Sleep complaints and sleep breathing disorders in upper and lower obstructive lung diseases
Introduction
Obstructive diseases of upper and lower airways, in particular rhinitis, asthma and chronic obstructive pulmonary diseases (COPD), influence the health status and quality of life of a large percentage of world population (1-3). Sleep represents one third of the human life and it modifies many physiological functions including breathing. The effects of changes during sleep on respiration can lead the symptomatic worsening in patients with chronic obstructive diseases. In addition, rhinitis, asthma, and COPD can lead to sleep fragmentation with consequent daytime fatigue, irritability, decreased mood, general malaise and cognitive impairment (4). Moreover, obstructive diseases of upper and lower airways may be involved in the pathogenesis of sleep breathing disorders such as obstructive sleep apnea syndrome (OSAS). This is a common disorder usually characterized by pharyngeal constriction during sleep, which causes sleep disruption, snoring, choking, frequent awakenings, and excessive daytime sleepiness. OSAS occurs in about 5–10% of the general population (5), and its prevalence increases with age. It is a heterogeneous disease, which causes chronic intermittent hypoxia (CIH) that drives the systemic inflammation that is the pathogenesis of the disease itself. Ioachimescu and Teodorescu have coined the acronym obstructive lung disease and obstructive sleep apnea syndrome (OLDOSA) to indicate the bidirectional links between obstructive lung diseases (OLD) and OSAS. The association between OSAS and COPD or “overlap syndrome” is well characterized, but the association between OSAS and asthma and rhinitis needs to be further investigated to clarify the cause and development of these conditions and the individualized therapeutic interventions (6).
The aim of this narrative review is to summarize the available knowledge about the link between OSAS and rhinitis, asthma, COPD. In particular, the sleep complaints resulting from the incomplete control of obstructive airways diseases and their relationship with OSAS will be discussed.
Sleep complaints and sleep-breathing disorders in rhinitis
In the relationship between sleep impairment and rhinitis, one could cause the other or they could share a common cause. That cause could be an additional medical condition that makes it more difficult to treat a concomitant disease, or these diseases could be independent from any kind of relationship. The latest ARIA guidelines [allergic rhinitis (AR) and its impact on asthma] classification considers rhinitis as a risk factor for sleep impairment and impaired sleep is a criterion to define the severity of the rhinitis. Studies analyzing the impact of rhinitis on sleep found that both in children and in adults almost 40–80% of patients with rhinitis complain of sleep impairment (7,8). Furthermore, Léger and colleagues published the results of an epidemiological study designed to assess the effects of rhinitis on sleep and to explore the relationship between AR duration and severity and the related sleep impairment (9). They studied 591 patients with AR of at least 1-year duration and 501 healthy controls. All the patients provided validated questionnaires assessing quality of life and sleepiness score. The results show a significant impact of AR on all dimensions of sleep quality and, consequently, a lower quality of life. Patients with AR, compared to the control group, had clinically relevant sleep complaints and sleep disorders. More than 40% of patients had snoring, non-restorative sleep, lack of sleep, nocturnal awakenings and difficulty falling asleep, and these complaints were more severe and persistent with increasing rhinitis severity and ARIA classification. A study performed in general practices on a large number of patients with AR (10), found that rhinitis severity was more important than its duration in causing sleep disturbances. Furthermore, the extrapolation of the DREAMS study results shows that rhinitis severity is mainly related to sleep complaints while the duration of rhinitis is associated with sleep apnea (11). Arousal and intermittent hypoxia can lead to a disruption of restorative sleep architecture with pre-cortical dysfunction that can cause problems in using information and leads to poor planning and haphazard execution of plans, disorganization, poor decision-making, rigid thinking, difficulty in maintaining attention and motivation, emotional liability (mood swings), overactivity and impulsivity (especially in children) (12). By mean of a specific neuropsychological assessment, the effect of rhinitis on a wide variety of cognitive processes and functions (attention, visual search and scanning, sequencing and shifting, psychomotor speed, abstraction, flexibility, ability to execute and modify a plan of action, and ability to maintain two trains of thought simultaneously) has been evaluated (13-15). The available results suggest that patients experience subtle slowing in the speed of cognitive processing (Marshall) and decreased efficiency. Also, AR patients may need to use more effort to reach the same performance as healthy subjects, with earlier exhaustion of their ability.
As mentioned before, rhinitis can be involved in sleep complaints as well as in the pathogenesis of sleep breathing disorders such as OSAS. Two physical principles may explain the relationship between rhinitis and OSAS. The Venturi principle states that air must pass faster through a small tube than through a larger one, if the volume of air and time to pass through are equal; the Bernoulli principle correlates the width of the airway to the risk of its collapsibility and the thinner the airways are, the greater the risk of collapsing and vice versa. Furthermore, upper airways act like a Starling resistor. A narrowing at the beginning (i.e., a blocked nose) causes the collapse of the lower tract (i.e., the pharynx) because of the collapsing forces it creates (16). Because of these physiologic principles, patients with chronic night-time rhinitis symptoms are 2 times more likely to snore than control subjects and this is the reason why about 50% of rhinitis patients with nasal congestion are 1.5 times more likely to snore than patients without nasal congestion (17). Several studies have shown this phenomenon. For example, Shedden and colleagues found that more than 80% of congested allergy sufferers have nasal obstruction as the cause of their sleep impairment (18). The logistic regression model estimating the association of congestion with snoring performed by Young, showed that individuals with chronic severe nasal congestion during the night have a 3.6-fold greater risk of habitual snoring at baseline and a 4.9-fold greater risk of habitual snoring at 5-year follow-up (19). Although the correlation between snoring and apnea needs to be better understood, nasal congestion is also associated with sleep apnea. The greater the increase of nasal resistance, the greater the increase in obstructive apnea. Moreover, apneas are longer and more frequent in patients with nasal congestion than those without it (20). This phenomenon seems strictly related to the cross sectional area of upper airways (21). Both nasal congestion and inflammation can contribute to sleep impairment in rhinitis patients. Several mediators and pro-inflammatory cytokines released in allergic inflammation act both in inducing mucosal edema and congestion and in altering sleep structure (22). Furthermore, the flow through a narrow upper airway could also be the cause of inflammation persistence through the vibration and trauma induced by snoring, and it is suggested that inflammation could alter the function of the pharyngeal reflex (23). In view of this information, it is clear that appropriate AR treatment is essential not only to ameliorate the typical rhinitis symptoms, but also to improve the related sleep and sleep breathing disorders. Several trials have been performed with different nasal steroid molecules. Hughes and colleagues showed that budesonide is able to improve sleep total score and to induce a refreshing and restorative sleeping (24). To support the value of corticosteroids, the efficacy of intranasal fluticasone in patients with OSAS and rhinitis has been assessed. It reduced the apnea-hypopnea index (AHI), in nasal congestion and improved daytime alertness (25,26). Similar results have been obtained using budesonide (24), flunisolide (27) and mometasone (28). To summarize, both symptoms and pathogenic mechanisms of rhinitis can induce sleep complaints. The anatomical features of rhinitis are involved in SDB development. Rhinitis treatment improves sleep impairments and seems crucial in facilitating the tolerability of continuous positive airway pressure (CPAP) in patients with OSAS (29,30).
Sleep disorders in asthma
As with rhinitis, asthma can be a cause of sleep complaints and be part to OSAS pathogenesis. In particular, OSAS and asthma can share a common cause or predisposing factors, they can be independent from each other or they can result from an add-on medical problem that makes it more difficult to control the concomitant pathology.
Asthma by itself may represent the cause of sleep complaints. GINA guidelines (Global Initiative for Asthma) consider sleep awakening due to asthma a sign of disease severity and a marker of uncontrolled disease (2). Sleep induces significant modification of breathing patterns (such as change in peak expiratory flow) (31) that are often overexpressed in asthmatics. In addition, sleep quality and daytime cognitive performance are impaired in patients with nocturnal asthma who are taking their usual maintenance medication compared to healthy controls. They had poorer scores in specific tests that assess concentration and attention, visual scanning, hand-eye coordination, and mental set flexibility (32). The correlation between OSAS and asthma was first investigated by Hudgel and Shrucard in the late 1970s (33). In asthmatic patients, OSAS contributes to poor asthma control (34) especially in patients who suffer most from nocturnal symptoms (35). It has been hypothesized that OSAS and asthma share most of their pathophysiological mechanisms; both often involve airways obstruction, gastroesophageal reflux, and obesity; moreover snoring could be a worsening factors for both the conditions. Teodorescu et al. (36) found a correlation between OSAS and uncontrolled asthma. Patients with obstructive apneas were 3.6 times more likely to have asthmatic symptoms despite receiving the correct treatment according to international guidelines. It is now accepted that the optimal management of either condition can ameliorate the perception and the treatment of the other (37). In fact, even in the late 1980s, Guilleminault and colleagues (38) noticed that asthmatic patients suffering from obstructive apneas who had CPAP therapy had less severe nocturnal asthmatic symptoms compared to patients who did not use CPAP. The beneficial effect of CPAP was concluded to be the reduction in vagal tone, which is increased in patients with OSAS during sleep, and consequently the reduction in episodes of nocturnal bronchoconstriction. Furthermore, according to the latest evidences, these improvements may be due to the recruitment of under-ventilated alveoli, the stabilization of the upper airway, and the improvement of inspiratory muscle activity (39,40). Furthermore, potential physiopathological correlations among asthma, rhinitis, and OSAS have been described (41). The increased upper airway collapsibility and the nasal obstruction that frequently accompany asthma may represent facilitating factors of OSAS onset in asthmatic patients. On the other hand some factors typical of OSAS, including neuroreceptorial mechanisms, gastroesophageal reflux disease (GERD), local and systemic inflammation, cardiac dysfunction, airways angiogenesis, leptin changes, and weight gain, can exacerbate nocturnal symptoms and negatively influence asthma control (42). The results of a real life survey (43) showed that the level of asthma control was inversely correlated with the presence of sleep disturbances. Well-controlled patients reported less frequent and less severe sleep disturbances compared to uncontrolled ones but, more interestingly, a significant percentage of subjects (11–20%) having achieved total control of asthma still reported sleep disturbances that contributed to increases in the impact of the disease and to impair quality of life. Nevertheless, although the correlation between asthma and obstructive sleep apneas is common, it is still underestimated and OSAS should be considered in patients with asthmatic symptoms, especially nocturnal, despite treatments based on international guidelines (44). US National Asthma Education and Prevention Program recommended to screen for OSAS all patients with asthma not fully controlled (45).
Moreover, obesity seems to play an important role in both conditions. Obesity is considered the most important risk factor for OSAS (46) because it increases upper airway collapsibility and alters sleep architecture through neurohormonal abnormalities, such as leptin-grelin hormonal changes. Leptin is a mediator of energy balance, which suppresses food intake and promotes weight loss. Obese patients have been found to be leptin-resistant, so plasma leptin levels are increased in these subjects. A novel hypothesis focuses on the potential pathogenic role of leptin in asthma exacerbations. Leptin receptors might be upregulated in the airways of patients suffering from obstructive lung diseases (47). Shore et al. found that the administration of leptin to mice induces bronchial hyperreactivity (BHR), mast cell activation, and increases IgE serum levels (48). Serum leptin levels have been found to be higher in non-obese asthmatic children compared to non-obese non-asthmatic controls (49), and in asthmatic adults compared to obese asthmatic controls (50). Leptin is also increased in OSAS: obese patients with OSAS have higher serum leptin levels than obese patients without OSAS and this might be due to the intermittent hypoxia although the exact mechanism is still unknown (51,52). This hormone could play an important role in asthma exacerbations in patients affected by the “alternative overlap syndrome”. Another relevant pathogenic factor common to both OSAS and asthma is GERD. GERD may exacerbate an underlying asthma directly via microaspirations of both acid and basic materials, gastric or duodenal, and indirectly via enhancing reflex bronchospasm. Thus, GERD interferes with achievement and maintenance of asthma control. The prevalence of GERD in OSAS patients is very high, as suggested by Valipour and Green in their studies, which estimated the presence of this comorbidity as 58% and 62% of patients with OSAS (53,54). The most important risk factor for GERD in OSAS patients has been identified as transient lower esophageal sphincter relaxations (TLESR) that occur during the arousal episodes that fragment sleep structure (55-58). Emilsson et al. conducted a longitudinal study on a general population assessing the prevalence of GERD in patients with sleep disordered breathing. They found that people with nocturnal reflux were 2 times more likely to have asthma and new onset of respiratory symptoms when compared to controls without nocturnal reflux (59). An indirect evidence of this comorbidity is the decreasing of nocturnal reflux symptoms, nocturnal asthma exacerbations, and amelioration of nighttime asthmatic symptoms during CPAP treatment (60,61). Both patients with OSAS and asthmatic patients have increased serum levels of vascular endothelial growth factor (VEGF), a hypoxia-stimulated protein that enhances neoangiogenesis, as compared to control subjects. Angiogenesis is a keystone in airway remodeling that occurs in asthma. Hoshino and colleagues found that airway remodeling and BHR were correlated with the expression of VEGF by airway cells (62,63). Moreover, studies conducted on OSAS patients highlighted that VEGF levels correlate with AHI and nocturnal hemoglobin desaturation (64,65) and that CPAP therapy has been shown to reduce nocturnal hypoxia and consequently also plasma VEGF and leptin levels (66). Nevertheless, at the present time little information about VEGF levels in asthmatic patients with OSAS is available.
Sleep complaints and sleep breathing disorders in COPD
It is well-known that sleep affects breathing by variations in airways resistance, muscular contractility, and central respiratory controls. This generally has no significant consequences for healthy subjects but might produce complications in patient with COPD who suffer from episodes of hypoxemia, especially nocturnal. In fact, in 1962 Trask and colleagues documented the worsening of hypoxemia during sleep in patients with COPD (67). Sleep effects on respiration include reduced responsiveness to cortical inputs, reduced chemoreceptors sensitivity (68,69), and respiratory muscles threshold response, in particular of accessory muscles including the intercostals which COPD patients are particularly dependent on (70,71), and increased airway resistance through nocturnal bronchoconstriction (72). Furthermore, during sleep, and in particular during REM sleep, there is a reduction of functional residual capacity (FRC), which is even more reduced in patients with COPD with subsequent mucus accumulation and worsening of ventilation/perfusion mismatch. Despite the severity of these premises, it was generally accepted that routine apposite sleep assessment was not indicated in patients with COPD with respiratory insufficiency (73). Due to the above-mentioned effect of sleep on airways patency and breathing pattern, COPD symptoms can be consistently present during the night (74). A quantitative internet-based interview of 803 COPD patients, including 289 patients with severe disease, showed that more than 30% of severe patients and more than 20% of overall population find night the most troublesome period for COPD symptoms (75). Other authors showed that overnight symptoms, assessed with the Jenkins sleep scale, are bothering respectively about 50%, 60%, 70% and 80% of mild, moderate, severe and very severe COPD patients (76). In a large observational study, the presence of nocturnal dyspnea was significantly predicted by lower FEV1, higher day-time dyspnea score (mMRC), more chronic bronchitis, more weezhing, and atrial fibrillation. Moreover night-time symptoms were strongly related with hospital admission, exacerbations, and decreased survival (77). An open question was used to assess the presence of an association of COPD with sleep breathing disorders such as OSAS. A large multivariate retrospective analysis conducted by Lacedonia and colleagues aimed at assessing the prevalence of OSAS and/or COPD in a sample of 720 patients, showed this association in almost 25% of patients (78). However, Bednarek and colleagues found OSAS approximately in 1% of patients (79). The results of several other studies are reported in Table 1.
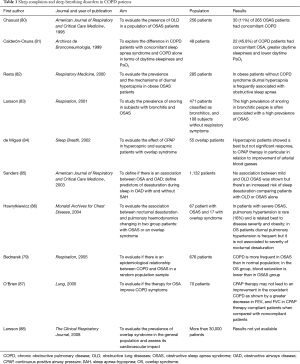
Full table
Several pathophysiological factors in COPD might predispose to OSAS. Redolfi and colleagues demonstrated that rostral fluid shift from legs into the neck in the supine position could predispose to obstructive sleep apneas (OSA), incrementing pharynx collapsibility (89). Both in COPD and asthma patients, the use of corticosteroids might predispose to OSA, particularly in COPD patients requiring long-term oral corticosteroids, inducing the accumulation of fat in parapharyngeal tissues and myopathy which might increase upper airway collapsibility (90). A keystone in the Overlap Syndrome is the presence of cardiovascular diseases; OSAS episodes of hypoxemia have been associated with increased risk of cardiovascular diseases and death due to systemic inflammation and augmented production of cytokines playing an important role in vascular and cardiac dysfunctions. Both OSAS and COPD have an underlying base of oxidative stress which, together with an increased sympathetic activation, leads to endothelial dysfunction via increased levels of NF-κB, C-reactive protein (CRP), IL-6, TNF-α and IL-8 and to the enlargement of atherosclerotic plaques. Furthermore, hypoxia induces the expression of two important transcription factors: NF-κB regulates the production of TNF-α and IL-8 which are promoters of atherosclerosis through the expression of several adhesion molecules (91); HIF-1 regulates the transcription of several genes involved in the formation of new vessels in poorly-oxygenated tissues (92). Both NF-κB and HIF-1 have been found to be elevated in patients with severe OSAS and COPD who suffer from CIH. Furthermore, circulating plasma levels of NF-κB are significantly correlated with OSAS severity (93). Similarly, CRP and IL-6 levels have been found to be increased both in OSAS and COPD patients (94-96) and contribute to atherosclerosis promoting the expression of vary adhesion molecules. According to the latest evidences, both increased levels of CRP and IL-6 are predictors of cardiovascular events (97,98). The clinical burden of these pathogenetic pathways has been assessed in the NHANES III study where a correlation between CRP levels and myocardial ischemia has been found (99). Despite this evidence, there is little proof that CPAP therapy can reduce plasma levels of these two mediators. In fact, a randomized controlled trial conducted by Kohler and colleagues didn’t find any differences in their plasmatic levels after a 4-week CPAP-therapy period (100). Viceversa, NF-κB, a mediator involved in cardiovascular inflammatory damage-related chronic exposure to intermittent hypoxia and reoxygenation (101), was significantly reduced by CPAP therapy (93).
Conclusions
Rhinitis, asthma and COPD are causes of sleep complaints that may be limited with optimization of their management. Furthermore, they represent a risk factor for both pathogenesis and worsening of sleep breathing disorders such as OSAS. Moreover, if OSAS treatment by CPAP permits a better control of obstructive airways diseases, the optimization of airways patency is necessary to facilitate nocturnal obstruction control. Nowadays we cannot manage sleep disordered breathing like a single pathology. It is necessary to study the patient’s overall condition. Therefore, in order to further improve SDB and OLD responses to their appropriate therapies, a holistic approach to the patient should be taken. In particular, it’s advisable for physicians to evaluate the presence of OSAS in patients with difficult to control asthma and vice versa to check for rhinitis, asthma and COPD in patients with OSAS presenting with daytime and seasonal symptoms, keeping in mind the importance of the relationships with disease severity and the undeniable value of making the diagnosis.
Acknowledgements
The authors thank Associazione Ricerca Malattie Immunologiche ed Allergiche (ARMIA), Associazione Pazienti Disturbi Respiratori nel Sonno (ASPADIRES) for the support in developing the study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Allergic Rhinitis and its Impact on Asthma. 2008. Available online: http://www.whiar.org
- Global Initiative for Asthma. 2008. Available online: www.ginasthma.com
- Global Initiative for Chronic Obstructive Lung Disease. 2015. Available online: http://www.goldcopd.com
- Randerath WJ, Sanner BM, Somers VK. editors. Sleep apnea: current diagnosis and treatment. vol.35. Karger, Basel: Progress in respiratory research, 2006:244.
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008;5:136-43. [Crossref] [PubMed]
- Ioachimescu OC, Teodorescu M. Integrating the overlap of obstructive lung disease and obstructive sleep apnoea: OLDOSA syndrome. Respirology 2013;18:421-31. [Crossref] [PubMed]
- Sears MR. Adverse effects of beta-agonists. J Allergy Clin Immunol 2002;110:S322-8. [Crossref] [PubMed]
- Scadding GK, Punekar S. EAACI Abstract Book. 2006.211. Abstract 741.
- Léger D, Annesi-Maesano I, Carat F, et al. Allergic rhinitis and its consequences on quality of sleep: An unexplored area. Arch Intern Med 2006;166:1744-8. [Crossref] [PubMed]
- Bousquet J, Neukirch F, Bousquet PJ, et al. Severity and impairment of allergic rhinitis in patients consulting in primary care. J Allergy Clin Immunol 2006;117:158-62. [Crossref] [PubMed]
- Bousquet J, Annesi-Maesano I, Carat F, et al. Characteristics of intermittent and persistent allergic rhinitis: DREAMS study group. Clin Exp Allergy 2005;35:728-32. [Crossref] [PubMed]
- Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest 2012;141:1601-10. [Crossref] [PubMed]
- Marshall PS, O'Hara C, Steinberg P. Effects of seasonal allergic rhinitis on selected cognitive abilities. Ann Allergy Asthma Immunol 2000;84:403-10. [Crossref] [PubMed]
- Kremer B, den Hartog HM, Jolles J. Relationship between allergic rhinitis, disturbed cognitive functions and psychological well-being. Clin Exp Allergy 2002;32:1310-5. [Crossref] [PubMed]
- Wilken JA, Berkowitz R, Kane R. Decrements in vigilance and cognitive functioning associated with ragweed-induced allergic rhinitis. Ann Allergy Asthma Immunol 2002;89:372-80. [Crossref] [PubMed]
- Georgalas C. The role of the nose in snoring and obstructive sleep apnoea: an update. Eur Arch Otorhinolaryngol 2011;268:1365-73. [Crossref] [PubMed]
- Young T, Finn L, Kim H. Nasal obstruction as a risk factor for sleep-disordered breathing. The University of Wisconsin Sleep and Respiratory Research Group. J Allergy Clin Immunol 1997;99:S757-62. [Crossref] [PubMed]
- Shedden A. Impact of nasal congestion on quality of life and work productivity in allergic rhinitis: findings from a large online survey. Treat Respir Med 2005;4:439-46. [Crossref] [PubMed]
- Young T, Finn L, Palta M. Chronic nasal congestion at night is a risk factor for snoring in a population-based cohort study. Arch Intern Med 2001;161:1514-9. [Crossref] [PubMed]
- McNicholas WT, Tarlo S, Cole P, et al. Obstructive apneas during sleep in patients with seasonal allergic rhinitis. Am Rev Respir Dis 1982;126:625-8. [PubMed]
- Houser SM, Mamikoglu B, Aquino BF, et al. Acoustic rhinometry findings in patients with mild sleep apnea. Otolaryngol Head Neck Surg 2002;126:475-80. [Crossref] [PubMed]
- Sabato R, Guido P, Salerno FG, et al. Airway inflammation in patients affected by obstructive sleep apnea. Monaldi Arch Chest Dis 2006;65:102-5. [PubMed]
- Paulsen FP, Steven P, Tsokos M, et al. Upper airway epithelial structural changes in obstructive sleep-disordered breathing. Am J Respir Crit Care Med 2002;166:501-9. [Crossref] [PubMed]
- Hughes K, Glass C, Ripchinski M, et al. Efficacy of the topical nasal steroid budesonide on improving sleep and daytime somnolence in patients with perennial allergic rhinitis. Allergy 2003;58:380-5. [Crossref] [PubMed]
- Kiely JL, Nolan P, McNicholas WT. Intranasal corticosteroid therapy for obstructive sleep apnoea in patients with co-existing rhinitis. Thorax 2004;59:50-5. [PubMed]
- Craig TJ, Mende C, Hughes K, et al. The effect of topical nasal fluticasone on objective sleep testing and the symptoms of rhinitis, sleep, and daytime somnolence in perennial allergic rhinitis. Allergy Asthma Proc 2003;24:53-8. [PubMed]
- Craig TJ, Teets S, Lehman EB, et al. Nasal congestion secondary to allergic rhinitis as a cause of sleep disturbance and daytime fatigue and the response to topical nasal corticosteroids. J Allergy Clin Immunol 1998;101:633-7. [Crossref] [PubMed]
- Baiardini I, Villa E, Rogkakou A, et al. Effects of mometasone furoate on the quality of life: a randomized placebo-controlled trial in persistent allergic rhinitis and intermittent asthma using the Rhinasthma questionnaire. Clin Exp Allergy 2011;41:417-23. [Crossref] [PubMed]
- Strobel W, Schlageter M, Andersson M, et al. Topical nasal steroid treatment does not improve CPAP compliance in unselected patients with OSAS. Respir Med 2011;105:310-5. [Crossref] [PubMed]
- Ballard RD, Gay PC, Strollo PJ. Interventions to improve compliance in sleep apnea patients previously non-compliant with continuous positive airway pressure. J Clin Sleep Med 2007;3:706-12. [PubMed]
- Catterall JR, Rhind GB, Stewart IC, et al. Effect of sleep deprivation on overnight bronchoconstriction in nocturnal asthma. Thorax 1986;41:676-80. [Crossref] [PubMed]
- Fitzpatrick MF, Engleman H, Whyte KF, et al. Morbidity in nocturnal asthma: sleep quality and daytime cognitive performance. Thorax 1991;46:569-73. [Crossref] [PubMed]
- Hudgel DW, Shucard DW. Coexistence of sleep apnea and asthma resulting in severe sleep hypoxemia. JAMA 1979;242:2789-90. [Crossref] [PubMed]
- Gutierrez MJ, Zhu J, Rodriguez-Martinez CE, et al. Nocturnal phenotypical features of obstructive sleep apnea (OSA) in asthmatic children. Pediatr Pulmonol 2013;48:592-600. [Crossref] [PubMed]
- Salles C, Terse-Ramos R, Souza-Machado A, et al. Obstructive sleep apnea and asthma. J Bras Pneumol 2013;39:604-12. [Crossref] [PubMed]
- Teodorescu M, Polomis DA, Hall SV, et al. Association of obstructive sleep apnea risk with asthma control in adults. Chest 2010;138:543-50. [Crossref] [PubMed]
- Teodorescu M, Consens FB, Bria WF, et al. Predictors of habitual snoring and obstructive sleep apnea risk in patients with asthma. Chest 2009;135:1125-32. [Crossref] [PubMed]
- Guilleminault C, Quera-Salva MA, Powell N, et al. Nocturnal asthma: snoring, small pharynx and nasal CPAP. Eur Respir J 1988;1:902-7. [PubMed]
- Wysocki M, Antonelli M. Noninvasive mechanical ventilation in acute hypoxaemic respiratory failure. Eur Respir J 2001;18:209-20. [Crossref] [PubMed]
- Martin JG, Shore SA, Engel LA. Mechanical load and inspiratory muscle action during induced asthma. Am Rev Respir Dis 1983;128:455-60. [Crossref] [PubMed]
- Alkhalil M, Schulman E, Getsy J. Obstructive sleep apnea syndrome and asthma: what are the links? J Clin Sleep Med 2009;5:71-8. [PubMed]
- Julien JY, Martin JG, Ernst P, et al. Prevalence of obstructive sleep apnea-hypopnea in severe versus moderate asthma. J Allergy Clin Immunol 2009;124:371-6. [Crossref] [PubMed]
- Braido F, Baiardini I, Ghiglione V. Sleep disturbances and asthma control: a real life study. Asian Pac J Allergy Immunol 2009;27:27-33. [PubMed]
- ten Brinke A, Sterk PJ, Masclee AA, et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J 2005;26:812-8. [Crossref] [PubMed]
- National Heart, Lung, and Blood Institute. Bethesda: National Institutes of Health. 2013. Available online: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf
- Basner M, Griefahn B, Berg Mv. Aircraft noise effects on sleep: mechanisms, mitigation and research needs. Noise Health 2010;12:95-109. [Crossref] [PubMed]
- Bruno A, Chanez P, Chiappara G, et al. Does leptin play a cytokine-like role within the airways of COPD patients? Eur Respir J 2005;26:398-405. [Crossref] [PubMed]
- Shore SA, Schwartzman IN, Mellema MS, et al. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol 2005;115:103-9. [Crossref] [PubMed]
- Guler N, Kirerleri E, Ones U, et al. Leptin: does it have any role in childhood asthma? J Allergy Clin Immunol 2004;114:254-9. [Crossref] [PubMed]
- Holguin F, Rojas M, Brown LA, et al. Airway and plasma leptin and adiponectin in lean and obese asthmatics and controls. J Asthma 2011;48:217-23. [Crossref] [PubMed]
- Phillips BG, Kato M, Narkiewicz K, et al. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol 2000;279:H234-7. [PubMed]
- Tatsumi K, Kasahara Y, Kurosu K, et al. Sleep oxygen desaturation and circulating leptin in obstructive sleep apnea-hypopnea syndrome. Chest 2005;127:716-21. [Crossref] [PubMed]
- Valipour A, Makker HK, Hardy R, et al. Symptomatic gastroesophageal reflux in subjects with a breathing sleep disorder. Chest 2002;121:1748-53. [Crossref] [PubMed]
- Green BT, Broughton WA, O'Connor JB. Marked improvement in nocturnal gastroesophageal reflux in a large cohort of patients with obstructive sleep apnea treated with continuous positive airway pressure. Arch Intern Med 2003;163:41-5. [Crossref] [PubMed]
- Berg S, Hoffstein V, Gislason T. Acidification of distal esophagus and sleep-related breathing disturbances. Chest 2004;125:2101-6. [Crossref] [PubMed]
- Kuribayashi S, Kusano M, Kawamura O, et al. Mechanism of gastroesophageal reflux in patients with obstructive sleep apnea syndrome. Neurogastroenterol Motil 2010;22:611-e172. [Crossref] [PubMed]
- Kuribayashi S, Massey BT, Hafeezullah M, et al. Upper esophageal sphincter and gastroesophageal junction pressure changes act to prevent gastroesophageal and esophagopharyngeal reflux during apneic episodes in patients with obstructive sleep apnea. Chest 2010;137:769-76. [Crossref] [PubMed]
- Shepherd K, Hillman D, Holloway R, et al. Mechanisms of nocturnal gastroesophageal reflux events in obstructive sleep apnea. Sleep Breath 2011;15:561-70. [Crossref] [PubMed]
- Emilsson ÖI, Bengtsson A, Franklin KA, et al. Nocturnal gastro-oesophageal reflux, asthma and symptoms of OSA: a longitudinal, general population study. Eur Respir J 2013;41:1347-54. [Crossref] [PubMed]
- Kerr P, Shoenut JP, Steens RD, et al. Nasal continuous positive airway pressure. A new treatment for nocturnal gastroesophageal reflux? J Clin Gastroenterol 1993;17:276-80. [Crossref] [PubMed]
- Shepherd KL, James AL, Musk AW, et al. Gastro-oesophageal reflux symptoms are related to the presence and severity of obstructive sleep apnoea. J Sleep Res 2011;20:241-9. [Crossref] [PubMed]
- Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol 2001;107:295-301. [Crossref] [PubMed]
- Simcock DE, Kanabar V, Clarke GW, et al. Proangiogenic activity in bronchoalveolar lavage fluid from patients with asthma. Am J Respir Crit Care Med 2007;176:146-53. [Crossref] [PubMed]
- Schulz R, Hummel C, Heinemann S, et al. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Respir Crit Care Med 2002;165:67-70. [Crossref] [PubMed]
- Imagawa S, Yamaguchi Y, Higuchi M, et al. Levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea--hypopnea syndrome. Blood 2001;98:1255-7. [Crossref] [PubMed]
- Sanner BM, Kollhosser P, Buechner N, et al. Influence of treatment on leptin levels in patients with obstructive sleep apnoea. Eur Respir J 2004;23:601-4. [Crossref] [PubMed]
- Trask CH, Cree EM. Oximeter studies on patients with chronic obstructive emphysema, awake and during sleep. N Engl J Med 1962;266:639-42. [Crossref] [PubMed]
- Phillipson EA. Control of breathing during sleep. Am Rev Respir Dis 1978;118:909-39. [PubMed]
- Gothe B, Altose MD, Goldman MD, et al. Effect of quiet sleep on resting and CO2-stimulated breathing in humans. J Appl Physiol Respir Environ Exerc Physiol 1981;50:724-30. [PubMed]
- Tusiewicz K, Moldofsky H, Bryan AC, et al. Mechanics of the rib cage and diaphragm during sleep. J Appl Physiol Respir Environ Exerc Physiol 1977;43:600-2. [PubMed]
- Johnson MW, Remmers JE. Accessory muscle activity during sleep in chronic obstructive pulmonary disease. J Appl Physiol Respir Environ Exerc Physiol 1984;57:1011-7. [PubMed]
- Hetzel MR, Clark TJ. Comparison of normal and asthmatic circadian rhythms in peak expiratory flow rate. Thorax 1980;35:732-8. [Crossref] [PubMed]
- Connaughton JJ, Catterall JR, Elton RA, et al. Do sleep studies contribute to the management of patients with severe chronic obstructive pulmonary disease? Am Rev Respir Dis 1988;138:341-4. [Crossref] [PubMed]
- Miravitlles M, Worth H, Soler Cataluña JJ, et al. Observational study to characterise 24-hour COPD symptoms and their relationship with patient-reported outcomes: results from the ASSESS study. Respir Res 2014;15:122. [Crossref] [PubMed]
- Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Curr Med Res Opin 2009;25:2043-8. [Crossref] [PubMed]
- Agusti A, Hedner J, Marin JM, et al. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev 2011;20:183-94. [Crossref] [PubMed]
- Lange P, Marott JL, Vestbo J, et al. Prevalence of night-time dyspnoea in COPD and its implications for prognosis. Eur Respir J 2014;43:1590-8. [Crossref] [PubMed]
- Lacedonia D, Carpagnano GE, Aliani M, et al. Daytime PaO2 in OSAS, COPD and the combination of the two (overlap syndrome). Respir Med 2013;107:310-6. [Crossref] [PubMed]
- Bednarek M, Plywaczewski R, Jonczak L, et al. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population study. Respiration 2005;72:142-9. [Crossref] [PubMed]
- Chaouat A, Weitzenblum E, Krieger J, et al. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med 1995;151:82-6. [Crossref] [PubMed]
- Calderón-Osuna E, Carmona Bernal C, Arenas Gordillo M, et al. A comparative study of patients with chronic obstructive pulmonary disease with and without obstructive sleep apnea syndrome. Arch Bronconeumol 1999;35:539-43. [PubMed]
- Resta O, Foschino-Barbaro MP, Bonfitto P, et al. Prevalence and mechanisms of diurnal hypercapnia in a sample of morbidly obese subjects with obstructive sleep apnoea. Respir Med 2000;94:240-6. [Crossref] [PubMed]
- Larsson LG, Lindberg A, Franklin KA, et al. Obstructive sleep apnoea syndrome is common in subjects with chronic bronchitis. Report from the Obstructive Lung Disease in Northern Sweden studies. Respiration 2001;68:250-5. [Crossref] [PubMed]
- de Miguel J, Cabello J, Sánchez-Alarcos JM, et al. Long-term effects of treatment with nasal continuous positive airway pressure on lung function in patients with overlap syndrome. Sleep Breath 2002;6:3-10. [Crossref] [PubMed]
- Sanders MH, Newman AB, Haggerty CL, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med 2003;167:7-14. [Crossref] [PubMed]
- Hawryłkiewicz I, Sliwiński P, Górecka D, et al. Pulmonary haemodynamics in patients with OSAS or an overlap syndrome. Monaldi Arch Chest Dis 2004;61:148-52. [Crossref] [PubMed]
- O'Brien A, Whitman K. Lack of benefit of continuous positive airway pressure on lung function in patients with overlap syndrome. Lung 2005;183:389-404. [Crossref] [PubMed]
- Larsson LG, Lindberg A. Concomitant obstructive sleep apnea and chronic obstructive pulmonary disease: study design--the OLIN OSAS-COPD study. Clin Respir J 2008;2 Suppl 1:120-2. [Crossref] [PubMed]
- Redolfi S, Yumino D, Ruttanaumpawan P, et al. Relationship between overnight rostral fluid shift and Obstructive Sleep Apnea in nonobese men. Am J Respir Crit Care Med 2009;179:241-6. [Crossref] [PubMed]
- Yigla M, Tov N, Solomonov A, et al. Difficult-to-control asthma and obstructive sleep apnea. J Asthma 2003;40:865-71. [Crossref] [PubMed]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685-95. [Crossref] [PubMed]
- Garvey JF, Taylor CT, McNicholas WT. Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur Respir J 2009;33:1195-205. [Crossref] [PubMed]
- Htoo AK, Greenberg H, Tongia S, et al. Activation of nuclear factor kappaB in obstructive sleep apnea: a pathway leading to systemic inflammation. Sleep Breath 2006;10:43-50. [Crossref] [PubMed]
- Yao M, Tachibana N, Okura M, et al. The relationship between sleep-disordered breathing and high-sensitivity C-reactive protein in Japanese men. Sleep 2006;29:661-5. [PubMed]
- Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 2003;107:1129-34. [Crossref] [PubMed]
- Gan WQ, Man SF, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004;59:574-80. [Crossref] [PubMed]
- Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336:973-9. [Crossref] [PubMed]
- Luc G, Bard JM, Juhan-Vague I, et al. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME Study. Arterioscler Thromb Vasc Biol 2003;23:1255-61. [Crossref] [PubMed]
- Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003;107:1514-9. [Crossref] [PubMed]
- Kohler M, Ayers L, Pepperell JC, et al. Effects of continuous positive airway pressure on systemic inflammation in patients with moderate to severe obstructive sleep apnoea: a randomised controlled trial. Thorax 2009;64:67-73. [Crossref] [PubMed]
- Greenberg H, Ye X, Wilson D, et al. Chronic intermittent hypoxia activates nuclear factor-kappaB in cardiovascular tissues in vivo. Biochem Biophys Res Commun 2006;343:591-6. [Crossref] [PubMed]


