cell lung cancer: a meta-analysis. J Cardiothorac Surg 2022;17:107. [Crossref] [PubMed]
- Nomori H, Yamazaki I, Machida Y, et al. Lobectomy versus segmentectomy: a propensity score-matched comparison of postoperative complications, pulmonary function and prognosis. Interact Cardiovasc Thorac Surg 2022;34:57-65. [Crossref] [PubMed]
- Tao H, Tanaka T, Hayashi T, et al. Influence of stapling the intersegmental planes on lung volume and function after segmentectomy. Interact Cardiovasc Thorac Surg 2016;23:548-52. [Crossref] [PubMed]
- Chen X, Jin R, Xiang J, et al. Methods for Dissecting Intersegmental Planes in Segmentectomy: A Randomized Controlled Trial. Ann Thorac Surg 2020;110:258-64. [Crossref] [PubMed]
- Yazawa T, Igai H, Numajiri K, et al. Comparison of stapler and electrocautery for division of the intersegmental plane in lung segmentectomy. J Thorac Dis 2021;13:6331-42. [Crossref] [PubMed]
- Fuzhi Y, Dongfang T, Wentao F, et al. Rapid Recovery of Postoperative Pulmonary Function in Patients With Lung Cancer and Influencing Factors. Front Oncol 2022;12:927108. [Crossref] [PubMed]
- Lim E, Seif K, Goetz T, et al. Agreement between observed and predicted postoperative forced expiratory volume in one second, forced vital capacity, and diffusing capacity for carbon monoxide after anatomic lung resection. J Thorac Dis 2024;16:247-52. [Crossref] [PubMed]
- Kobayashi K, Saeki Y, Kitazawa S, et al. Three-dimensional computed tomographic volumetry precisely predicts the postoperative pulmonary function. Surg Today 2017;47:1303-11. [Crossref] [PubMed]
- Fan Z, Zhao S, Wang L, et al. Comparison between functional lung volume measurement and segment counting for predicting postoperative pulmonary function after pulmonary resection in lung cancer patients. BMC Pulm Med 2023;23:6. [Crossref] [PubMed]


