
Tumor immune microenvironment remodeling and prognosis of patients with esophageal squamous cell carcinoma after neoadjuvant chemotherapy with and without immunotherapy: a retrospective cohort study
Highlight box
Key findings
• Neoadjuvant chemotherapy with and without immunotherapy may upregulate the programmed cell death ligand-1 (PD-L1) protein expression level, increase tumor-infiltrating lymphocytes, and remodel the tumor immune microenvironment in patients with esophageal squamous cell carcinoma (ESCC). Neoadjuvant immunochemotherapy (nICT) could more significantly upregulate PD-L1, CD3+ T cells, and CD8+ T cells. Pretreatment tumor differentiation and PD-L1 level could be predictive of major pathological response (MPR).
What is known and what is new?
• Immunotherapy has changed the treatment pattern of various cancer types, including ESCC.
• As immunotherapy is only effective in a subset of patients, it remains an unmet clinical need to identify which patients are most likely to respond to and benefit from immunotherapy.
• This study focuses on analyzed clinical information and examined the tumor microenvironment features and immune antigen-related biomarkers in patients’ histopathology specimens before and after treatment, with the aim to explore the factors related to tumor immune microenvironment remodeling and patient prognosis.
What is the implication, and what should change now?
• The study indicates the degree of tumor differentiation and PD-L1 expression are not only correlated with MPR but also associated with patient prognosis. nICT might be recommended as the preferred treatment for locally advanced resectable ESCC.
Introduction
Esophageal cancer is the 7th most common cancer worldwide, and its incidence and mortality rates are steadily increasing with significant geographic disparities. For locally advanced, resectable disease, neoadjuvant chemotherapy (nCT) or concurrent chemoradiotherapy is currently the standard of care for esophageal squamous cell carcinoma (ESCC) (1-4).
Recent years, the addition of anti-programmed death-1 (PD-1) inhibitors has led to improvement in overall survival in patients with metastatic disease based on several phase III trials, such as KEYNOTE-590 (5-9). The combination of chemotherapy and immune checkpoint inhibitors has become the standard first-line treatment for patients with recurrent metastatic esophageal cancer. In locally advanced esophageal cancer, there are also a large number of clinical trials being conducted to explore more optimized treatment models. In resectable esophageal cancer, neoadjuvant concurrent chemoradiotherapy combined with immunotherapy or nCT combined with immunotherapy are the focus of exploration (10-12). At present, based on the results of small sample or phase II clinical studies (11-13), nCT combined with immunotherapy for locally advanced resectable esophageal cancer has shown promising efficacy and high safety. However, due to the lack of long-term survival data for large-scale cases, the role of immunotherapy in locally advanced resectable esophageal cancer has not yet been established. Similarly, the synergistic mechanism of combined chemotherapy and immunotherapy, its impact on the tumor immune microenvironment, and predictive factors for efficacy still need further exploration. Based on the above background, we collected patients with ESCC who received nCT or immunochemotherapy at the Fourth Hospital of Hebei Medical University from December 2019 to March 2022. We tested the tumor microenvironment (TME) indicators and immune antigen-related biomarkers of the tumor specimens before and after neoadjuvant therapy, and collected information on host-related biomarkers. The purpose of this study was to investigate the outcomes of nCT and neoadjuvant immunochemotherapy (nICT) in TME remodeling among patients with ESCC and to evaluate the prognostic value of immune-related biomarkers and clinicopathological characteristics. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-828/rc).
Methods
Study design and participant selection
This study is a retrospectively cohort study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The Fourth Hospital of Hebei Medical University (No. 2024KS074). Informed consent was waived due to the retrospective study design. We collected data from patients with locally advanced ESCC who underwent neoadjuvant therapy followed by esophagectomy at the Department of Thoracic Surgery at the Fourth Hospital of Hebei Medical University between December 2019 and March 2022. The study procedure is shown in Figure 1.
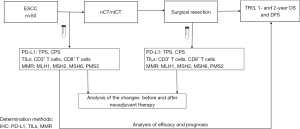
The inclusion criteria for patients were as follows: (I) patients with pathologically confirmed resectable non-metastatic thoracic ESCC; (II) patients received nCT with or without immunotherapy; (III) patients underwent open or minimally invasive esophagectomy; and (IV) available pre-treatment biopsy. Patients were excluded if they had (I) incomplete clinical, pathological, or imaging records; (II) metastatic disease; or (III) history of other malignancy.
Neoadjuvant therapy
All patients received two to three cycles of nCT with or without immune checkpoint inhibitors every 3 weeks. The chemotherapy regimen included albumin-bound paclitaxel or docetaxel combined with cisplatin or carboplatin. Immune checkpoint inhibitors included anti PD-1 antibodies sintilimab, camrelizumab, or pembrolizumab at 200 mg/3 weeks.
Endoscopy, histopathological specimens, clinical information, and surgical resection
All patients underwent endoscopic evaluation prior to treatment. The specimens were collected during the endoscopy and post treatment sample was collected during surgery. Immunohistochemistry was used to determine the expression of programmed cell death ligand-1 (PD-L1), tumor infiltrating lymphocytes (TILs), and MMR in tumor tissues. Evaluation of the TME included PD-L1 tumor proportion score (TPS), PD-L1 combined positive score (CPS), and tumor-infiltrating lymphocytes (CD3+ T cells and CD8+ T cells). Immune antigen-related biomarkers included mismatch repair (MMR) genes: human MutL homolog 1 (MLH1), MutS homolog 2 (MSH2), MutS homolog 6 (MSH6), and PMS1 homolog 2 (PMS2). Tumors were classified as proficient expression [MMR-proficient (pMMR)] or deficient expression [MMR-deficient (dMMR)] when there was a loss of more than one protein. Patient laboratory data of interest were absolute lymphocyte, neutrophil, and platelet counts and the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR).
Clinical and pathological stages based on the tumor-node-metastasis (TNM) staging system of the American Joint Committee on Cancer/Union for International Cancer Control Eighth Edition Cancer Staging Manual (14). The clinicopathological information collected included the gender, age, family history, tumor site, tumor differentiation, tumor maximum length under endoscopic evaluation, tumor maximal diameter on computed tomography (CT) scan, clinical TNM (cTNM) stage, types of neoadjuvant therapy, and the interval between neoadjuvant therapy and surgical resection.
Patients received right thoracotomy with a two-incision (Ivor Lewis) or three-incision (McKeown) operation or minimally invasive radical esophagectomy, with two-field or three-field lymph node dissections. Patients with lower segment cancers without lymph node metastasis to the upper mediastinum on the preoperative evaluation received left thoracotomy with incomplete two-field lymph node dissection. The tumor regression grade (TRG) was reported according to the College of American Pathologists (CAP): TRG 0, no viable cancer cells (complete response); TRG 1, single or small clusters of cancer cells (moderate response); TRG 2, residual cancer cells with interstitial fibrosis (mild response); and TRG 3, little or no tumor regression changes with extensive residual cancer cells (poor response) (15). Patients with TRG 0 or TRG 1 were classified as major pathological response (MPR) group, whereas patients with grades TRG 2 and TRG 3 were classified as non-MPR group. In addition, pathological complete response (pCR) was defined as a postoperative esophageal specimen with no cancer residue in the lymph nodes and a postoperative stage of ypT0N0M0.
Immunohistochemical examination of histopathology specimens
The mouse anti-human DAKO anti-PD-L1 (22C3) polyclonal antibody was acquired from Merck & Co., Inc. (Rahway, NJ, USA), an Alcian blue periodic acid Schiff (AB-PAS) staining kit was acquired from Beijing Solarbio Technology Co., Ltd. (Beijing, China), a DAKO Link 48 Autostainer was obtained from Agilent Technologies Co., Ltd. (Santa Clara, CA, USA), antigen repair solution (pH 8.0) was acquired from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd. (Beijing, China), and xylene was purchased from the Beijing Chemical Reagents Company (Beijing, China).
In routine fashion, the specimens were fixed, dehydrated, cleared, embedded in paraffin, sectioned into 4-µm slices, placed on glass microscope slides, and baked at 56 ℃. The slides were routinely stained with the hematoxylin and eosin staining. Staining of PD-L1 22C3 was conducted using the DAKO Link 48 Autostainer. Placenta tissue was used as an external control to verify the adequacy of the PD-L1 staining reaction. The TPS and CPS were calculated. TPS was defined as the percentage of tumor cells with any intensity PD-L1 membrane staining (TPS = any intensity of PD-L1 membrane staining positive tumor cells/total number of tumor cells × 100%). PD-L1 staining was consider negative if TPS <1% and positive for TPS ≥1%. CPS was defined as the percentage of positive live tumor cells (partial or complete membrane staining of any intensity), lymphocytes, and macrophages (membrane or cytoplasmic staining of any intensity) in all live tumor cells [CPS = (PD-L1 membrane staining positive tumor cells + lymphocytes + macrophages)/total number of tumor cells × 100%]. PD-L1 was consider negative if CPS <1, and positive for CPS ≥1.
Clinical follow-up and outcome measures
Patients were followed every 3 months after surgery for 2 years. The collection of prognosis outcomes mainly included patients follow-up visits and telephone follow-ups. Evaluations included physical exam, thoracic and abdominal CT, upper gastrointestinal barium meal/iohexol radiography, and supraclavicular ultrasound. Other examinations were ordered as indicated clinically.
The primary goal of the study was to study the correlation between the changes in TME, immune antigen-related biomarkers, before and after neoadjuvant therapy. Secondary outcomes were to correlate TME changes with pathological outcome (pCR rate and MPR rate).
Statistical analysis
Statistical analyses were performed using SPSS 26.0 (IBM Corp., Armonk, NY, USA). Patients were assigned to either a nCT group or nICT group. Continuous data are presented as the mean ± standard deviation (SD) or as the median with interquartile range (IQR) and were compared with the independent samples t-test or Mann-Whitney test depending on the normality test results. Categorical data are presented as numbers and percentages and were compared using the Chi-squared test. Cox regression analysis model was used for univariate and multivariate analysis of prognosis outcomes. Covariates included patient clinical pathological factors, TME indicators, TILs, and neoadjuvant therapy modes. Forward stepwise method was used to screen covariates. The Kaplan-Meier method was used to create survival curves, the log-rank test was used for survival analysis. P<0.05 in a two-sided test was considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 50 patients were included in this study; 14 and 36 patients in the nCT and nICT groups, respectively (Table 1). Age ranges from 43–77 years old (median 64 years). Cancer was located in the upper, middle and lower esophagus in 4, 28, and 18 patients respectively; 6, 32, and 12 (9 patients with cT3N2M0 and 3 patients with cT4aN2M0) had stage II, III, and IVa disease respectively. Patients received 2–3 cycles of neoadjuvant therapy (2 cycles in 45 patients and 3 cycles in 5 patients), surgery was performed 3.4–12.9 weeks after finishing treatment.
Table 1
| Characteristic | nCT (n=14) | nICT (n=36) | χ2 | P value |
|---|---|---|---|---|
| Gender | 3.024 | 0.08 | ||
| Female | 8 (57.1) | 11 (30.6) | ||
| Male | 6 (42.9) | 25 (69.4) | ||
| Age (years) | 0.397 | 0.53 | ||
| <65 | 6 (42.9) | 19 (52.8) | ||
| ≥65 | 8 (57.1) | 17 (47.2) | ||
| Median [range] | 64 [55–72] | 64 [43–77] | ||
| Family history | 0.000 | >0.99 | ||
| No | 10 (71.4) | 27 (75.0) | ||
| Yes | 4 (28.6) | 9 (25.0) | ||
| Tumor location | 1.814 | 0.40 | ||
| Upper | 0 | 4 (11.1) | ||
| Middle | 8 (57.1) | 20 (55.6) | ||
| Lower | 6 (42.9) | 12 (33.3) | ||
| Tumor length under endoscope† | 0.242 | 0.62 | ||
| <5 cm | 6 (42.9) | 12 (33.3) | ||
| ≥5 cm | 8 (57.1) | 22 (61.1) | ||
| Tumor diameter | 0.099 | 0.75 | ||
| <1.8 cm | 6 (42.9) | 19 (52.8) | ||
| ≥1.8 cm | 8 (57.1) | 17 (47.2) | ||
| Tumor differentiation | 3.921 | 0.16 | ||
| Poor | 9 (64.3) | 16 (44.4) | ||
| Moderate | 5 (35.7) | 12 (33.3) | ||
| High | 0 | 8 (22.2) | ||
| cT stage | 0.426 | 0.51 | ||
| cT1 + cT2 | 3 (21.4) | 5 (13.9) | ||
| cT3 + cT4 | 11 (78.6) | 31 (86.1) | ||
| cN stage | 0.066 | 0.80 | ||
| cN0 | 8 (57.1) | 22 (61.1) | ||
| cN+ | 6 (42.9) | 14 (38.9) | ||
| Duration from neoadjuvant therapy and surgery | ||||
| Median [range] | 5.8 [4.3–8.7] | 4.8 [3.4–12.9] | 3.571 | 0.06 |
| <5.2 weeks | 4 (28.6) | 21 (58.3) | ||
| ≥5.2 weeks | 10 (71.4) | 15 (41.7) | ||
Data are presented as n (%) or median [range]. †, in the neoadjuvant immunochemotherapy group, two patients had no measurements due to failed endoscopic examination from esophageal stenosis. nCT, neoadjuvant chemotherapy; nICT, neoadjuvant immunochemotherapy.
Surgical and pathologic results
R0 resection was achieved in all 50 patients. In total, 9, 6, 7, and 28 patients had TRG 0, TRG 1, TRG 2, and TRG 3 tumors, respectively. In the nCT and nICT groups, the pCR rates were 7.1% and 22.2% (χ2=0.699; P=0.40) respectively, while the MPR rates were 7.1% and 38.9% (χ2=4.837; P=0.03), respectively (Table 2).
Table 2
| Outcome | Patients, n (%) | P value | |
|---|---|---|---|
| nCT (n=14) | nICT (n=36) | ||
| R0 resection | 14 (100.0) | 36 (100.0) | >0.99 |
| TRG stage | 0.01 | ||
| 0 | 1 (7.1) | 8 (22.2) | |
| 1 | 0 | 6 (16.7) | |
| 2 | 0 | 7 (19.4) | |
| 3 | 13 (92.9) | 15 (41.7) | |
| pCR | 0.40 | ||
| Yes | 1 (7.1) | 8 (22.2) | |
| No | 13 (92.9) | 28 (77.8) | |
| MPR | 0.03 | ||
| Yes | 1 (7.1) | 14 (38.9) | |
| No | 13 (92.9) | 22 (61.1) | |
| Lymph node | 0.49 | ||
| ypN0 | 6 (42.9) | 22 (61.1) | |
| ypN1 | 6 (42.9) | 9 (25.0) | |
| ypN2 | 0 | 2 (5.6) | |
| ypN3 | 2 (14.3) | 3 (8.3) | |
| ypTNM | 0.17 | ||
| I | 2 (14.3) | 16 (44.4) | |
| II | 6 (42.9) | 8 (22.2) | |
| III | 4 (28.6) | 6 (16.7) | |
| IV | 2 (14.3) | 6 (16.7) | |
Data are presented as n (%). nCT, neoadjuvant chemotherapy; nICT, neoadjuvant immunochemotherapy; TRG, tumor regression grade; pCR, pathological complete response; MPR, major pathological response.
TME feature changes before and after neoadjuvant therapy
A total of 36 patients had sufficient available data for the analysis of TME features before and after neoadjuvant therapy (Table 3). Nine patients achieved a pCR, while five patients had insufficient tissue of preneoadjuvant therapy.
Table 3
| Features and biomarkers | pCR group preneoadjuvant therapy (n=9) | Non-pCR group (n=36) | |||
|---|---|---|---|---|---|
| Preneoadjuvant therapy | Postneoadjuvant therapy | z | P value | ||
| Tumor immune microenvironment features | |||||
| PD-L1 TPS | |||||
| Negative | 2 (22.2) | 30 (66.7) | 15 (33.3) | 13.333 | <0.001 |
| Positive | 7 (77.8) | 6 (22.2) | 21 (77.8) | ||
| M [P25, P75] | 20 [2.9–50] | 0 [0–0.8] | 1 [0–2.75] | −3.638 | <0.001 |
| PD-L1 CPS | |||||
| Negative | 1 (11.1) | 16 (59.3) | 11 (40.7) | 1.481 | 0.22 |
| Positive | 8 (88.9) | 20 (44.4) | 25 (55.6) | ||
| M [P25, P75] | 30 [4.5–60] | 1 [0.8–1] | 3 [0.8–8] | −3.520 | <0.001 |
| CD3+ T cell expression | |||||
| <5% | 2 (22.2) | 16 (61.5) | 10 (38.5) | 2.167 | 0.14 |
| ≥5% | 7 (77.8) | 20 (43.5) | 26 (56.5) | ||
| M [P25, P75] | 5.0 [3.5–30] | 5 [2–10] | 12.5 [3–23.75] | −3.613 | <0.001 |
| CD8+ T cell expression | |||||
| <1% | 3 (33.3) | 14 (63.6) | 8 (36.4) | 2.356 | 0.13 |
| ≥1% | 6 (66.7) | 22 (44.0) | 28 (56.0) | ||
| M [P25, P75] | 1 [0.4–20] | 1 [0.2–3.75] | 3 [1–10] | −3.740 | <0.001 |
| Patient-related biomarkers | |||||
| NE (×109/L) | 4.22 [3.60–5.01] | 4.30 [3.34–5.46] | 3.51 [2.84–4.73] | −2.129 | 0.03 |
| LY (×109/L) | 1.40 [1.21–1.88] | 1.75 [1.25–2.06] | 1.52 [1.28–1.75] | −1.885 | 0.06 |
| PLT (×109/L) | 259.0 [173.0–318.5] | 247.5 [197.5–275.7] | 229.0 [188.5–279.3] | −1.571 | 0.12 |
| NLR | 3.44 [1.99–3.72] | 2.46 [1.91–3.13] | 2.28 [1.69–3.36] | −0.644 | 0.52 |
| PLR | 155.8 [106.7–232.3] | 142.4 [116.0–185.3] | 153.1 [105.7–210.2] | −0.628 | 0.53 |
Data are presented as n (%) or M [P25, P75]. pCR, pathological complete response; PD-L1, programmed cell death ligand 1; M, median; TPS, tumor proportion score; CPS, combined positive score; NE, neutrophil; LY, lymphocyte; PLT, platelet; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
In the baseline condition of preneoadjuvant therapy, 45 patients were available for analysis of the expression status of microenvironmental markers (five patients had insufficient tissue of preneoadjuvant therapy), among which 11 cases were MPR and 34 non-MPR. The positive expression rates of PD-L1 TPS/CPS were 81.8%, 11.8%, and 90.9%, 52.9%, respectively, P<0.001, P=0.057. The expression rates of CD3+ T cells ≥5% were 81.8% and 52.9% respectively, with P=0.18, while the expression rates of CD8+ T cells ≥1% were 63.6% and 61.8% respectively, with P>0.99.
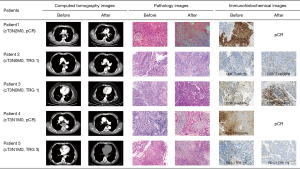
Compared to pre-treatment biopsy, there was a statistically significant increase in PD-L1 TPS (Z=−3.638; P<0.001), PD-L1 CPS (Z=−3.520; P<0.001), CD3+ T cells expression rate (Z=−3.613; P<0.001), and CD8+ T cells expression rate (Z=−3.740; P<0.001). The difference in pre-post NLR and PLR was not statistically significant (P>0.05). Only one patient had a dMMR tumor [also microsatellite instability (MSI)], with low PD-L1 expression (TPS 1%, CPS 2) and had an ypT3N3M0 tumor at surgery (TRG 3). Figure 2 shows CT images, pathology, and microenvironmental immunohistochemical images before and after neoadjuvant therapy in five patients.
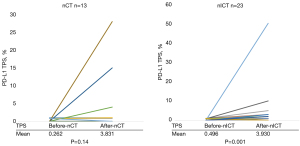
After treatment, the PD-L1 TPS and PD-L1 CPS were increased in 30.8% (4/13) and 46.2% (6/13) of patients in the nCT group and in 65.2% (15/23) and 87.0% (20/23) of patients in the nICT group, respectively. Upregulation was more pronounced in the nICT group (χ2=3.955; P=0.047) compared to the nCT group (χ2=5.009; P=0.03). Patients in the nICT group also had a high upregulation of CD3+, CD8+ T cells expression, and PLR (P<0.05) (Table 4 and Figure 3).
Table 4
| Feature | nCT (n=13) | nICT (n=23) | χ2 | P value |
|---|---|---|---|---|
| PD-L1 TPS | 3.955 | 0.047 | ||
| Not upregulated | 9 (69.2) | 8 (34.8) | ||
| Upregulated | 4 (30.8) | 15 (65.2) | ||
| PD-L1 CPS | 5.009 | 0.03 | ||
| Not upregulated | 7 (53.8) | 3 (13.0) | ||
| Upregulated | 6 (46.2) | 20 (87.0) | ||
| CD3+ T cells | 4.108 | 0.04 | ||
| Not upregulated | 8 (61.5) | 5 (21.7) | ||
| Upregulated | 5 (38.5) | 18 (78.3) | ||
| CD8+ T cells | 4.108 | 0.04 | ||
| Not upregulated | 8 (61.5) | 5 (21.7) | ||
| Upregulated | 5 (38.5) | 18 (78.3) | ||
| NLR | 0.002 | 0.97 | ||
| Not upregulated | 8 (61.5) | 14 (60.9) | ||
| Upregulated | 5 (38.5) | 9 (39.1) | ||
| PLR | 4.760 | 0.03 | ||
| Not upregulated | 10 (76.9) | 9 (39.1) | ||
| Upregulated | 3 (23.1) | 14 (60.9) |
Data are presented as n (%). nCT, neoadjuvant chemotherapy; nICT, neoadjuvant immunochemotherapy; PD-L1, programmed cell death ligand 1; TPS, tumor proportion score; CPS, combined positive score; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
Factors associated with outcome
Fifteen patients achieved MPR after neoadjuvant treatment. The rate of MPR was 66.7% and 14.3% in PD-L1 TPS-positive and -negative patients, respectively (χ2=11.338; P=0.001). The correlation between clinicopathological characteristics and MPR was analyzed in the binary logistic regression analysis in these 50 patients. The independent variables included: the maximum tumor length under endoscopy (<5 vs. ≥5 cm), tumor diameter (<1.8 vs. ≥1.8 cm), cT staging (cT1 + cT2 vs. cT3 + cT4), cN staging (cN0 vs. cN+), degree of differentiation (poorly differentiated vs. moderately-to-well differentiated), interval between neoadjuvant therapy and surgery (<5.2 vs. ≥5.2 weeks), PD-L1 expression (negative vs. positive), CD3+ T cell expression rate (<5% vs. ≥5%), CD8+ T cell expression rate (<1% vs. ≥1%), and neoadjuvant therapy (nCT vs. nICT). Univariate analysis showed that tumor differentiation, PD-L1 TPS, PD-L1 CPS, and neoadjuvant therapy correlated with postoperative MPR. Among these factors, moderately-to-well differentiated tumors, high TPS score, and nICT had positive correlations with the postoperative MPR. After adjustment of covariates, the variables retained in the model included tumor differentiation and PD-L1 TPS (Table 5). The MPR rates were 44.0% or 16.0% in tumors with moderately-to-well or poor differentiation, respectively [odds ratio (OR) =17.608; 95% confidence interval: 3.160–98.101; P=0.001]. The negative and positive PD-L1 TPS subgroups had MPR rates of 14.3% and 66.7%, (OR =6.887; 95% confidence interval: 1.204–39.413; P=0.03). The similar results were observed in the nICT subgroup.
Table 5
| Characteristic | n | MPR rate (%) | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | ||||
| Tumor length under endoscope# | 0.38 | ||||||||
| <5 cm | 18 | 38.9 | 1 | ||||||
| ≥5 cm | 30 | 26.7 | 0.571 | 0.164–1.987 | |||||
| Tumor diameter | 0.76 | ||||||||
| <1.8 cm | 25 | 28.0 | 1 | ||||||
| ≥1.8 cm | 25 | 32.0 | 1.210 | 0.360–4.065 | |||||
| cT stage | 0.62 | ||||||||
| cT1 + cT2 | 8 | 37.5 | 1 | ||||||
| cT3 + cT4 | 42 | 28.6 | 0.667 | 0.137–3.237 | |||||
| cN stage | 0.21 | ||||||||
| cN0 | 30 | 36.7 | 1 | ||||||
| cN+ | 20 | 20.0 | 0.432 | 0.115–1.622 | |||||
| Tumor differentiation | 0.04 | 0.03 | |||||||
| Poor | 25 | 16.0 | 1 | 1 | |||||
| Moderately-to-well | 25 | 44.0 | 4.125 | 1.092–15.585 | 6.887 | 1.204–39.413 | |||
| Interval between neoadjuvant therapy and surgery | 0.76 | ||||||||
| <5.2 weeks | 25 | 28.0 | 1 | ||||||
| ≥5.2 weeks | 25 | 32.0 | 1.210 | 0.360–4.065 | |||||
| PD-L1 TPS | 0.001 | 0.001 | |||||||
| Negative | 35 | 14.3 | 1 | 1 | |||||
| Positive | 15 | 66.7 | 12.000 | 2.868–50.212 | 17.608 | 3.160–98.101 | |||
| PD-L1 CPS | 0.04 | ||||||||
| Negative | 18 | 11.1 | 1 | ||||||
| Positive | 32 | 40.6 | 5.474 | 1.072–27.951 | |||||
| CD3+ T-cell expression rate | 0.10 | ||||||||
| <5% | 19 | 15.8 | 1 | ||||||
| ≥5% | 31 | 38.7 | 3.368 | 0.807–14.066 | |||||
| CD8+ T-cell expression rate | 0.66 | ||||||||
| <1% | 21 | 33.3 | 1 | ||||||
| ≥1% | 29 | 27.6 | 0.762 | 0.225–2.578 | |||||
| Treatment | 0.03 | ||||||||
| nCT | 14 | 7.1 | 1 | ||||||
| nICT | 36 | 38.9 | 6.960 | 1.214–39.890 | |||||
#, in the neoadjuvant immunochemotherapy group, two patients had no measurements due to failed endoscopic examination from esophageal stenosis. MPR, major pathological response; ESCC, esophageal squamous cell carcinoma; CI, confidence interval; PD-L1, programmed cell death ligand 1; TPS, tumor proportion score; CPS, combined positive score; nCT, neoadjuvant chemotherapy; nICT, neoadjuvant immunochemotherapy.
Survival analysis
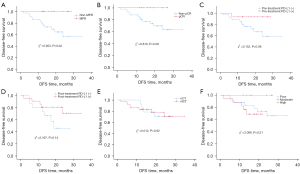
At a medium follow up of 32 months, all patients completed treatment and were alive. Twelve patients recurred, all in the non-MPR group, including 8 and 4 patients in the nICT and nCT groups, respectively. Most of these patients had negative (11 patients <1%) or low PD-L1 TPS (1 patient =1%) at diagnosis. In these 12 patients, 3, 3, 5, and 1 patients recurred in regional lymph node, distant metastasis, regional lymph node and distant metastasis, and tumor bed recurrence, respectively. The median disease-free survival (DFS) for all patients was 18.0 months, with 1- and 2-year DFS rates of 85.8% and 74.6%, respectively; moreover, the 1- and 2-year DFS rates were 100% and 100%, respectively, in the MPR group, versus 79.6% and 56.4%, respectively, in the non-MPR group (χ2=5.953; P=0.02). There were no significant differences in DFS in the subgroup analyses based on pCR, pretreatment PD-L1 TPS expression, posttreatment PD-L1 TPS expression, neoadjuvant therapy type, or different tumor differentiation degrees (P>0.05) (Figure 4). Cox regression model analysis showed that postoperative MPR was associated with DFS (P=0.03), whereas age, neoadjuvant therapy, tumor location, family history, tumor differentiation degree, tumor maximum length under endoscopy, tumor diameter, cT stage, cN stage, interval between neoadjuvant therapy and surgery, and tumor microenvironmental features before and after treatment (PD-L1 protein expression, CD3+ T cell expression, and CD8+ T cell expression) had no significant correlation with DFS (P>0.05).
Discussion
Esophageal cancer is a common gastrointestinal malignant tumor with high morbidity and mortality. China accounts for more than half of the incidence of esophageal cancer and more than half of the cancer related deaths (16-18). Currently, treatment of locally advanced esophageal cancer include chemotherapy alone or in combination with radiation therapy followed by surgery. The role of molecular targeted therapy and immune therapy is still being studied. However, the prognosis remains poor, and the most optimized model for new adjuvant therapy still awaits exploration. In this study, we aimed to compare nCT and immunochemotherapy in terms of the effect on the TME, immune biomarkers and clinicopathological characteristics in an attempt to identify biomarkers of response.
In this study, 15 patients achieved MPR after neoadjuvant therapy, with 14 being from the nICT group (as shown in Table 2). Observations of baseline microenvironment indicators indicated that these patients exhibited a higher PD-L1 TPS/CPS positive expression rate and CD3+/CD8+ T cell expression rate in numerical terms (81.8% vs. 11.8%, 90.9% vs. 52.9%, 81.8% vs. 52.9%, and 63.6% vs. 61.8%). However, in the subsequent binary logistic regression analysis, we did not identify the expression of CD3+/CD8+ T cells as an influencing factor for MPR. In contrast, PD-L1 TPS exhibited significant statistical significance in both univariate and multivariate analyses. Therefore, patients with positive PD-L1 TPS expression may be a superior population for nICT in patients with ESCC.
The study further observed the microenvironment indicators of non-pCR patients before and after neoadjuvant therapy, and found that both nCT and nICT hand an upregulatory effect on TME indicators, including PD-L1 TPS/CPS and CD3+/CD8+ T cell expression. Compared with nCT, nICT had a more pronounced upregulation effect on the four indicators (P<0.05). Additionally, we observed a more significant upregulation of the host microenvironment indicator PLR by nICT. Regarding TILs, we knew from previous studies (19-21) that their positive expression was associated with higher cancer specific survival (CSS) rate and DFS period, the higher the level of TIL count with high expression of CD3, CD8, and FOXP3 in the tumor, the greater the survival benefit for patients. Previous study has shown that after nCT in locally advanced ESCC, the expression of PD-L1 and infiltration of CD8+ T cells in tumor tissue were significantly increased (22), indicating that nICT may be more effective for ESCC. In our findings, patients exhibited heightened CD3+/CD8+ T cell expression post-nICT, which undoubtedly enhanced TME-mediated anti-tumor immunity, potentially contributing to the enhanced efficacy of neoadjuvant immunotherapy. Concerning the upregulation of PD-L1 TPS/CPS expression, the effect on patient outcomes varied across studies (23-25). Generally, more studies (24-26) suggested that high PD-L1 expression was a negative prognostic factor, which might be related to adaptive immune resistance caused by the activation of the PD-1 pathway. However, this was precisely the mechanism of anti-PD-1/PD-L1 monoclonal antibodies in exerting anti-tumor efficacy. From this point of view, it may be a more reasonable treatment strategy for patients with high PD-L1 expression after neoadjuvant therapy to receive adjuvant treatment with immune checkpoint inhibitors.
From the perspective of surgical results, compared with nCT, nICT achieved higher rates of pCR and MPR. The pCR rates in the two groups were 7.1% and 22.2% (P=0.40), and the MPR rates were 7.1% and 38.9% (P=0.03), respectively, the latter was obviously better than the former. This result aligned with prior studies. For instance, in two prospective studies (3,4) the pCR rate of nCT for ESCC was only 2.2% to 2.9%. Relatively speaking, although there are currently no large-scale, long-term results for nICT, multiple phase II trials (8,27-30) have reported pCR rates of over 20% (25% to 36%). Therefore, it appeared that nICT may yield superior pathological outcomes compared to chemotherapy alone (in the results of our binary logistic multivariate analysis, nICT was not an independent influencing factor of MPR. We considered this may be related to the study’s small sample size).
In the context of subgroup analysis related to DFS, the study revealed that whether MPR was achieved after neoadjuvant therapy serves as an independent influencing factor. Additionally, we analyzed the correlation between the expression of PD-L1 and prognosis before and after neoadjuvant therapy in patients. The DFS of the PD-L1 TPS-positive group prior to neoadjuvant therapy was higher at both 1- and 2-year marks, exhibiting an increasing trend compared to the PD-L1 TPS-negative group (85.5% vs. 80.0% and 81.0% vs. 45.3%, respectively, P=0.08). After neoadjuvant therapy, a similar trend was observed in the expression of PD-L1 TPS and survival outcomes. The 1- and 2-year DFS rates for the TPS-positive group compared to the TPS-negative group were 85.6% vs. 81.3% and 81.5% vs. 46.7%, respectively, P=0.14. Of course, the interfering factor present in this outcome was that only a proportion of patients received immunoadjuvant therapy postoperatively. We believe that it is worthwhile to further investigate the necessity of immunoadjuvant therapy for patients who have not achieved pCR after neoadjuvant therapy and have high PD-L1 expression, as well as the potential survival benefits associated with it.
Our study has certain limitations. First, we employed a single-center, retrospective design and a small sample size. The results of the study thus need to be verified in randomized multicenter, phase III, clinical trials with larger sample sizes. Second, only a small number of immune microenvironment markers were studied, and they could not fully capture the TME remodeling that occurs following nICT. In addition, our study has a short follow up and survival data are not available Further follow up in needed.
Conclusions
In conclusion, we found that neoadjuvant therapy could upregulate PD-L1 expression levels, increase the abundance of tumor-infiltrating lymphocytes, and remodel the tumor immune microenvironment in patients with ESCC. nICT may exert a more significant remodeling effect than may nCT. The degree of tumor differentiation and the tumor tissue PD-L1 expression level before treatment could be used to predict pathological remission in these patients after neoadjuvant therapy and was found to be indirectly associated with patient prognosis. Our preliminary results suggest that nICT might be superior to nCT for treating patients with ESCC.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-828/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-828/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-828/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-828/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The Fourth Hospital of Hebei Medical University (No. 2024KS074). Informed consent was waived due to the retrospective study design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 2018;36:2796-803. [Crossref] [PubMed]
- Kato K, Ito Y, Daiko H, et al. A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study. J Clin Oncol 2022;40:238.
- Tang H, Wang H, Fang Y, et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy followed by minimally invasive esophagectomy for locally advanced esophageal squamous cell carcinoma: a prospective multicenter randomized clinical trial. Ann Oncol 2023;34:163-72. [Crossref] [PubMed]
- Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 2021;398:759-71. [Crossref] [PubMed]
- Yoon HH, Kato K, Raymond E, et al. LBA-1 RATIONALE-306: Randomized, global, placebo-controlled, double-blind phase 3 study of tislelizumab plus chemotherapy versus chemotherapy as first-line treatment for advanced or metastatic esophageal squamous cell carcinoma (ESCC). Ann Oncol 2022;33:S375. [Crossref] [PubMed]
- Wang ZX, Cui C, Yao J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell 2022;40:277-288.e3. [Crossref] [PubMed]
- Lu Z, Wang J, Shu Y, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ 2022;377:e068714. [Crossref] [PubMed]
- Luo H, Lu J, Bai Y, et al. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA 2021;326:916-25. [Crossref] [PubMed]
- Wang Z, Shao C, Wang Y, et al. Efficacy and safety of neoadjuvant immunotherapy in surgically resectable esophageal cancer: A systematic review and meta-analysis. Int J Surg 2022;104:106767. [Crossref] [PubMed]
- Liu J, Yang Y, Liu Z, et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer 2022;10:e004291. [Crossref] [PubMed]
- Qiao Y, Zhao C, Li X, et al. Efficacy and safety of camrelizumab in combination with neoadjuvant chemotherapy for ESCC and its impact on esophagectomy. Front Immunol 2022;13:953229. [Crossref] [PubMed]
- Huang B, Shi H, Gong X, et al. Comparison of efficacy and safety between pembrolizumab combined with chemotherapy and simple chemotherapy in neoadjuvant therapy for esophageal squamous cell carcinoma. J Gastrointest Oncol 2021;12:2013-21. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol 2017;12:36-42.
- Available online: https://www.cap.org/protocols-and-guidelines/cancer-reporting-tools/cancer-protocol-templates. Last accessed June 11, 2021: [EB/OL].
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Feng RM, Zong YN, Cao SM, et al. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond) 2019;39:22. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Sudo T, Nishida R, Kawahara A, et al. Clinical Impact of Tumor-Infiltrating Lymphocytes in Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 2017;24:3763-70. [Crossref] [PubMed]
- Noble F, Mellows T, McCormick Matthews LH, et al. Tumour infiltrating lymphocytes correlate with improved survival in patients with oesophageal adenocarcinoma. Cancer Immunol Immunother 2016;65:651-62. [Crossref] [PubMed]
- Stein AV, Dislich B, Blank A, et al. High intratumoural but not peritumoural inflammatory host response is associated with better prognosis in primary resected oesophageal adenocarcinomas. Pathology 2017;49:30-7. [Crossref] [PubMed]
- Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med 2021;384:1191-203. [Crossref] [PubMed]
- Zhang W, Pang Q, Zhang X, et al. Programmed death-ligand 1 is prognostic factor in esophageal squamous cell carcinoma and is associated with epidermal growth factor receptor. Cancer Sci 2017;108:590-7. [Crossref] [PubMed]
- Okadome K, Baba Y, Nomoto D, et al. Prognostic and clinical impact of PD-L2 and PD-L1 expression in a cohort of 437 oesophageal cancers. Br J Cancer 2020;122:1535-43. [Crossref] [PubMed]
- Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005;11:2947-53. [Crossref] [PubMed]
- Chen Z, Chen Q, Li S, et al. IL-12RB1: a novel immune prognostic biomarker for oral squamous cell carcinoma and linked to PD-1/PD-L1 expression in the tumor immune microenvironment. Ann Transl Med 2022;10:144. [Crossref] [PubMed]
- Yu YK, Meng FY, Wei XF, et al. Neoadjuvant chemotherapy combined with immunotherapy versus neoadjuvant chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2024; [Crossref]
- Yang P, Zhou X, Yang X, et al. Neoadjuvant camrelizumab plus chemotherapy in treating locally advanced esophageal squamous cell carcinoma patients: a pilot study. World J Surg Oncol 2021;19:333. [Crossref] [PubMed]
- Yang W, Xing X, Yeung SJ, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer 2022;10:e003497. [Crossref] [PubMed]
- Xing W, Zhao L, Zheng Y, et al. The Sequence of Chemotherapy and Toripalimab Might Influence the Efficacy of Neoadjuvant Chemoimmunotherapy in Locally Advanced Esophageal Squamous Cell Cancer-A Phase II Study. Front Immunol 2021;12:772450. [Crossref] [PubMed]


