
Recommendations for broadening eligibility criteria in esophagus cancer clinical trials: the mortality disparity of esophagus cancer as a first or second primary malignancy
Highlight box
Key findings
• Esophagus-2 patients have more favorable esophagus cancer-specific survival during the initial 5 years following diagnosis while non-cancer related mortality remains consistently higher. Consequently, short-term overall survival is actually comparable.
What is known and what is new?
• The presence of a prior malignancy preceding the diagnosis may wield a substantial impact on the prognosis of the disease. This potential influence on survival, compounded by other considerations, has precipitated the exclusion of individuals with a history of prior malignancies from the majority of clinical trials related to esophageal cancer. Nevertheless, the absence of studies estimating the impact of this factor on survival means that these trials may be influenced in terms of generalizability and accrual.
• This study is the first comprehensive investigation into the prognosis of esophagus-2, revealing a distinct and time-dependent disease trajectory of different causes of mortalities.
What is the implication, and what should change now?
• The disease course of esophagus-2 is not inherently more aggressive than that of esophagus-1. Therefore, there is no sufficient reason opting for conservative care solely based on a history of first malignancy. Esophagus cancer patients with a history of prior malignancies should not be uniformly excluded from clinical trials. The compromised non-cancer related survival observed in esopahgus-2 patients in the long term emphasizes the importance of active surveillance, prevention and management of the comorbidities and complications to optimize overall prognosis.
Introduction
Esophageal cancer (EC) ranks as the seventh most prevalent cancer globally and stands as the sixth leading cause of cancer-related mortality worldwide, with around 544,000 reported deaths in 2020 (1). For all stages of EC combined, the 5-year survival rate hovers around 20%. In the United States, EC ranks 14th in terms of common cancers, with an anticipated 21,560 new cases and 16,120 deaths in 2023. Men have a higher risk of developing EC compared to women (2). Treatment approaches for EC, which incorporate immunotherapy as a standard option for both early and advanced stages, have witnessed significant advancements (3). The two most prevalent histologic subtypes of EC are adenocarcinoma and squamous cell carcinoma.
The population of cancer survivors in the US continues to increase (4). A study based on Surveillance, Epidemiology, and End Results (SEER) database reported that 13.1% of adults newly diagnosed with EC in 2019 survived a previous cancer (5). The presence of a prior malignancy preceding the diagnosis may wield a substantial impact on the disease’s prognosis. This potential influence on survival, compounded by other considerations, has precipitated the exclusion of individuals with a history of prior malignancies from the majority of clinical trials related to EC (6,7). Two-thirds of trials supported by National Cancer Institute from 2018 to 2020 exclude patients with prior or concurrent cancers (8). Nevertheless, the absence of studies estimating the impact of prior cancers on survival means that these trials may be influenced in terms of clinical trial generalizability and accrual (9,10). Understanding the nature and impact of prior cancer is critical to improving equity in cancer care, disease outcomes, and patient experience.
Pan and Saad have respectively investigated the survival outcomes of stage I–III (N0M0) and stage IV esophagus carcinoma with a prior cancer (11,12). However, the hazard ratio (HR) for mortality of esophagus cancer as a second primary malignancy (esophagus-2) relative to patients developing first primary esophagus cancer (esophagus-1) may not be constant, and the markedly different incidence rates of non-cancer caused death between them have not been revealed. The subgroup with different characteristics of the first malignancy may exhibit divergent mortality risk. Hence, we took advantage of the well-established SEER database to investigate the time-varying hazards of esophagus cancer-specific, non-cancer related and overall mortality among patients with esophagus-2 versus esophagus-1, and whether characteristics of the first malignancy could potentially modify such risks. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1881/rc).
Methods
Study population
We built a population-based cohort composed of patients diagnosed as primary esophagus cancer between January 1, 1975 and December 31, 2019 in the United States, utilizing the National Cancer Institute’s SEER program. The SEER database, covering about 34.6% of the U.S. population, provides data on cancer incidence rates, patient characteristics, treatment modalities, and long-term follow-up (https://seer.cancer.gov/about/overview.html). We identified 89,887 primary esophagus cancer patients proved pathologically. The exclusion criteria were as follows: younger than 20 years old, without birth year, with no accurate follow-up, diagnosed from autopsy, with esophagus cancer as the third or subsequent primary malignancy. The research was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Ascertainment of first and second primary esophagus cancer
The SEER database maintains stringent criteria for delineating multiple primary neoplasms. Due to the prerequisite of a pathological diagnosis for inclusion, it is not probable that esophagus-2 cases in SEER database represent metastases from the initial primary cancer, unless the prior cancer itself is esophagus cancer. SEER guidelines dictate that cases of uncertainty are considered single primaries unless substantiated as new primary malignancies (13).
Out of the remaining 89,887 patients, 15,093 had a history of prior malignancies. Due to challenges in distinguishing esophagus-2 from esophagus-1 metastases and the complexity of ascertaining the cause of mortality, 273 patients with a previous esophagus cancer diagnosis were also excluded. Thus, esophagus-2 refers to primary esophagus cancer as a second malignancy after a previous non-esophagus malignancy. Ultimately, we identified 89,341 patients diagnosed as primary esophagus cancer, including 74,521 esophagus-1 and 14,820 esophagus-2. By connecting the prior malignancy records in SEER database, we identified patients with information on prior cancer (N=8,273) which were analyzed in subgroups by characteristics of the first malignancy.
Ascertainment of mortality
The primary outcome of interest was esophagus cancer-specific mortality, with non-cancer related and overall mortality considered as secondary outcomes. By direct patient contact or connecting with registries through healthcare institutions, we followed patients from cancer diagnosis until December 31, 2020 or death, whichever came first. To identify a single, disease-specific cause, we derived the cause of mortality from death certificate with algorithms that considers factors such as site of the cancer diagnosis, tumor sequence, and comorbidities (14).
Statistical analysis
First, we analyzed tumor and clinical characteristics between patients with esophagus-1 and esophagus-2 utilizing logistic regression model. Demographic features were covariate-controlled in the estimation of tumor characteristics, and tumor characteristics were additionally adjusted for in subgroup analysis of therapeutic regimens.
We depicted esophagus cancer-specific, non-cancer related and overall mortality from diagnosis to a decade thereafter for esophagus-1 and esophagus-2 respectively. As the proportional hazards hypothesis was rejected by our previous demonstration, we abandoned the Cox regression model. To enable HRs to vary over time, a flexible parametric survival model was utilized to derive the HRs and 95% confidence intervals (CIs) of mortality risk (15). To avoid the bias of misjudging deaths caused by the first primary malignancy attributed to esophagus-2 or vice versa, we further estimated the correlation with any cancer-specific mortality.
Given that variables may act as potential covariates to mediate the effect on mortality (e.g., esophagus-2 patients are more likely to be diagnosed at localized stage and consequently with better prognosis), the previous mentioned analyses were controlled for demographic features in model A and additionally adjusted for clinical and tumor characteristics in model B. Because we attempted to emphasize the correlations independent of clinical and tumor features, only model B was applied in the following analyses.
Considering the heterogeneous HRs over time, we separately conducted Cox proportional hazards models within different periods (0 to <1 year, 1 to <5 years, and 5 to 10 years after diagnosis). To address the concern of competing risk (e.g., other cancer) that may prevent individuals from dying from the cause of death we focused on, the HRs of esophagus cancer-specific mortality and non-cancer related mortality were estimated using Fine-Gray competing risk model. This approach allowed us to consider and adjust for the potential influence of competing risks on the observed outcomes (16).
We established flexible parametric survival model in STATA 17.0 (StataCorp LLC) while the other analyses were performed in R (version 3.6.3; The R Foundation). The significant level was set at P<0.05.
Results
Patients and clinical characteristics
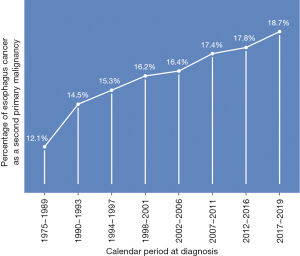
A total of 74,521 patients had esophagus-1, while 14,820 patients had esophagus-2. The median follow-up period was 0.67 years, ranging from 0 to 39.58 years. Esophagus-2 accounted for 12.1% of new cases between 1975 and 1989, and this proportion increased to 18.7% between 2017 and 2019 (Figure 1).
Compared to patients with esophagus-1, those with esophagus-2 were older and with more recent diagnoses (Table S1). They were more prone to be diagnosed at early stage, with squamous cell carcinoma, with tumors located in the upper and middle thirds of esophagus, and with well-to-moderately differentiated grade (Table S2). Conversely, patients with esophagus-2 were less likely to undergo surgery, radiation or chemotherapy, among whom the median duration from the prior cancer to esophagus-2 diagnosis was 3 years. The prostate, colon and rectum, and lung and bronchus were the most common sites of the first cancer (Figure 2).

Mortality risk of esophagus-2 relative to esophagus-1
Out of the total number of patients, 64,455 (86.5%) with esophagus-1 and 13,029 (87.9%) with esophagus-2 died during the follow-up period. Comparing the two groups, patients with esophagus-2 exhibited a lower cumulative mortality rate specifically attributed to esophagus cancer. However, they had a higher cumulative mortality rate associated with non-cancer causes from the time of diagnosis up to 10 years thereafter (Figure 3). Furthermore, the cumulative all-cause mortality rate was higher in esophagus-2 patients starting from one year after diagnosis and onwards. However, no notable distinction was observed between the two groups within the first year following diagnosis.

In the first 3 years after diagnosis, patients with esophagus-2 had a similar risk of overall mortality compared to those with esophagus-1 when controlling for tumor and clinical features (Figure 4). Yet, the HR of overall death increased thereafter, up to 10 years after esophagus cancer diagnosis. As for esophagus cancer-specific death, patients with esophagus-2 had a reduced risk until 6 years after diagnosis, but the risk became similar to that of esophagus-1 patients from 6 years after diagnosis onwards. It is important to note that even when ascribing cases dying of other cancers to esophagus cancer, patients with esophagus-2 still had a decreased HR of any cancer-specific mortality in the initial 5 years after diagnosis (Figure S1). On the other hand, esophagus-2 patients had a higher risk of non-cancer related mortality throughout the follow-up period. To provide practical estimates, HRs over time were calculated by dividing the follow-up period into three intervals (Table 1). These HRs strongly support the temporal pattern observed in Figure 4.

Table 1
| Variables | N (%) | From 0 to <1 year after diagnosis | From 1 to <5 years after diagnosis | From 5 to 10 years after diagnosis | |||||
|---|---|---|---|---|---|---|---|---|---|
| N (IR) | HR (95% CI)a | N (IR) | HR (95% CI)a | N (IR) | HR (95% CI)a | ||||
| Overall mortality | |||||||||
| First primary esophagus cancer | 74,521 | 41,690 (90.7) | 1.000 | 18,870 (21.9) | 1.000 | 2,520 (3.9) | 1.000 | ||
| Second primary esophagus cancer | 14,820 | 8,442 (94.0) | 0.978 (0.955–1.002) | 3,807 (23.8) | 1.006 (0.971–1.042) | 568 (5.6) | 1.131 (1.030–1.243) | ||
| Esophagus cancer-specific mortality | |||||||||
| First primary esophagus cancer | 74,521 | 34,008 (74.0) | 1.000 | 14,524 (16.9) | 1.000 | 928 (1.4) | 1.000 | ||
| Second primary esophagus cancer | 14,820 | 6,018 (67.0) | 0.847 (0.824–0.871) | 2,500 (15.6) | 0.878 (0.841–0.917) | 151 (1.5) | 0.893 (0.748–1.065) | ||
| Non-cancer related mortality | |||||||||
| First primary esophagus cancer | 74,521 | 4,295 (9.3) | 1.000 | 2,866 (3.3) | 1.000 | 1351 (2.1) | 1.000 | ||
| Second primary esophagus cancer | 14,820 | 1,804 (20.1) | 1.876 (1.773–1.984) | 1,006 (6.3) | 1.523 (1.414–1.639) | 372 (3.7) | 1.284 (1.140–1.446) | ||
a, HRs were adjusted for age and calendar period at diagnosis, sex, race, marital status, median household income in the county of residence, rural-urban continuum, tumor stage, histology, tumor grade, surgery, radiation therapy, and chemotherapy. CI, confidence interval; HR, hazard ratio; IR, incidence rate per 100 person-years; N, number of deaths.
The correlation with esophagus cancer-specific or non-cancer related mortality was reaffirmed in consideration of competing risks, such as death from cancers except esophagus and non-cancer causes, as demonstrated in Table S3. This analysis accounted for the potential influence of competing risks and further supported the findings regarding the mortality risk of esophagus-2 patients.
Factors of first malignancy that modified the risk of mortality
Regarding esophagus cancer-specific mortality, a consistent temporal pattern was observed among all esophagus-2 patients, except for those with a history of skin cancers, who exhibited similar mortality rates to esophagus-2 patients throughout the follow-up period (Table 2). Among esophagus-2 patients, those with a distant stage as their first malignancy experienced a greater reduction in esophagus cancer-specific death during the initial 5 years, especially within the first year after diagnosis. The earlier the diagnosis of esophagus-2 occurred following the first malignancy, the lower the esophagus cancer-specific mortality.
Table 2
| Variables | N (%) | From 0 to <1 year after diagnosis | From 1 to <5 years after diagnosis | From 5 to 10 years after diagnosis | |||||
|---|---|---|---|---|---|---|---|---|---|
| N (IR) | HR (95% CI)a | N (IR) | HR (95% CI)a | N (IR) | HR (95% CI)a | ||||
| By sites of first malignancy | |||||||||
| Prostate | 2,216 (26.8) | 867 (61.7) | 0.905 (0.845–0.969) | 400 (15.0) | 0.974 (0.881–1.077) | 20 (1.2) | 0.713 (0.455–1.119) | ||
| Colon and rectum | 802 (9.7) | 276 (54.7) | 0.787 (0.699–0.886) | 133 (13.4) | 0.845 (0.712–1.002) | 5 (0.8) | 0.578 (0.239–1.396) | ||
| Lung and bronchus | 774 (9.4) | 277 (69.2) | 0.611 (0.543–0.688) | 68 (13.1) | 0.676 (0.532–0.858) | 3 (1.3) | 1.212 (0.388–3.789) | ||
| Breast | 531 (6.4) | 206 (62.6) | 0.831 (0.723–0.954) | 88 (13.6) | 0.831 (0.672–1.028) | 5 (1.2) | 0.727 (0.300–1.761) | ||
| Skin | 450 (5.4) | 184 (65.2) | 1.016 (0.878–1.175) | 76 (14.7) | 0.971 (0.774–1.218) | 5 (1.7) | 1.084 (0.448–2.626) | ||
| Others | 3,500 (42.3) | 1,290 (60.8) | 0.758 (0.717–0.801) | 521 (14.9) | 0.880 (0.806–0.961) | 31 (1.6) | 0.935 (0.651–1.341) | ||
| By tumor stage of first malignancy | |||||||||
| Localized | 4,117 (50.0) | 1,547 (73.9) | 0.858 (0.815–0.904) | 689 (15.6) | 0.890 (0.824–0.962) | 30 (1.0) | 0.671 (0.465–0.970) | ||
| Regional | 2,375 (28.7) | 879 (46.0) | 0.747 (0.699–0.799) | 390 (13.1) | 0.933 (0.843–1.032) | 24 (1.5) | 0.934 (0.620–1.408) | ||
| Distant | 947 (11.4) | 320 (77.8) | 0.671 (0.601–0.749) | 87 (14.9) | 0.709 (0.574–0.876) | 2 (0.9) | 0.354 (0.088–1.423) | ||
| Unstaged | 834 (10.1) | 354 (56.4) | 0.805 (0.724–0.894) | 120 (14.0) | 0.902 (0.753–1.081) | 13 (2.6) | 0.972 (0.735–1.286) | ||
| By time elapsed from first malignancy (years) | |||||||||
| 0 to <2 | 2,664 (32.2) | 903 (58.4) | 0.662 (0.619–0.707) | 367 (14.0) | 0.869 (0.783–0.965) | 25 (1.5) | 0.824 (0.551–1.230) | ||
| 2 to <5 | 2,398 (29.0) | 917 (60.4) | 0.829 (0.776–0.885) | 425 (15.2) | 0.903 (0.819–0.995) | 20 (1.1) | 0.705 (0.451–1.102) | ||
| 5 to <10 | 2,167 (26.2) | 873 (64.0) | 0.890 (0.832–0.952) | 367 (14.6) | 0.897 (0.807–0.996) | 20 (1.3) | 0.902 (0.576–1.413) | ||
| ≥10 | 1,044 (12.6) | 407 (66.5) | 0.921 (0.834–1.017) | 127 (13.9) | 0.875 (0.733–1.043) | 4 (1.4) | 1.227 (0.454–3.315) | ||
| By radiation therapy of first malignancy | |||||||||
| Yes | 2,907 (35.1) | 1,049 (58.4) | 0.869 (0.783–0.965) | 475 (16.3) | 0.886 (0.808–0.972) | 27 (1.7) | 0.927 (0.631–1.361) | ||
| No/unknown | 5,366 (64.9) | 2,051 (63.2) | 0.829 (0.792–0.867) | 811 (13.7) | 0.767 (0.714–0.823) | 42 (1.1) | 0.650 (0.476–0.886) | ||
| By chemotherapy of first malignancy | |||||||||
| Yes | 1,739 (21.0) | 548 (50.9) | 0.622 (0.572–0.677) | 237 (14.2) | 0.729 (0.641–0.829) | 12 (1.6) | 0.914 (0.516–1.618) | ||
| No/unknown | 6,534 (79.0) | 2,552 (64.3) | 0.829 (0.796–0.863) | 1,049 (14.6) | 0.827 (0.776–0.881) | 57 (1.3) | 0.695 (0.532–0.910) | ||
a, HRs were adjusted for were adjusted for age and calendar period at diagnosis, sex, race, cohabitation status, median household income in the county of residence, rural-urban continuum, tumor stage, histology, tumor grade, surgery, radiation therapy, and chemotherapy. CI, confidence interval; HR, hazard ratio; IR, incidence rate per 100 person-years; N, number of deaths.
Concerning overall mortality, a similar temporal pattern was observed among all esophagus-2 patients, except for the 5 years afterwards, which may be attributed to the small sample size and limited statistical power during that timeframe (Table 3). Distant stage of the prior cancer was correlated with worse overall mortality during the first 5 years but superior overall mortality thereafter. In contrast, esophagus-2 patients with a less advanced stage of the first malignancy exhibited superior overall mortality during the first 5 years but poorer overall mortality thereafter. Additionally, the time elapsed since the first primary malignancy diagnosis also clearly influenced the association with overall mortality. For patients diagnosed within 2 years of their first malignancy, mortality worsened starting from 1 year, but not 5 years, after being diagnosed as esophagus-2.
Table 3
| Variables | N (%) | From 0 to <1 year after diagnosis | From 1 to <5 years after diagnosis | From 5 to 10 years after diagnosis | |||||
|---|---|---|---|---|---|---|---|---|---|
| N (IR) | HR (95% CI)a | N (IR) | HR (95% CI)a | N (IR) | HR (95% CI)a | ||||
| By sites of first malignancy | |||||||||
| Prostate | 2,216 (26.8) | 1,161 (82.6) | 0.971 (0.915–1.030) | 574 (21.6) | 1.052 (0.967–1.145) | 85 (5.0) | 1.058 (0.849–1.320) | ||
| Colon and rectum | 802 (9.7) | 417 (82.6) | 1.001 (0.909–1.103) | 215 (21.7) | 1.038 (0.907–1.188) | 27 (4.1) | 1.240 (0.846–1.817) | ||
| Lung and bronchus | 774 (9.4) | 528 (132.0) | 0.971 (0.890–1.058) | 155 (29.8) | 1.173 (1.001–1.376) | 13 (5.5) | 1.610 (0.925–2.803) | ||
| Breast | 531 (6.4) | 279 (84.8) | 0.922 (0.819–1.039) | 128 (19.9) | 0.915 (0.767–1.092) | 24 (5.8) | 1.256 (0.836–1.886) | ||
| Skin | 450 (5.4) | 226 (80.1) | 1.025 (0.899–1.169) | 104 (20.1) | 1.004 (0.827–1.218) | 17 (5.9) | 1.376 (0.850–2.228) | ||
| Others | 3,500 (42.3) | 1,984 (93.5) | 0.968 (0.925–1.013) | 863 (24.6) | 1.101 (1.028–1.180) | 111 (5.7) | 1.219 (1.005–1.479) | ||
| By tumor stage of first malignancy | |||||||||
| Localized | 4,117 (50.0) | 2,075 (99.1) | 0.938 (0.897–0.981) | 1,044 (23.7) | 1.004 (0.943–1.070) | 140 (4.8) | 1.139 (0.956–1.356) | ||
| Regional | 2,375 (28.7) | 1,334 (69.8) | 0.937 (0.887–0.989) | 626 (21.0) | 1.149 (1.060–1.245) | 88 (5.5) | 1.219 (0.981–1.514) | ||
| Distant | 947 (11.4) | 633 (153.8) | 1.128 (1.043–1.221) | 186 (31.8) | 1.160 (1.004–1.342) | 13 (5.6) | 0.779 (0.450–1.349) | ||
| Unstaged | 834 (10.1) | 553 (88.2) | 1.042 (0.957–1.134) | 183 (21.3) | 1.053 (0.910–1.220) | 36 (7.3) | 1.793 (1.284–2.505) | ||
| By time elapsed from first malignancy (years) | |||||||||
| 0 to <2 | 2,664 (32.2) | 1,604 (103.7) | 0.988 (0.939–1.039) | 632 (24.1) | 1.138 (1.051–1.233) | 93 (5.4) | 1.116 (0.904–1.378) | ||
| 2 to <5 | 2,398 (29.0) | 1,294 (85.2) | 0.961 (0.909–1.016) | 661 (23.6) | 1.060 (0.981–1.147) | 100 (5.6) | 1.226 (1.000–1.503) | ||
| 5 to <10 | 2,167 (26.2) | 1,152 (84.4) | 0.953 (0.898–1.011) | 543 (21.7) | 0.993 (0.911–1.083) | 77 (5.1) | 1.308 (1.037–1.651) | ||
| ≥10 | 1,044 (12.6) | 545 (89.1) | 0.991 (0.910–1.080) | 203 (22.2) | 1.052 (0.914–1.210) | 7 (2.5) | 0.783 (0.370–1.656) | ||
| By radiation therapy of first malignancy | |||||||||
| Yes | 2,907 (35.1) | 1,622 (90.2) | 0.900 (0.856–0.946) | 761 (26.1) | 1.080 (1.004–1.162) | 100 (6.3) | 1.230 (1.004–1.506) | ||
| No/unknown | 5,366 (64.9) | 2,973 (91.6) | 0.985 (0.948–1.022) | 1278 (21.6) | 1.103 (1.042–1.169) | 177 (4.8) | 0.949 (0.813–1.107) | ||
| By chemotherapy of first malignancy | |||||||||
| Yes | 1,739 (21.0) | 964 (89.5) | 0.917 (0.860–0.978) | 443 (26.6) | 1.037 (0.944–1.140) | 46 (6.1) | 1.305 (0.972–1.752) | ||
| No/unknown | 6,534 (79.0) | 3,631 (91.5) | 0.982 (0.932–1.041) | 1,596 (22.2) | 1.057 (1.004–1.114) | 231 (5.1) | 0.991 (0.864–1.135) | ||
a, HRs were adjusted for age and calendar period at diagnosis, sex, race, cohabitation status, median household income in the county of residence, rural-urban continuum, tumor stage, histology, tumor grade, surgery, radiation therapy, and chemotherapy. CI, confidence interval; HR, hazard ratio; IR, incidence rate per 100 person-years; N, number of deaths.
The HRs for non-cancer related mortality were significantly elevated among esophagus-2 patients compared to esophagus-1 patients, except for those with a history of breast cancer and skin neoplasms (Table 4). The temporal pattern shows that the HR was highest during the initial 2 years after diagnosis and slowly declined thereafter (Figure 4E,4F). Notably, esophagus-2 patients who had a previous lung and bronchus malignancy had an extremely high HR compared to esophagus-1 patients (Table 4). The severity of the first malignancy stage had a direct impact on non-cancer related mortality, with more advanced stages associated with worse outcomes in each period. Particularly, esophagus-2 patients diagnosed within 2 years after their first malignancy exhibited the highest HR, indicating a higher frequency of complications leading to death when individuals experienced multiple carcinomas within a short time. Esophagus-2 patients receiving chemotherapy or radiation therapy for first malignancies also suffered higher risk of non-cancer related mortality.
Table 4
| Variables | N (%) | From 0 to <1 year after diagnosis | From 1 to <5 years after diagnosis | From 5 to 10 years after diagnosis | |||||
|---|---|---|---|---|---|---|---|---|---|
| N (IR) | HR (95% CI)a | N (IR) | HR (95% CI)a | N (IR) | HR (95% CI)a | ||||
| By sites of first malignancy | |||||||||
| Prostate | 2,216 (26.8) | 209 (14.9) | 1.585 (1.376–1.826) | 130 (4.9) | 1.400 (1.171–1.675) | 60 (3.5) | 1.294 (0.992–1.689) | ||
| Colon and rectum | 802 (9.7) | 110 (21.8) | 2.348 (1.940–2.840) | 64 (6.5) | 1.727 (1.345–2.216) | 19 (2.9) | 1.579 (0.999–2.496) | ||
| Lung and bronchus | 774 (9.4) | 223 (55.7) | 3.340 (2.913–3.831) | 77 (14.8) | 3.223 (2.560–4.058) | 10 (4.2) | 1.953 (1.026–3.719) | ||
| Breast | 531 (6.4) | 60 (18.2) | 1.815 (1.398–2.357) | 27 (4.2) | 1.010 (0.687–1.486) | 18 (4.4) | 1.724 (1.073–2.770) | ||
| Skin | 450 (5.4) | 26 (9.2) | 1.046 (0.710–1.540) | 19 (3.7) | 1.116 (0.709–1.757) | 10 (3.5) | 1.434 (0.764–2.690) | ||
| Others | 3,500 (42.3) | 476 (22.4) | 2.029 (1.843–2.234) | 246 (7.0) | 1.745 (1.529–1.993) | 68 (3.5) | 1.319 (1.029–1.69) | ||
| By tumor stage of first malignancy | |||||||||
| Localized | 4,117 (50.0) | 384 (18.3) | 1.541 (1.385–1.714) | 263 (6.0) | 1.344 (1.181–1.530) | 98 (3.4) | 1.418 (1.147–1.753) | ||
| Regional | 2,375 (28.7) | 333 (17.4) | 2.062 (1.842–2.308) | 183 (6.1) | 2.030 (1.744–2.362) | 56 (3.5) | 1.325 (1.006–1.744) | ||
| Distant | 947 (11.4) | 235 (57.1) | 3.527 (3.086–4.031) | 70 (12.1) | 2.655 (2.088–3.376) | 11 (4.7) | 1.066 (0.585–1.943) | ||
| Unstaged | 834 (10.1) | 152 (24.2) | 2.316 (1.965–2.729) | 47 (5.5) | 1.661 (1.241–2.221) | 20 (4.1) | 1.909 (1.218–2.990) | ||
| By time elapsed from first malignancy (years) | |||||||||
| 0 to <2 | 2,664 (32.2) | 502 (32.4) | 2.691 (2.451–2.956) | 208 (7.9) | 2.181 (1.891–2.514) | 61 (3.5) | 1.307 (1.004–1.700) | ||
| 2 to <5 | 2,398 (29.0) | 285 (18.8) | 1.840 (1.630–2.076) | 169 (6.0) | 1.516 (1.296–1.774) | 71 (4.0) | 1.504 (1.177–1.922) | ||
| 5 to <10 | 2,167 (26.2) | 215 (15.8) | 1.573 (1.368–1.808) | 130 (5.2) | 1.291 (1.079–1.544) | 50 (3.3) | 1.487 (1.111–1.990) | ||
| ≥10 | 1,044 (12.6) | 102 (16.7) | 1.577 (1.290–1.929) | 56 (6.1) | 1.631 (1.245–2.137) | 3 (1.1) | 0.586 (0.187–1.840) | ||
| By radiation therapy of first malignancy | |||||||||
| Yes | 2,907 (35.1) | 432 (24.0) | 2.145 (1.941–2.371) | 214 (7.3) | 1.752 (1.523–2.016) | 64 (4.0) | 1.409 (1.092–1.819) | ||
| No/unknown | 5,366 (64.9) | 672 (20.7) | 1.959 (1.804–2.127) | 349 (5.9) | 1.432 (1.280–1.603) | 121 (3.3) | 1.139 (0.942–1.377) | ||
| By chemotherapy of first malignancy | |||||||||
| Yes | 1,739 (21.0) | 305 (28.3) | 2.524 (2.243–2.841) | 153 (9.2) | 2.158 (1.831–2.543) | 30 (4.0) | 1.540 (1.067–2.222) | ||
| No/unknown | 6,534 (79.0) | 799 (20.1) | 1.892 (1.753–2.043) | 410 (5.7) | 1.393 (1.255–1.547) | 155 (3.5) | 1.170 (0.988–1.386) | ||
a, HRs were adjusted for age and calendar period at diagnosis, sex, race, cohabitation status, median household income in the county of residence, rural-urban continuum, tumor stage, histology, tumor grade, surgery, radiation therapy, and chemotherapy. CI, confidence interval; HR, hazard ratio; IR, incidence rate per 100 person-years; N, number of deaths.
Factors of esophagus cancer that modified the risk of mortality
When comparing esophagus-2 to esophagus-1, lower HRs for esophagus cancer-specific mortality were observed from diagnosis up to 10 years afterwards among patients under the age of 65 and patients who did not undergo surgery, radiation therapy, or chemotherapy (Table S4). Male gender consistently showed an association with poorer esophagus cancer-specific death. The HRs for overall death in subgroup analysis were consistent with the previous findings, except for the 5 years afterwards, which may be attributed to the small sample size and limited statistical power during that timeframe (Table S5). As expected, overall mortality rates for both esophagus-2 and esophagus-1 patients have gradually improved in recent years. Higher HRs for non-cancer related mortality were observed among patients with distant stage, undifferentiated grade, squamous histology, and those under the age of 65 years (Table S6). Other factors related to esophagus-2, such as race, did not significantly modify the association with mortality risks.
Discussion
Utilizing a large population-based cohort, this study represents the first comprehensive investigation into the prognosis of esophagus-2 patients, revealing a distinct and time-dependent disease trajectory. Our findings challenge that esophagus cancer-specific survival is not necessarily inferior to esophagus-1, especially within the initial 5 years after diagnosis. In contrast, regardless of the post-diagnosis period, the risk of non-cancer related mortality remains consistently higher in esophagus-2 compared to esophagus-1. Consequently, the improved overall mortality risk in esophagus-2 is limited to the period more than 5 years after diagnosis, despite short-term overall survival is actually comparable. Given the increasing incidence of esophagus-2 recently, our findings offer timely guides for clinical assessment and decision making.
Our discovery does not support the notion that esophagus-2 is generally more invasive than esophagus-1, despite the evidence suggesting that second primary cancers may have biological differences compared to the initial malignancy. For instance, previous research has shown that among breast cancer survivors, the risk of EC is significantly increased (8.3-fold) when the radiation dose to the esophageal tumor location reaches or exceeds 35 Gy (17). Additionally, a study of colorectal cancer in Hodgkin lymphoma survivors who underwent radiation therapy revealed a higher frequency of microsatellite instability in these tumors due to somatic mutations in mismatch repair genes (18). Interestingly, in therapy-related EC compared to sporadic cancer, no significant differences were found in the frequency of microsatellite instability or loss of heterozygosity. However, specific regions investigated, such as D17S1327 (17q21.31), showed a lower frequency of loss of heterozygosity in metachronous tumors compared to sporadic ones (19). It is important to note that the occurrence of second primary cancers resulting from treatment for the initial primary cancer is not common, with only a 6% incidence rate among breast cancer survivors (20). Therefore, it is unlikely that the presumed biological differences in esophagus-2 attributed to treatment would essentially render worse survival, if any such association exists.
Based on our study findings of lower esophagus cancer-specific mortality and similar overall mortality within the initial 5 years after diagnosis, we argue against adopting a conservative treatment for esophagus-2 simply because of a cancer history. Just as our conjecture, esophagus-2 patients actually receive more conservative management, as they are 10% less likely to undergo surgical treatment even after adjusting for clinical and tumor characteristics. This could be attributed to limitations in dosage or comorbidities resulting from prior cancer treatment. Radiotherapy and chemotherapy are also administered less frequently to esophagus-2 patients (Table S2) (21-23).
Given that the median time from the prior cancer to esophagus-2 diagnosis is approximately 3 years, it is plausible that most cases of esophagus-2 are detected during routine follow-up of the prior cancer or by “screening”, benefiting from better access to healthcare. This detection method may introduce a “lead-time bias” in which the cancer detected earlier would lead to the perception of improved outcomes. For instance, the greatest reduction was observed in esophagus cancer-specific death among esophagus-2 patients within 2 years after their prior cancer. This observation is further verified by our findings that esophagus-2 patients tend to be diagnosed at an early stage and with well/moderately differentiated grade. However, even after carefully adjusting for clinical characteristics and treatment modalities, the association between esophagus-2 and improved cancer-specific survival remains robust. This suggests that the favorable outcomes observed in esophagus-2 patients are independent of the aforementioned prognostic factors.
Prior to our study, the time-varying disease course of esophagus-2 patients had not been previously investigated. Pan and Saad separately examined the survival outcomes of stage I–III (N0M0) and stage IV esophageal carcinoma with a prior cancer, respectively (11,12). They both reached the conclusion that esophagus cancer-specific survival was better for esophagus-2 patients, while overall survival did not show a significant difference between esophagus-1 and esophagus-2. To some extent, this conclusion aligns with our findings, but it overlooks the dynamic HRs that can be appropriately explained by our approach of unraveling temporal patterns. By taking into account the changing HRs over time, our findings provide a thorough understanding of the prognosis of esophagus-2 patients, shedding light on the distinct disease course experienced by this population.
Our innovative findings reveal that non-cancer related mortality in esophagus-2 patients remains consistently higher than that of esophagus-1 patients, even after adjusting for clinical and tumor characteristics, as well as therapeutic modalities. Malnutrition and cachexia syndrome, commonly observed in about half of all cancer patients, can contribute to increased non-cancer related mortality. These conditions are characterized by anorexia, malnutrition, loss of adipose tissue, and sarcopenia (loss of muscle mass) (24). Moderate to severe malnutrition is an independent risk factor for respiratory and cardiovascular complications. Parameters such as serum albumin and total cholesterol, which reflect nutritional status, can negatively affect tissue repair and resistance to illness, potentially leading to higher incidence of postoperative complications (25). Sarcopenia, resulting from an imbalance between insufficient food intake and increased tumor metabolism, is also identified as a risk factor for major postoperative complications, including anastomotic leaks and pulmonary complications (26). Furthermore, complications related to the treatment of the first primary cancer can contribute to the higher non-cancer related mortality observed in esophagus-2 patients. Certain contemporary cancer treatments, such as alkylating chemotherapies, anthracycline-based chemotherapies, and radiation targeting the chest area, can increase the susceptibility of patients to developing heart disease (27). Neutropenia caused by aggressive chemotherapy regimens, exposure to invasive procedures or medical devices, as well as hematopoietic stem cell transplantation, can significantly raise the vulnerability of patients to infections, which are substantial contributors to non-cancer related mortality (28,29). The high mortality rate associated with prior lung and bronchus malignancy may attribute to its progressive nature and thus high rate of receiving chemotherapy and radiotherapy.
Additionally, previous research has indicated that psychological stress may prompt cardiovascular mortality (30), especially in a vulnerable cohort. Stress is implicated in heightened risk for cardiac regulatory alterations (31), potentially via the sympathetic nervous system and hemostatic activation (32,33). The experience of being diagnosed as and living with cancer imposes significant stress. Substantial evidence has demonstrated that risks for cardiovascular mortality (34), suicide (35-37) and mental health disorders (38) increase sharply after an initial malignancy diagnosis. Su et al. conducted a study which revealed that patients with multiple primary cancers had a higher mortality rate from suicides compared to those with a single primary cancer (39). The risk of cardiovascular mortality within the first month proceeding a cancer diagnosis is doubled compared to individuals without cancer (40), underlying the acute and significant impact of stress on the cardiovascular health induced by such a diagnosis. Thus, it is not difficult to explain why the non-cancer mortality rate was extremely high in the initial 2 years after an esophagus-2 diagnosis.
Therefore, the higher rate of suicide may also contribute to the increased non-cancer related mortality observed in esophagus-2 patients. These factors collectively highlight the complex interplay between cancer, its treatment, nutritional status, and overall health, which contribute to the higher non-cancer related mortality seen in esophagus-2 patients.
The study findings indicate that the noninferior overall survival observed in esophagus-2 patients is limited to the first 5 years following diagnosis, however, in the long term, they experience poorer overall survival compared to esophagus-1 ones. This could be attributed to the progression of the prior cancer, which may contribute to the increased risks. The study supports this hypothesis by noting that the increased risk of overall mortality among esophagus-2 patients with a prior cancer diagnosed at a distant stage occurs much earlier compared to those diagnosed at a localized or regional stage. For esophagus-2 patients with a history of lung and bronchus cancer, the overall survival worsens starting from the second year after diagnosis, rather than 5 years onward. This can be explained by the fact that esophagus cancer patients in lung and bronchus cancer survivors have the highest non-cancer related mortality rate (Table 4). The study also highlights that these patients have the highest prior cancer-related death rate and the lowest esophagus cancer-related death rate (11). Furthermore, the findings demonstrate that esophagus-2 in lung and bronchus survivors has the worst 5-year survival rate, which further supports the notion that factors apart from esophagus-2, such as the presence of the first primary malignancy, can significantly impact the overall mortality of esophagus-2 cases (Figure S2).
Conflicting results are yielded by previous studies investigating esophagus-2 in cancer survivors from different cancer sites. While some studies have reported worse overall and disease-specific survival of gastrointestinal cancers, including esophagus cancer, in Hodgkin lymphoma survivors compared to de novo cases (41), the survival difference was not confirmed for specific subsites (e.g., esophagus) of gastrointestinal cancer due to insufficient statistical power. It is important to consider the heterogeneity of carcinogenesis among the subsites of gastrointestinal cancer diagnosed in Hodgkin lymphoma survivors (18,19). Therefore, it is not possible to draw a conclusive statement regarding the survival trends for esophagus-2 in this specific population. Further research with larger cohorts focusing on the esophagus subgroup in Hodgkin lymphoma survivors is necessary to provide a more convincing conclusion.
Many cancer clinical trials exclude patients with a prior cancer (8). For example, in 1,103 early phase trials identified on CT.gov (clinicaltrials.gov), 86% of trials restricted patients with a history of prior cancers (42). As our study shows, a previous cancer did not appear to adversely affect survival, which provides further support for our argument against exclusion of these patients. Table 3 reflects that, for most cancer types of previous malignancy (except for lung and bronchus cancer), esophagus-2 patients maintained similar overall mortality with esophagus-1 patients until 5 years after diagnosis. As most clinical trials use 5-year overall survival rate (5-year OS rate) as the primary endpoint, it is unreasonable to exclude patients with prior cancer from clinical trials.
Determining the impact of prior cancer exclusion criteria on trial accrual requires disease-specific and protocol-specific details, including stage and timing of prior cancer diagnoses (43). We note that esophagus-2 patients with a prior cancer less than 2 years preceding esophagus cancer, suffered higher risk of overall mortality compared to esophagus-1 patients for 1 year after diagnosis (Table 3), which raises the question of whether the lead time from previous cancer impacts on the chances of recurrence. Some trials use a 5-year exclusion window (i.e., only prior cancers occurred in this window were excluded) (44). However, due to the higher overall mortality of esophagus-2 patients with a distant stage or lung and bronchus cancer type, we appreciate the point at which a patient may be considered “cured” of a cancer is variable—late recurrences of indolent cancers such as hormone-sensitive breast cancer may not be rare, whereas more aggressive lung and bronchus cancer usually recurs in 5 years. This clearly adds to the complexity of any proposal for including patients with previous malignancies in trials, but does not necessarily render the problem unsolvable. Due to the exponentially decreased risk of recurrence for most high-grade cancers after 3 years, this would seem a very reasonable timeframe to suggest as a cancer-free baseline.
The strengths of our study lie in its large-scale, population-based prospective cohort of patients with primary esophagus cancer, which minimizes common biases like selection and surveillance biases. Our comprehensive analyses of the temporal pattern of mortality risk help elucidate inconsistent results in previous literature. However, there were several limitations. Firstly, competing risks do exist among different cause of death, but it is noteworthy that analysis using the Fine-Gray model led to even stronger correlations (Table S3). Secondly, there is a possibility of incorrect recording of the cause of death; however, a validation study demonstrated a reasonably satisfactory concordance rate of esophagus cancer-specific mortality (45). Additionally, our subsequent analysis on any cancer-specific survival reflected that even if we attribute deaths from all the other cancers to esophagus cancer, esophagus-2 patients still exhibit a decreased risk of any cancer-specific death within the initial 5 years (Figure S1). Thirdly, certain factors associated with survival, such as tobacco use and comorbidities, were not recorded in our study. However, such factors are more influential in non-cancer related mortality rather than cancer-specific mortality. Lastly, we excluded esophagus-2 cases with esophagus cancer as their prior cancer due to the difficulty in distinguishing esophagus-2 cases from esophageal metastases. Despite these limitations, our study provides valuable insights into the mortality risks and characteristics of esophagus-2 patients, contributing to a better understanding of this specific population.
Conclusions
In summary, the study findings indicate that esophagus-2 patients have better esophagus cancer-specific survival within the initial 5 years following diagnosis compared to esophagus-1 patients. However, beyond the 5-year mark, the survival outcomes for esophagus-2 patients worsen. Furthermore, those with esophagus-2 experience worse non-cancer related mortality throughout their disease course. This association remains significant even after accounting for clinical characteristics and treatment modalities.
These findings indicate that the disease course of esophagus-2 is not inherently more aggressive than that of esophagus-1. Therefore, there is no sufficient reason choosing conservative treatment solely based on a history of first malignancy. Esophagus-2 patients should not be entirely excluded from clinical trial participation, and we suggested a 3-year exclusion window for them. The compromised non-cancer related survival observed in esophagus-2 patients in the long term emphasizes the necessity of active surveillance, prevention and management of the comorbidities and complications to optimize overall prognosis.
Acknowledgments
The abstract has been published in Proceedings of the American Association for Cancer Research Annual Meeting 2024; Part 1 (Regular Abstracts); 2024 Apr 5-10; San Diego, CA. Philadelphia (PA): AACR; Cancer Res 2024;84(6_Suppl): Abstract nr 4860. The authors express sincere gratitude for the efforts of the SEER Program cancer registries in the creation of the SEER database.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1881/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1881/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1881/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morgan E, Soerjomataram I, Rumgay H, et al. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates From GLOBOCAN 2020. Gastroenterology 2022;163:649-658.e2. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Patel MA, Kratz JD, Lubner SJ, et al. Esophagogastric Cancers: Integrating Immunotherapy Therapy Into Current Practice. J Clin Oncol 2022;40:2751-62. [Crossref] [PubMed]
- Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin 2022;72:409-36. [Crossref] [PubMed]
- Murphy CC, Tortolero GA, Gerber DE, et al. An Updated Report on the Prevalence of Prior Cancer Among Persons Newly Diagnosed With Cancer in the Surveillance, Epidemiology, and End Results Program. JAMA Oncol 2023;9:1147-50. [Crossref] [PubMed]
- Ji Y, Du X, Zhu W, et al. Efficacy of Concurrent Chemoradiotherapy With S-1 vs Radiotherapy Alone for Older Patients With Esophageal Cancer: A Multicenter Randomized Phase 3 Clinical Trial. JAMA Oncol 2021;7:1459-66. [Crossref] [PubMed]
- Luo H, Lu J, Bai Y, et al. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA 2021;326:916-25. [Crossref] [PubMed]
- Denicoff AM, Ivy SP, Tamashiro TT, et al. Implementing Modernized Eligibility Criteria in US National Cancer Institute Clinical Trials. J Natl Cancer Inst 2022;114:1437-40. [Crossref] [PubMed]
- Tournoux C, Katsahian S, Chevret S, et al. Factors influencing inclusion of patients with malignancies in clinical trials. Cancer 2006;106:258-70. [Crossref] [PubMed]
- Van Spall HG, Toren A, Kiss A, et al. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA 2007;297:1233-40. [Crossref] [PubMed]
- Pan D, Xu W, Gao X, et al. Survival outcomes in esophageal cancer patients with a prior cancer. Medicine (Baltimore) 2021;100:e24798. [Crossref] [PubMed]
- Saad AM, Al-Husseini MJ, Elgebaly A, et al. Impact of prior malignancy on outcomes of stage IV esophageal carcinoma: SEER based study. Expert Rev Gastroenterol Hepatol 2018;12:417-23. [Crossref] [PubMed]
- SEER. 2007 Multiple primary and histology coding rules. [cited 2023 August 22]. Available online: https://seer.cancer.gov/tools/mphrules/
- SEER. SEER cause-specific death classification. 2023 [cited 2023 August 22]. Available online: https://seer.cancer.gov/causespecific/
- Lambert P, Royston P. Further Development of Flexible Parametric Models for Survival Analysis. The Stata Journal 2009;9:265-90. [Crossref]
- Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 1999;94:496-509. [Crossref]
- Morton LM, Gilbert ES, Hall P, et al. Risk of treatment-related esophageal cancer among breast cancer survivors. Ann Oncol 2012;23:3081-91. [Crossref] [PubMed]
- Rigter LS, Snaebjornsson P, Rosenberg EH, et al. Double somatic mutations in mismatch repair genes are frequent in colorectal cancer after Hodgkin's lymphoma treatment. Gut 2018;67:447-55. [Crossref] [PubMed]
- Boldrin E, Rumiato E, Fassan M, et al. Genetic features of metachronous esophageal cancer developed in Hodgkin's lymphoma or breast cancer long-term survivors: an exploratory study. PLoS One 2015;10:e0117070. [Crossref] [PubMed]
- Berrington de Gonzalez A, Curtis RE, Gilbert E, et al. Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br J Cancer 2010;102:220-6. [Crossref] [PubMed]
- Khanna L, Prasad SR, Yedururi S, et al. Second Malignancies after Radiation Therapy: Update on Pathogenesis and Cross-sectional Imaging Findings. Radiographics 2021;41:876-94. [Crossref] [PubMed]
- Meinardi MT, Gietema JA, van Veldhuisen DJ, et al. Long-term chemotherapy-related cardiovascular morbidity. Cancer Treat Rev 2000;26:429-47. [Crossref] [PubMed]
- Khakoo AY, Yeh ET. Therapy insight: Management of cardiovascular disease in patients with cancer and cardiac complications of cancer therapy. Nat Clin Pract Oncol 2008;5:655-67. [Crossref] [PubMed]
- Suzuki H, Asakawa A, Amitani H, et al. Cancer cachexia--pathophysiology and management. J Gastroenterol 2013;48:574-94. [Crossref] [PubMed]
- Horinouchi T, Yoshida N, Harada K, et al. A retrospective study of preoperative malnutrition based on the Controlling Nutritional Status score as an associated marker for short-term outcomes after open and minimally invasive esophagectomy for esophageal cancer. Langenbecks Arch Surg 2022;407:3367-75. [Crossref] [PubMed]
- Fang P, Zhou J, Xiao X, et al. The prognostic value of sarcopenia in oesophageal cancer: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2023;14:3-16. [Crossref] [PubMed]
- Curigliano G, Cardinale D, Dent S, et al. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin 2016;66:309-25. [Crossref] [PubMed]
- Zembower TR. Epidemiology of infections in cancer patients. Cancer Treat Res 2014;161:43-89. [Crossref] [PubMed]
- Donnelly JP, Blijlevens NM, van der Velden WJ. Host impairments in patients with neoplastic diseases. Cancer Treat Res 2014;161:1-41. [Crossref] [PubMed]
- Kivimäki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol 2018;15:215-29. [Crossref] [PubMed]
- Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol 2008;51:1237-46. [Crossref] [PubMed]
- Wittstein IS. The Sympathetic Nervous System in the Pathogenesis of Takotsubo Syndrome. Heart Fail Clin 2016;12:485-98. [Crossref] [PubMed]
- Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539-48. [Crossref] [PubMed]
- Shen Q, Ma Y, Jöud A, et al. Psychiatric Disorders and Cardiovascular Diseases During the Diagnostic Workup of Suspected Prostate Cancer. JNCI Cancer Spectr 2021;5:pkaa108. [Crossref] [PubMed]
- Henson KE, Brock R, Charnock J, et al. Risk of Suicide After Cancer Diagnosis in England. JAMA Psychiatry 2019;76:51-60. [Crossref] [PubMed]
- Lu D, Fall K, Sparén P, et al. Suicide and suicide attempt after a cancer diagnosis among young individuals. Ann Oncol 2013;24:3112-7. [Crossref] [PubMed]
- Liu Q, Wang X, Kong X, et al. Subsequent risk of suicide among 9,300,812 cancer survivors in US: A population-based cohort study covering 40 years of data. EClinicalMedicine 2022;44:101295. [Crossref] [PubMed]
- Carreira H, Williams R, Müller M, et al. Associations Between Breast Cancer Survivorship and Adverse Mental Health Outcomes: A Systematic Review. J Natl Cancer Inst 2018;110:1311-27. [Crossref] [PubMed]
- Su C, Wang Y, Wu F, et al. Suicide and Cardiovascular Death Among Patients With Multiple Primary Cancers in the United States. Front Cardiovasc Med 2022;9:857194. [Crossref] [PubMed]
- Fang F, Fall K, Mittleman MA, et al. Suicide and cardiovascular death after a cancer diagnosis. N Engl J Med 2012;366:1310-8. [Crossref] [PubMed]
- Rigter LS, Schaapveld M, Janus CPM, et al. Overall and disease-specific survival of Hodgkin lymphoma survivors who subsequently developed gastrointestinal cancer. Cancer Med 2019;8:190-9. [Crossref] [PubMed]
- Duma N, Kothadia SM, Azam TU, et al. Characterization of Comorbidities Limiting the Recruitment of Patients in Early Phase Clinical Trials. Oncologist 2019;24:96-102. [Crossref] [PubMed]
- Gerber DE, Pruitt SL, Halm EA. Should criteria for inclusion in cancer clinical trials be expanded? J Comp Eff Res 2015;4:289-91. [Crossref] [PubMed]
- Gerber DE, Laccetti AL, Xuan L, et al. Impact of prior cancer on eligibility for lung cancer clinical trials. J Natl Cancer Inst 2014;106:dju302. [Crossref] [PubMed]
- Hu CY, Xing Y, Cormier JN, et al. Assessing the utility of cancer-registry-processed cause of death in calculating cancer-specific survival. Cancer 2013;119:1900-7. [Crossref] [PubMed]


