
The safety and prognostic evaluation of subsequent aortic surgery after thoracic endovascular aortic repair: a retrospective cohort study
Highlight box
Key findings
• Aortic surgery after thoracic endovascular aortic repair (TEVAR) is a safe and effective.
What is known and what is new?
• A proportion of patients who underwent TEVAR may require aortic surgery because of retrograde type A aortic dissection occurring at the proximal end of the TEVAR stent, as confirmed by computed tomography angiography (RTAD).
• This group of people should be regarded as having type A aortic dissection and require aggressive surgical procedures.
What is the implication, and what should change now?
• For patients who develop RTAD after TEVAR, it is crucial to actively pursue surgical intervention, which does not pose additional risks to the patients. Furthermore, for the existing TEVAR stents, it is necessary to properly trim the proximal crown portion of the stent graft. This modification creates an optimal area for anastomosis, thereby enhancing the reliability and effectiveness of the anastomosis.
Introduction
The treatment of aortic diseases involves meticulous clinical decision-making, requiring a delicate balance between surgical risks and patient prognosis. With the continuous advancement of thoracic endovascular aortic repair (TEVAR), an increasing number of patients are opting for this treatment modality (1). However, some patients who have undergone TEVAR may need to undergo open aortic surgery postoperatively, which can be caused by various reasons (2). Among these, retrograde type A aortic dissection occurring at the proximal end of the TEVAR stent, as confirmed by computed tomography angiography (CTA) (RTAD) is the focus of discussion in this article. In the current clinical treatment process, there is not yet a unified diagnostic and treatment plan for this group of patients. This requires us to conduct more in-depth research. Investigating the safety of open aortic surgery after TEVAR provides valuable insights for clinical practice, assisting surgeons in better assessing patient outcomes and formulating personalized treatment plans, ultimately contributing to the improvement of patient prognosis. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-71/rc).
Methods
Patients
From September 2016 to August 2020, Guangdong Provincial People’s Hospital treated 21 patients who underwent repeat aortic surgery after TEVAR, with all cases confirmed through aortic CTA. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Review Committee of Guangdong Provincial People’s Hospital (KY-Z-2022-218-01) and individual consent for this retrospective analysis was waived.
Definitions and follow-up
RTAD refers to aortic dissection occurring at the proximal end of the TEVAR stent, as confirmed by CTA. Myocardial dysfunction refers to a condition indicated by transthoracic echocardiography showing a left ventricular ejection fraction of less than 50%. Dialysis is required when kidney function falls below a critical level, typically when the glomerular filtration rate (GFR) is less than 15 mL/min/1.73 m2 or when serum creatinine levels are significantly elevated above normal values. Core temperature refers to rectal temperature.
Patients are followed up annually by telephone, focusing on symptoms and their corresponding management.
Statistical analysis
Continuous variables were described as mean ± standard deviation or median (range), and categorical values as number (%). The Kaplan-Meier method was used to evaluate survival, and the “survival” package in R was used for plotting. Due to the limited number of cases in this study, descriptive statistics are mostly used rather than other statistical methods. This is an observational cohort retrospective study.
Operative techniques
The surgery was reported previously (3). Specially, during the exploration of the arch, the proximal anchoring area of the TEVAR stent was appropriately trimmed. In some patients where the stent was positioned too close to the proximal end (Figure 1A), a section of the covered stent was judiciously excised (Figure 1B,1C). Figure 1D shows the condition of the aortic arch computed tomography (CT) of an RTAD patient before undergoing aortic surgery. The aortic arch was then trimmed to the proximal end of the left common carotid artery opening, and a frozen elephant trunk (FET) stent was implanted distally (Figure 2A). The diameter of the elephant trunk stent was selected based on the diameter of the distal artery and the TEVAR stent. Four-branch artificial blood vessels [Terumo (Tokyo, Japan) or Maquet (Rastatt, Germany)] were utilized to reconstruct the arch (Figure 2B). After completion of the distal anastomosis between the four branch vessels and the autologous aorta, one branch vessel was selected for arterial perfusion to restore distal circulation. After the distal stumps of the left subclavian artery, left carotid artery, and innominate artery were anastomosed to branch vessels, the rewarming process was commenced. The extracorporeal circulation pipeline was gradually weaned, and the operation was concluded (Figure 2C). Figure 2D shows the CT of an RTAD patient after undergoing aortic surgery. Table 1 provides information related to the surgery.
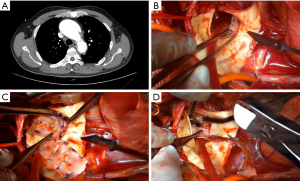

Table 1
| Surgical-related information | Value |
|---|---|
| Acute phase (≤14 days) | 19 (90.5) |
| Ascending aorta replacement + TAR + FET technique | 17 (81.0) |
| Ascending aorta replacement + TAR | 1 (4.8) |
| Ascending aorta + right half arch replacement | 3 (14.3) |
| Additional procedures | |
| Aortic root replacement (Bentall procedure) | 6 (28.6) |
| Aortic root replacement (Wheat procedure) | 1 (4.8) |
| Aortic valve commissure resuspension | 5 (23.8) |
| MVR | 1 (4.8) |
| Ascending aorta-abdominal aortic bypass | 1 (4.8) |
| Cardiopulmonary bypass time (min) | 246.1±55.5 |
| Aortic cross-clamp time (min) | 130.0±38.6 |
| Selective cerebral perfusion time (min) | 8.5±7.7 |
Data are presented as n (%) or mean ± standard deviation. TAR, total arch replacement; FET, frozen elephant trunk; MVR, mitral valve replacement; SD, standard deviation.
Results
The average age of the patients was 53±11.41 years, and 20 individuals (95.2%) were male (demographic data presented in Table 2). The indications for the index TEVAR were 19 cases of type B aortic dissection (TBAD) and two cases of penetrating aortic ulcer. In five patients, TEVAR and chimney grafting implantation were simultaneously performed, with one case involving the left carotid artery and five cases involving the left subclavian artery. The median interval between the initial TEVAR surgery and RTAD was 5.0 months (range, 0.25–96 months). Table 3 summarizes the relevant information of the TEVAR procedure.
Table 2
| Preoperative data | Value |
|---|---|
| Age (years) | 53±11.41 |
| Male gender | 20 (95.2) |
| Smoker | 6 (28.6) |
| Hypertension | 15 (71.4) |
| Coronary artery disease | 2 (9.5) |
| Abdominal aortic aneurysm | 1 (4.8) |
| Aortic regurgitation ≥ moderate | 5 (23.8) |
| Pericardial effusion | 4 (19.0) |
| Symptoms | |
| Chest pain | 9 (42.9) |
| Headache | 2 (9.5) |
| Asymptomatic | 12 (57.1) |
| Hemoglobin content (g/L) | 113.3±22.7 |
| White blood cell count (109/L) | 9.1±3.1 |
| Platelet count (109/L) | 203.2±6.0 |
| D-dimer (ng/mL) | 4,139.5±2,824.7 |
| Serum creatinine (μmol/L) | 89.3±28.2 |
Data are presented as n (%) or mean ± standard deviation.
Table 3
| TEVAR-related information | Value |
|---|---|
| TEVAR indications | |
| Type B aortic dissection | 19 (90.5) |
| Penetrating aortic ulcer | 2 (9.5) |
| Proximal landing zone | |
| 1 | 17 (81.0) |
| 2 | 3 (14.2) |
| 3 | 1 (4.8) |
| Time interval from TEVAR to RTAD (months) | 5 [0.25–96] |
| ≤14 days | 1 (4.8) |
| >14 days–3 months | 8 (38.1) |
| >3–12 months | 7 (33.3) |
| >12 months | 5 (23.8) |
| Chimney graft implantation | |
| Left common carotid artery | 1 (4.8) |
| Left subclavian artery | 5 (23.8) |
Data are presented as n (%) or median [range]. TEVAR, thoracic endovascular aortic repair; RTAD, retrograde type A aortic dissection occurring at the proximal end of the TEVAR stent, as confirmed by CTA; CTA, computed tomography angiography.
Among all admitted patients, 19 individuals (90.5%) received treatment during the acute phase (within 14 days). Seventeen patients underwent ascending aorta replacement plus total arch replacement with elephant trunk stent implantation, one patient underwent only ascending aorta replacement plus total arch replacement, and three patients underwent ascending aorta plus right hemiarch replacement. Among them, 12 cases required aortic root repair, including six cases of Bentall procedure, one case of Wheat procedure, and five cases of aortic sinus repair. Additional procedures included one case of mitral valve replacement, two cases of mitral valvuloplasty, and one case of ascending aorta-femoral artery bypass. The mean cardiopulmonary bypass (CPB) time, aortic cross-clamp time, and selective cerebral perfusion (SCP) time were 246.1±55.5, 130.0±38.6, and 8.5±7.7 minutes, respectively. The median duration of mechanical ventilation was 2 days (range, 1–21 days). The mean duration of postoperative intensive care unit stay was 6.4±5.4 days and in-hospital stay was 20.0±10.9 days. Adverse events (death, cardiac insufficiency, renal failure requiring dialysis) occurred in four cases (19.0%), including two cases of death during hospitalization, one case of cardiac insufficiency, and one case of renal failure requiring dialysis. Additionally, three patients were readmitted to the operating room for treatment twice due to anastomotic stenosis of aortic branch vessels, large postoperative pleural fluid volume, and delayed chest closure. Among the 19 surviving patients, one was lost to follow-up, while the follow-up data for the remaining 18 were available (Figure 3). The follow-up duration ranged from 38 to 85 months, with an average of 53.7±16.1 months. During the follow-up period, one patient experienced headache, one reported dizziness, one had renal dysfunction, and one developed a cerebral infarction. Specific details are listed in Table 4.
Table 4
| Postoperative Information | Value |
|---|---|
| Ventilation time (days) | 2 [1–21] |
| Intensive care unit duration (days) | 6.4±5.4 |
| Postoperative in-hospital stay (days) | 20.0±10.9 |
| Adverse events | |
| Death | 2 (9.5) |
| Myocardial dysfunction | 1 (4.8) |
| Renal failure necessitating dialysis | 1 (4.8) |
| Re-exploration for operation | 4 (19.0) |
| Survival number after discharge | 19 (90.5) |
| Adverse events after discharge | |
| Headache | 1 (4.8) |
| Vertigo | 1 (4.8) |
| Renal failure | 1 (4.8) |
| Stroke | 1 (4.8) |
Data are presented as n (%), mean ± standard deviation or median [range].
Discussion
Although RTAD is a rare complication after TEVAR, it poses significant and often fatal risks to patients. In the event of RTAD, nearly all patients require repeat aortic surgery, with an incidence of 0.9–6.8%, and the time interval between the initial TEVAR and the occurrence of RTAD varies (4-9). Apart from uncontrolled blood pressure, the inflammatory response induced by invasive procedures in the aorta appears to be a major factor (4). In this study, the shortest time interval between TEVAR and RTAD was less than one week, while the longest was 96 months, indicating that inflammation may persist in the mended aorta postoperatively, potentially spanning several years.
Among the patients who underwent TEVAR, five presented with chest and back pain as the initial symptom of RTAD, while another five exhibited ischemia of the central nervous system, renal, and digestive system. The remaining cases were identified during follow-up aortic CTA. This suggests that procedures and devices related to TEVAR carry the risk of causing RTAD. First, factors associated with TEVAR may lead to damage of the aorta. For example, during the delivery of guide wires and stents, the aorta may be susceptible to injury. The balloon dilation process following stent placement can also result in damage to the intima of the aorta, thereby exacerbating aortic inflammation and making it more susceptible to developing RTAD (10). Second, the choice of stent used during surgery is also an important contributing factor to the formation of RTAD (11). Typically, the selection of stent graft models is based on preoperative CTA and intraoperative imaging findings. To enhance the closure of the aortic entry tear and promote thrombosis of the false lumen, the chosen model is usually 10% larger or more than the measured diameter. However, oversized stent grafts impose greater wall stress on the inflamed and fragile aorta, making patients undergoing TEVAR more susceptible to adverse aortic-related events postoperatively (10,12). Previous studies have indicated that selecting stents with diameters ranging from 0–5% larger than the diameter measured in the anchoring zone on preoperative CTA has achieved favorable outcomes in preventing the occurrence of RTAD (13,14). For asymptomatic RTAD patients, upon reviewing CTA during follow-up, we observed that a considerable number of patients exhibited varying degrees of aortic atherosclerosis. This suggests that potential aortic pathology is also a significant factor in RTAD (11,15).
Due to the extremely high mortality rate associated with type A aortic dissection, almost all patients included in this study underwent emergency surgery (≤14 days). The standard surgical approach involved a conventional ascending aorta replacement combined with total arch replacement through a midline thoracotomy. Additionally, 17 patients in this study underwent simultaneous implantation of a FET stent. One patient, with no dissection occurring in the aorta adjacent to the TEVAR stent, underwent only ascending aorta and aortic arch replacement. The remaining three cases, where the dissection was confined to the ascending aorta, did not undergo total arch replacement; instead, they underwent ascending aorta replacement combined with right hemiarch replacement. Previous studies have indicated that aortic arch replacement with FET implantation is highly advantageous for patients with RTAD, especially those at high surgical risk. This not only reduces procedural complexity but also significantly shortens the overall surgical duration (6,11,16,17). However, due to the presence of TEVAR stents and the complex anatomy of the aortic arch, adopting this technique has become more challenging, inevitably leading to prolonged CPB and surgical duration. Consequently, there is a significantly increased likelihood of postoperative complications, such as paraplegia, renal impairment, and gastrointestinal complications. In our study, following RTAD repair, one patient required renal replacement therapy through hemodialysis due to elevated blood creatinine levels, and two patients died during hospitalization. One case was attributed to uncontrollable infection resulting from a pre-existing aortic-esophageal fistula, while the other was due to postoperative cardiac dysfunction.
Based on our past experience, patients who develop RTAD after TEVAR surgery need to undergo surgery as soon as possible, which is consistent with the results of a previous single-center study (18). However, compared to patients who have not undergone TEVAR, there are several additional aspects that require attention during the aortic surgical procedure. First, it is necessary to remove the portion of the TEVAR stent proximal anchor, as the rigid and exposed proximal part is likely to abrade the anastomotic line of the distal end of the four-branch artificial blood vessel during aortic surgery, leading to the occurrence of anastomotic fistula. In addition, we only trim the exposed portion near the proximal end of the TEVAR stent while preserving the remaining distal part to maintain the effect of TEVAR. An increase in procedures involving the aortic arch typically requires longer periods of SCP. At this point, the strategy for cerebral protection becomes crucial. When necessary, bilateral antegrade cerebral perfusion can be implemented to ensure brain protection. Simultaneously, monitoring the patient’s cerebral protection status through methods such as electroencephalography is advisable (19).
This study had certain limitations. As Guangdong Provincial People’s Hospital is the largest cardiovascular disease center in South China, many surgical patients are transferred from other hospitals. In this study, only three patients underwent TEVAR at Guangdong Provincial People’s Hospital. Consequently, we were unable to obtain relevant information for patients who underwent TEVAR procedures at other hospitals. Establishing a separate research cohort for this subgroup of patients was also not feasible. Therefore, in this study, we only conducted descriptive analyses on patients with RTAD, and could not predict risk factors.
Conclusions
This study showed that aortic surgery after TEVAR is a safe and effective treatment method. However, multi-center large-sample studies and long-term follow-up are needed to enhance safety and durability to ensure that patients receive optimal treatment and prognosis.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-71/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-71/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-71/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-71/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Review Committee of Guangdong Provincial People’s Hospital (KY-Z-2022-218-01) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yurekli I, Kestelli M, Cakir H. About Timing of TEVAR in Dissection. Ann Thorac Surg 2023;115:795. [Crossref] [PubMed]
- Dun Y, Shi Y, Guo H, et al. The surgical management of retrograde type A aortic dissection after thoracic endovascular aortic repair. Interact Cardiovasc Thorac Surg 2020;30:732-8. [Crossref] [PubMed]
- Sun LZ, Ma WG, Zhu JM, et al. Sun's procedure for chronic type A aortic dissection: total arch replacement using a tetrafurcate graft with stented elephant trunk implantation. Ann Cardiothorac Surg 2013;2:665-6. [PubMed]
- Eggebrecht H, Thompson M, Rousseau H, et al. Retrograde ascending aortic dissection during or after thoracic aortic stent graft placement: insight from the European registry on endovascular aortic repair complications. Circulation 2009;120:S276-81. [Crossref] [PubMed]
- Wang GQ, Qin YF, Shi ST, et al. Retrograde type A aortic dissection during or after thoracic endovascular aortic repair: a single center 16-year experience. Front Cardiovasc Med 2023;10:1160142. [Crossref] [PubMed]
- Li B, Pan XD, Ma WG, et al. Stented elephant trunk technique for retrograde type A aortic dissection after endovascular stent graft repair. Ann Thorac Surg 2014;97:596-602. [Crossref] [PubMed]
- Canaud L, Ozdemir BA, Patterson BO, et al. Retrograde aortic dissection after thoracic endovascular aortic repair. Ann Surg 2014;260:389-95. [Crossref] [PubMed]
- Chen Y, Zhang S, Liu L, et al. Retrograde Type A Aortic Dissection After Thoracic Endovascular Aortic Repair: A Systematic Review and Meta-Analysis. J Am Heart Assoc 2017;6:e004649. [Crossref] [PubMed]
- Cheng L, Xiang D, Zhang S, et al. Reintervention after Thoracic Endovascular Aortic Repair of Uncomplicated Type B Aortic Dissection. J Clin Med 2023;12:1418. [Crossref] [PubMed]
- Bellos JK, Petrosyan A, Abdulamit T, et al. Retrograde type A aortic dissections after endovascular stent-graft placement for type B dissection. J Cardiovasc Surg (Torino) 2010;51:85-93. [PubMed]
- Ali-Hasan-Al-Saegh S, Halloum N, Scali S, et al. A systematic review and meta-analysis of retrograde type A aortic dissection after thoracic endovascular aortic repair in patients with type B aortic dissection. Medicine (Baltimore) 2023;102:e32944. [Crossref] [PubMed]
- Ma T, Dong ZH, Wang S, et al. Computational investigation of interaction between stent graft and aorta in retrograde type A dissection after thoracic endovascular aortic repair for type B aortic dissection. J Vasc Surg 2018;68:14S-21S.e2.
- Liu L, Zhang S, Lu Q, et al. Impact of Oversizing on the Risk of Retrograde Dissection After TEVAR for Acute and Chronic Type B Dissection. J Endovasc Ther 2016;23:620-5. [Crossref] [PubMed]
- Wen Q, Wu G, Ji Y, et al. Physician-Modified Endografts for the Treatment of Thoracic Aortic Pathologies Involving the Aortic Arch. J Endovasc Ther 2023; Epub ahead of print. [Crossref] [PubMed]
- Ma T, Dong ZH, Fu WG, et al. Incidence and risk factors for retrograde type A dissection and stent graft-induced new entry after thoracic endovascular aortic repair. J Vasc Surg 2018;67:1026-1033.e2. [Crossref] [PubMed]
- An Z, Song Z, Tang H, et al. Retrograde Type A Dissection after Thoracic Endovascular Aortic Repair: Surgical Strategy and Literature Review. Heart Lung Circ 2018;27:629-34. [Crossref] [PubMed]
- Zhou J, Yao X, Guo B, et al. Surgical Treatment of Retrograde Type A Aortic Dissection After Thoracic Endovascular Aortic Repair. Int Heart J 2022;63:286-92. [Crossref] [PubMed]
- Roselli EE, Abdel-Halim M, Johnston DR, et al. Open aortic repair after prior thoracic endovascular aortic repair. Ann Thorac Surg 2014;97:750-6. [Crossref] [PubMed]
- Kamenskaya OV, Klinkova AS, Chernyavsky AM, et al. Deep Hypothermic Circulatory Arrest vs. Antegrade Cerebral Perfusion in Cerebral Protection during the Surgical Treatment of Chronic Dissection of the Ascending and Arch Aorta. J Extra Corpor Technol 2017;49:16-25. [Crossref] [PubMed]



