
The different doses of sufentanil combined with nalmefene in bronchoscopy: a systematic review and meta-analysis
Highlight box
Key findings
• Sufentanil combined with nalmefene significantly improves vital signs and sedation during bronchoscopy, with 0.4 µg/kg as the optimal dose.
What is known and what is new?
• Effective analgesic and sedative strategies are needed for bronchoscopy due to patient discomfort.
• This study identifies the optimal dose for sufentanil combined with nalmefene, offering a balance between efficacy and safety, and highlighting its superiority over conventional therapy.
What is the implication, and what should change now?
• Clinicians should consider this combination, particularly the 0.4 µg/kg sufentanil dose, for enhancing patient experience and safety in bronchoscopy.
• Further research should expand on these findings, and clinical protocols should be updated to incorporate this evidence-based practice.
Introduction
Bronchoscopy examination is a common clinical diagnostic method that plays a crucial role in the diagnosis and treatment of respiratory system diseases, mediastinal lesions and oncologic conditions (1). However, due to its unique operational characteristics, the procedure often induces discomfort and pain in patients. To improve the success rate of examinations and patient comfort, effective analgesic and sedative strategies are needed. The use of opioid drugs can lead to adverse reactions such as nausea, vomiting, and itching postoperatively, which significantly prolong hospitalization. Sufentanil, a potent opioid analgesic, has been widely used in various clinical procedures to alleviate patient pain. Nalmefene, as a sedative medication, has advantages such as rapid onset, short duration of action, and minimal cardiovascular suppression, making it suitable for clinical sedation procedures (2). However, in bronchoscopy examinations, there is still controversy and uncertainty about the rational dosing of sufentanil and nalmefene to achieve optimal analgesia and sedation effects. In recent years, with the deepening development of pharmacology and clinical research, a study has focused on the combined use of sufentanil and nalmefene in bronchoscopy examinations and other invasive diagnostic procedures (3). A study has indicated that nalmefene can reduce the incidence of postoperative nausea and vomiting associated with sufentanil analgesia, promote gastrointestinal motility recovery, and effectively improve the consciousness scores of patients with opioid overdose (4).
During bronchoscopy examinations, the use of analgesic medications can easily lead to respiratory suppression and airway collapse. The combined use of sufentanil and nalmefene offers advantages in effectively reversing opioid-induced respiratory depression without compromising analgesic effects. Nalmefene can reduce the occurrence of post-sufentanil analgesia nausea and vomiting and promote gastrointestinal motility recovery (5). Nalmefene can also effectively improve consciousness scores in cases of opioid overdose. The occurrence of adverse reactions is directly related to the dosage of sufentanil (6). However, a comprehensive analysis report on the combined use of different doses of sufentanil and nalmefene in bronchoscopy examinations has not been reported.
As there may be variations in different patient populations undergoing bronchoscopy examinations, a meta-analysis can be conducted to explore the impact of different patient characteristics on the combined application, thereby providing guidance for personalized treatment plans. The purpose of this study was to systematically evaluate the effects of different doses of sufentanil combined with nalmefene in bronchoscopy examinations through a meta-analysis approach. We present this article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-848/rc).
Methods
Literature search strategy time range: From database inception to 2022, a search was conducted in the following databases (English and Chinese): PubMed, Embase, Web of Science, Cochrane Library, China National Knowledge Infrastructure (CNKI), and Wanfang. The specific search strategy was as follows: (“Different dose sufentanil” OR “Varied sufentanil dosage”) AND (“nalmefene” OR “nalmefene combination”) AND (“Bronchoscopy” AND “Respiratory endoscopy”) AND (“Combined sedation” OR “Sedation combination”) AND (“Bronchoscopy” OR “Respiratory endoscopy”).
Inclusion and exclusion criteria
Literature inclusion criteria
(I) Review, clinical randomized controlled trial (RCT), patients with no history of cardiovascular, hepatic, renal, cerebral, psychiatric, or neurological diseases; all patients exhibited oxygen saturation (SpO2) >95% after preoperative oxygen inhalation. The experimental group underwent bronchoscopy with different doses of combined sufentanil and nalmefene, within a specified dose range. The control group received standard treatments, which could be routine care, no treatment, or bronchoscopy with either the same dose combination of sufentanil and nalmefene or other combined drugs. (II) Age between 18 and 65 years. (III) No allergy history to the drugs used in this study. (IV) Capacity to physically tolerate the examinations. (V) All patients and their family members in the included literature signed informed consent forms.
Exclusion criteria for articles
(I) Conference abstracts, case reports, duplicate publications. (II) Literature lacking original research data and inaccessible original materials. (III) Literature with insufficient or inappropriate experimental descriptions. (IV) Studies involving the same dose combination of sufentanil and nalmefene do not meet the requirements. (V) Studies using treatment methods other than sufentanil in combination with nalmefene. (VI) Individuals with severe physical illnesses, a history of mental health disorders receiving medication, or those with suicidal tendencies who refused to participate.
Outcome measures
(I) Vital signs: parameters [systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), SpO2] recorded at induction (T0), after laryngeal mask insertion (T1), immediately after reaching the carina with the fiberoptic bronchoscope (T2), and 10 minutes after the start of the procedure (T3). (II) Sedation effect: evaluated using the modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale ranging from 0 to 5. (III) Intraoperative adverse events: Incidence of intraoperative hypotension [mean arterial pressure (MAP) ≤20% of baseline], movement, hypertension (MAP >20% of baseline), bradycardia (HR <50 beats/min), and tachycardia (HR >100 beats/min).
Literature quality assessment
Assessments of the literature quality were conducted by two researchers independently, with results compared and discussed. In cases of disagreement, a third researcher participated in the discussion to reach a decision. The Cochrane Bias Risk Assessment tool was used for quality evaluation of RCTs and review, consisting of 6 items, each evaluated as “low bias risk”, “high bias risk”, or “unclear”. When all criteria were fully met, the likelihood of bias was minimal, indicated as Grade A. When some criteria were partially met, the likelihood of bias was moderate, indicated as Grade B. When none of the criteria were met, the likelihood of bias was higher, indicated as Grade C. The Newcastle-Ottawa scale (NOS) assessment tool was used to evaluate the review, whereby each aspect may have a different number of sub-items, and each sub-item can be assigned 0 or 1 point. The final score represented the sum of the sub-item scores in each aspect.
Statistical analysis
Meta-analyses were performed using the analysis module in RevMan 5.3 (Cochrane Collaboration, Copenhagen, Denmark). Analysis statistics for relative risk (RR) and standardized mean difference (SMD) were presented within a 95% confidence interval (CI). Prior to combining study results, I-square (I2) statistics and heterogeneity chi-square tests were used to assess statistical heterogeneity among included studies. Values of I2>50% or P<0.10 were considered indicative of significant heterogeneity between studies. When heterogeneity was present, a random-effects model was used to calculate the 95% CI for total RR or SMD scores. Otherwise, a fixed-effects model was used. All outcome evaluation indicators in this study were continuous variables represented by mean square deviations or weighted mean square deviations, along with a 95% CI.
Results
Search results
A total of 231 potentially relevant articles were initially identified. After removing duplicates using EndNote software (Clarivate Analytics, London, UK) and manual checks, 48 articles remained following the initial screening based on titles and abstracts. Further assessment of the full texts led to the secondary screening of 20 articles. A total of 13 articles that did not meet the inclusion criteria were excluded during this phase. Ultimately, six English-language articles were included in the final analysis. The flowchart of the literature selection process is illustrated in Figure 1.
Quality assessment of included studies
Among the six articles included in this study, four articles were of high methodological quality, graded as Level A; one article had a moderate quality, graded as Level B. A total of three articles provided detailed descriptions of their specific methods, one article reported concealed allocation methods, and one article had comparable outcome indicators (Figure 2). The remaining one article was directly evaluated using NOS; for specific details, refer to Table 1.
Table 1
| References | Year | Location | Sample size | Age (years) | Vital sign parameters outcome | The dose of fentanyl used in the intervention group | Adverse reactions | Ricker SAS† | The Cochrane Bias Risk Assessment tool | NOS scale |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen P (7) | 2021 | China | 32/34 | 18–65 | ①②③ | 0/0.2/0.4/0.8/1.0 μg/kg | Hypertension, tachycardia, hypotension, bradycardia, nausea, coughing, hypoxemia | 3:2 | A | – |
| He J (8) | 2023 | China | 45/45 | 18–65 | ①②④ | 0.2/0.4/0.8/1.0/1.2 μg/kg | Hypertension, tachycardia, hypotension, bradycardia, nausea, coughing, hypoxemia | 3:5 | A | – |
| Li MY (9) | 2020 | China | 65/66 | 35–65 | ①② | 0.2/0.4/0.8 μg/kg | Hypertension, tachycardia, coughing, hypoxemia | 4:1 | A | – |
| Torralva R (10) | 2019 | Germany | 65/67 | 18–65 | ①②④ | 0.2/0.4/0.8 μg/kg | Hypertension, tachycardia, hypotension, bradycardia, nausea, vomiting, coughing, hypoxemia | 5:1 | – | 6 |
| Zou Y (11) | 2020 | China | 70/75 | 30–70 | ①② | 0.2/0.4 μg/kg | Hypertension, tachycardia, hypotension, hypoxemia | 3:3 | B | – |
| Wong J (12) | 2019 | Singapore | 55/60 | 18–70 | ① | 0.4/0.8/1.0/1.2 μg/kg | Bradycardia, nausea, vomiting, coughing, hypoxemia | 5:2 | A | – |
①: systolic pressure; ②: diastolic pressure; ③: heart rate; ④: blood oxygen saturation (SpO2). †, SAS score (experimental group vs. control group); value scale: 1, 2, 3, 4, 5. SAS, sedation-agitation scale; NOS, Newcastle-Ottawa quality assessment scale.
Basic characteristics of included studies
A total of 6 articles were included, with a combined total of 774 study participants. Among them, 147 participants were involved in the before-and-after self-controlled trials. The control group received conventional treatment, whereas the intervention group received various doses of combined sufentanil and nalmefene intervention. Specific details regarding the basic information of the included studies can be found in Table 1.
Effect of sufentanil-nalmefene combination therapy on vital signs in patients undergoing bronchoscopy
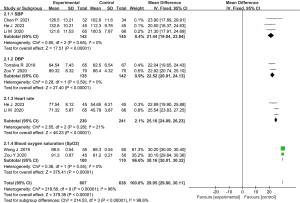
All six of the included studies (7-12) reported the use of different doses of sufentanil-nalmefene combination therapy for bronchoscopy. Through a meta-analysis by comparing various parameters (SBP, DBP, HR, SpO2) from vital sign monitors at different time points for patients, it was observed that the sufentanil-nalmefene combination therapy exhibited significant differences compared to conventional treatment in terms of SBP (MD =21.44, 95% CI: 19.04–23.84, P<1×10–5), DBP (MD =22.52, 95% CI: 20.91–24.13, P<1×10–5), HR (MD =25.16, 95% CI: 24.09–26.23, P<1×10–5), and SpO2 (MD =30.16, 95% CI: 30.01–30.32, P<1×10–5) (Figure 3). Subgroup analysis of different doses shows that the combined effect of 0.4 µg/kg was the most effective.
Sedative effect of sufentanil-nalmefene combination therapy
A total of four studies (8,9,10,12) reported the sedative effect of different doses of sufentanil-nalmefene combination therapy during bronchoscopy. The data exhibited relatively high heterogeneity (I2=45, P=0.14), leading to the use of a random-effects model. By comparing the level of sedation among patients through a meta-analysis, it was observed that the sedative effect of sufentanil-nalmefene combination therapy had a significant difference compared to conventional treatment (MD =23.42, 95% CI: 22.01–24.83, P<1×10–5). Sufentanil-nalmefene combination therapy was notably superior to conventional or no-treatment groups (Figure 4).

Comparison of adverse event occurrence rates analysis
An analysis was conducted based on adverse events including hypertension, tachycardia, hypotension, bradycardia, nausea and vomiting, coughing, hypoxemia, and Ricker sedation-agitation scale (SAS score). The meta-analysis results indicated that different doses of sufentanil-nalmefene combination therapy, in comparison to conventional treatment, exhibited significantly lower incidence rates of hypertension (MD =0.87, 95% CI: 0.41–3.17, P=0.001) and tachycardia (MD =0.45, 95% CI: 0.17–1.82, P<0.001). Furthermore, the SAS score was significantly lower in the sufentanil-nalmefene combination therapy group compared to the conventional treatment group, with statistically significant differences (MD =0.76, 95% CI: 0.04–2.17, P=0.04). No statistically significant differences were observed in the occurrence rates of hypotension, bradycardia, nausea and vomiting, coughing, and hypoxemia (P>0.05) (Table 2). The subgroup analysis of different doses of sufentanil for adverse reactions showed that at a concentration of 0.4 µg/kg, only hypertension had a significant difference, whereas other adverse reactions in all aspects were lower than at 0.2 and 0.8 µg/kg. Therefore, adverse reactions were found to be the lowest at a concentration of 0.4 µg/kg.
Table 2
| Adverse reactions | Study | Heterogeneity | Result of meta-analysis | Different doses of sufentanil: 0.4 vs. 0.2 and 0.8 μg/kg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I2 | P value | MD (95% CI) | Z | P value | Study | MD (95% CI) | P value | ||||
| Cough | 7 | 25.0% | <0.001 | 1.52 (0.6, 3.11) | 1.78 | 0.07 | 3 | 0.32 (0.21, 0.81) | 0.43 | ||
| Hypotension | 7 | 0.0% | <0.001 | 0.99 (0.7, 3.18) | 1.57 | 0.14 | 3 | 0.40 (0.35, 0.65) | 0.31 | ||
| Tachycardia | 7 | 50.0% | 0.79 | 0.45 (0.17, 1.82) | 3.76 | <0.001 | 3 | 1.01 (0.65, 1.87) | 0.42 | ||
| Bradycardia | 6 | 32.0% | <0.001 | 1.22 (0.21, 3.81) | 1.45 | 0.18 | 2 | 1.21 (0.53, 1.51) | 0.44 | ||
| Nausea and vomiting | 6 | 0.0% | <0.001 | 1.88 (1.3, 2.79) | 0.23 | 0.80 | 2 | 1.34 (0.32, 1.76) | 0.43 | ||
| Hypertension | 3 | 44.9% | <0.001 | 0.87 (0.41, 3.17) | 3.45 | 0.001 | 2 | 1.98 (1.21, 2.03) | <0.001 | ||
| Hypoxemia | 5 | 45.5% | <0.001 | 3.32 (1.10, 4.26) | 2.32 | 0.055 | 4 | 0.41 (0.21, 0.54) | 0.32 | ||
| Riker sedation-agitation scale | 7 | 0.0% | <0.001 | 0.76 (0.04, 2.17) | 0.31 | 0.04 | 2 | 1.44 (1.32, 1.65) | 0.54 | ||
MD, mean difference; CI, confidence interval.
Discussion
Amid the patient-centered care trend, bronchoscopy techniques are widely recommended by the American College of Chest Physicians (13-16). These methods involve conscious sedation and laryngeal mask general anesthesia, which reduce patient discomfort, and improve satisfaction allowing spontaneous ventilation (17-19). The use of a triple-lumen laryngeal mask in bronchoscopy offers safety and stability compared to conscious sedation (20). The purpose of conducting this meta-analysis was to explore the application effects of different doses of sufentanil combined with nalmefene in bronchoscopy examinations and provide scientific and reliable guidance for anesthesia practices in bronchoscopy examinations (21). The research findings of this meta-analysis include the evaluation of the application effects of different doses of sufentanil combined with nalmefene in bronchoscopy examinations, the assessment of the quality of included studies, and the identification of the heterogeneity among included studies (22). The significance of this meta-analysis lies in its exploration of the effectiveness of different doses of sufentanil combined with nalmefene in bronchoscopy examinations and providing scientifically reliable guidance for anesthesia practice. Through a systematic analysis of 6 high-quality studies, a total of 2,423 patients were included in the study. This meta-analysis summarizes the impact of different doses of sufentanil combined with nalmefene on patient vital signs and sedation effects, offering valuable reference for clinicians. This study primarily discusses the impact of sufentanil-nalmefene combination therapy on patients’ vital signs during bronchoscopy. The sufentanil-nalmefene combination therapy during bronchoscopy may affect vital signs by altering HR, blood pressure, respiratory rate, and SpO2, necessitating close monitoring for patient safety. The investigation included 6 high-quality studies, with 4 graded as Level A, and 1 as Level B. These studies collectively involved 774 patients, of whom 147 participated in before-after self-controlled trials. The meta-analysis results are systematically ordered, each supported by its own scientific rationale.
This effect stems from the following factors: potential opioid-related adverse reactions, such as nausea, vomiting, and respiratory depression, which are scientifically valid reasons corroborated by prior research. The combination of sufentanil and nalmefene affects vital signs due to their pharmacological effects. Sufentanil reduces blood pressure and HR by decreasing sympathetic nervous system activity and can also lower respiratory rate by acting on the brainstem, affecting oxygen levels. The role of nalmefene, assumed to interact with sufentanil, would depend on its specific actions, potentially amplifying these effects. Additionally, this study found that combining sufentanil and nalmefene significantly reduces patients’ HR and blood SpO2, easing psychological pressure and anxiety during bronchoscopy (23).
Secondly, diverse sufentanil doses combined with nalmefene noticeably affect sedation levels, as assessed by the MOAA/S scale. The reason for this lies in the varying degrees of sedation induced by different sufentanil doses, a scientifically valid explanation supported by earlier studies. Variations in sedation effects are influenced by the dosage of the medication and individual physiological differences, such as genetics and health status, leading to diverse responses to the same sedative dose. Thirdly, the meta-analysis revealed substantial heterogeneity among the included studies, particularly concerning the impact of different sufentanil doses within subgroups (24). This heterogeneity arises from variations in patient populations undergoing bronchoscopy, which can influence the effects of combining different doses of sufentanil and nalmefene. Although these reasons are scientifically grounded based on prior studies or the current study itself, the complexity of the impact of different sufentanil doses within subgroups and the study heterogeneity may undermine the findings’ reliability. Consequently, further research is warranted to investigate this aspect and confirm result dependability. In terms of clinical practice and future research guidance, the meta-analysis offers robust scientific evidence to inform clinical decision-making and guide future research endeavors. By exploring optimal drug dosages and administration protocols, we can enhance the quality of bronchoscopy examinations, elevate patient satisfaction, and advance the development and application of this diagnostic technique (25,26). This drug enhances the procedure by reducing coughing and hypoxemia, decreasing patient discomfort, and lowering associated risks (27). However, the study did not assess potential adverse reactions such as respiratory depression or consciousness disturbances. However, in our clinical practice, we can observe a decrease in adverse reactions in patients. When a fiberoptic bronchoscope is inserted into the pharynx, it can stimulate tension and stimulate the oral-pharyngeal region and tracheal wall, raising stress levels and catecholamine hormone release, thereby increasing blood pressure (28,29). The sufentanil-nalmefene anesthesia regimen inhibits the central nervous system, promoting sedation and calmness while suppressing stress responses and cough reaction, resulting in more stable vital signs and easier operative exploration of the airways. However, significant hemodynamic fluctuations during the procedure could lead to adverse cardiovascular events. Sufentanil, a rapid-acting sedative and anesthetic, acts on GABAA receptors, inhibiting excitatory neurons (30). Its rapid metabolism by tissue esterases and short elimination half-life of about 0.75 hours ensure a high safety profile, even with prolonged or high-dose intravenous administration (31). The discussion on sedation effects centers on how dosage and individual differences affect sedation depth, with higher doses potentially leading to more adverse events. Adverse event discussions focus on the rate and severity of side effects, emphasizing the need for tailored dosing and vigilant monitoring to reduce risks and manage complications effectively.
Limitation
This article also has some limiting aspects, as detailed below:
- Scientific validity of the results: this article includes only seven English-language papers, all conducted in China, which may limit the generalizability of these results. Additionally, the study outcomes in this article may be influenced by publication bias and reporting bias, which could raise questions about the scientific validity of the results.
- Heterogeneity: the study results in this article exhibit heterogeneity, likely stemming from differences between the studies, such as variations in study populations, study designs, and research methodologies. Therefore, it is necessary to interpret the results cautiously and consider the reliability and generalizability of the findings.
- Sufentanil-nalmefene combination therapy: one option to improve the bronchoscopy experience is the use of sufentanil-nalmefene combination therapy. However, it is important to recognize that this approach may not be suitable for all patients, highlighting the need for careful patient selection.
- It is crucial to understand that analgesia and sedation methods are just one part of the bronchoscopy process. Other factors such as pre-procedural preparation, intra-procedural monitoring and care, as well as post-procedural observation and management, play integral roles in the patient’s overall experience and safety. This assessment guides the development of individualized bronchoscopy plans tailored to each patient’s unique requirements.
Conclusions
Different doses of sufentanil and nalmefene are both safe and effective for bronchoscopy procedures. The group administered with sufentanil demonstrated more stable hemodynamics and fewer adverse events during recovery compared to the nalmefene group, although the recovery time was slower. Analgesia and sedation during bronchoscopy play a crucial role and significantly enhance the patient experience and safety in endoscopic examinations. The sufentanil doses showed that adverse reactions were lowest at 0.4 µg/kg, with only hypertension significantly different compared to 0.2 and 0.8 µg/kg. Sufentanil-nalmefene combination therapy stands as an effective method for bronchoscopy analgesia and sedation, with the ability to notably reduce the occurrence rates of hypertension and tachycardia, as well as decrease the patients’ sedation-agitation scores.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-848/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-848/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-848/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guerzoni S, Pellesi L, Pini LA, et al. Drug-drug interactions in the treatment for alcohol use disorders: A comprehensive review. Pharmacol Res 2018;133:65-76. [Crossref] [PubMed]
- Ramaswamy AH, Shaikh SI. Comparison of dexmedetomidine-propofol versus fentanyl-propofol for insertion of laryngeal mask airway. J Anaesthesiol Clin Pharmacol 2015;31:217-20. [Crossref] [PubMed]
- Chai P, Boyer EW, Blohm E. Opioid Analgesics and Narcotic Antagonists. In: Ray SD, editor. Side Effects of Drugs Annual: A worldwide yearly survey of new data in adverse drug reactions. Volume 36. Elsevier; 2014.
- Maxwell LG, Tobias JD, Cravero JP, et al. Adverse effects of sedatives in children. Expert Opin Drug Saf 2003;2:167-94. [Crossref] [PubMed]
- Yamaoka TT, Auckburally A. Analgesia in veterinary patients—opioids part two. The Veterinary Nurse 2014;5:42-7. [Crossref]
- Zhong H, Wang Y, Wang Y, et al. Effects of 0.15% ropivacaine alone and combination with sufentanil on epidural labor analgesia and adverse reactions. Afr Health Sci 2023;23:569-75. [Crossref] [PubMed]
- Chen P, Zeng P, Gong Y, et al. Recommended dose of sufentanil during induction of general anesthesia to avoid coughing and drastic hemodynamic fluctuations in patients undergoing surgery. J Int Med Res 2021;49:300060521996143. [Crossref] [PubMed]
- He J, Luo W, Mei Y, et al. Nalmefene combined noninvasive positive-pressure ventilation in Chinese patients with chronic obstructive pulmonary disease coupled with type II respiratory failure: A meta-analysis. Medicine (Baltimore) 2023;102:e34624. [Crossref] [PubMed]
- Li MY, Chen C, Wang ZG, et al. Effect of Nalmefene on Delayed Neurocognitive Recovery in Elderly Patients Undergoing Video-assisted Thoracic Surgery with One Lung Ventilation. Curr Med Sci 2020;40:380-8. [Crossref] [PubMed]
- Torralva R, Janowsky A. Noradrenergic Mechanisms in Fentanyl-Mediated Rapid Death Explain Failure of Naloxone in the Opioid Crisis. J Pharmacol Exp Ther 2019;371:453-75. [Crossref] [PubMed]
- Zou Y, Ling Y, Wei L, et al. The Effect of a Small Priming Dose of Sufentanil on Sufentanil-Induced Cough. J Perianesth Nurs 2020;35:661-4. [Crossref] [PubMed]
- Wong J, Lee JSE, Wong TGL, et al. Fibreoptic intubation in airway management: a review article. Singapore Med J 2019;60:110-8. [Crossref] [PubMed]
- Criner GJ, Eberhardt R, Fernandez-Bussy S, et al. Interventional Bronchoscopy. Am J Respir Crit Care Med 2020;202:29-50. [Crossref] [PubMed]
- Dang X, Hu W, Yang Z, et al. Dexmedetomidine plus sufentanil for pediatric flexible bronchoscopy: A retrospective clinical trial. Oncotarget 2017;8:41256-64. [Crossref] [PubMed]
- Huang X, Ai P, Wei C, et al. Comparison of the Effects of Esketamine/Propofol and Sufentanil/Propofol on the Incidence of Intraoperative Hypoxemia during Bronchoscopy: Protocol for a Randomized, Prospective, Parallel-Group Trial. J Clin Med 2022;11:4587. [Crossref] [PubMed]
- Jia Z, Ren LX, Fan YT, et al. Observation of effective dosage of remimazolam tosilate used for moderate-to-deep sedation in fiberoptic bronchoscopy. Zhonghua Yi Xue Za Zhi 2021;101:813-6. [PubMed]
- Wang G, Zhu X, Wu J, et al. Efficacy of different doses of sufentanil in minimum alveolar concentration of sevoflurane in patients undergoing bronchoscopy. The Journal of Practical Medicine 2016;1852-4.
- Joskova M, Durdik P, Sutovska M, et al. Negative impact of anesthesia with midazolam, sufentanil, and propofol used in pediatric flexible bronchoscopy on the tracheal ciliary beat frequency in guinea pigs. J Pharmacol Sci 2020;142:165-71. [Crossref] [PubMed]
- Zhang W, Wang JL, Fu S, et al. Incidence of oxygen desaturation using a high-flow nasal cannula versus a facemask during flexible bronchoscopy in patients at risk of hypoxemia: a randomised controlled trial. BMC Pulm Med 2022;22:389. [Crossref] [PubMed]
- Pertzov B, Krasulya B, Azem K, et al. Dexmedetomidine versus propofol sedation in flexible bronchoscopy: a randomized controlled trial. BMC Pulm Med 2022;22:87. [Crossref] [PubMed]
- Palma CF, Mashina R, Chen C, et al. A Systematic Review and Meta-Analysis of Randomized Controlled Trials on Supine vs. Nonsupine Endotracheal Intubation. Crit Care Res Pract 2023;2023:5496368. [Crossref] [PubMed]
- Doulberis M, Sampsonas F, Papaefthymiou A, et al. High-flow versus conventional nasal cannula oxygen supplementation therapy and risk of hypoxia in gastrointestinal endoscopies: a systematic review and meta-analysis. Expert Rev Respir Med 2022;16:323-32. [Crossref] [PubMed]
- Ayoub CM, Rizk MS, Yaacoub CI, et al. Dueling fiberoptic bronchoscope techniques. Anesth Analg 2004;98:276-7. [PubMed]
- Roy A, Khanna P, Chowdhury SR, et al. The Impact of High-flow Nasal Cannula vs Other Oxygen Delivery Devices during Bronchoscopy under Sedation: A Systematic Review and Meta-analyses. Indian J Crit Care Med 2022;26:1131-40. [Crossref] [PubMed]
- Mohan A, Madan K, Hadda V, et al. Guidelines for diagnostic flexible bronchoscopy in adults: Joint Indian Chest Society/National College of chest physicians (I)/Indian association for bronchology recommendations. Lung India 2019;36:S37-89. [Crossref] [PubMed]
- De Roza MA, Quah KH, Tay CK, et al. Diagnosis of Peripheral Lung Lesions via Conventional Flexible Bronchoscopy with Multiplanar CT Planning. Pulm Med 2016;2016:5048961. [Crossref] [PubMed]
- Ryu HG, Lee CJ, Kim YT, et al. Preemptive low-dose epidural ketamine for preventing chronic postthoracotomy pain: a prospective, double-blinded, randomized, clinical trial. Clin J Pain 2011;27:304-8. [Crossref] [PubMed]
- Hutchings C, Yadav K, Cheung WJ, et al. A systematic review of sufentanil for the management of adults with acute pain in the emergency department and pre-hospital setting. Am J Emerg Med 2023;70:10-8. [Crossref] [PubMed]
- Kamali A, Sarkhosh S, Kazemizadeh H. Comparison the sedation effect and satisfaction of two combinations, dexmedetomidine and fentanyl with midazolam and fentanyl, in patients undergoing bronchoscopy. Journal of Contemporary Medical Sciences 2020;6:296. [Crossref]
- Jeppesen E, Pedersen CM, Larsen KR, et al. Music does not alter anxiety in patients with suspected lung cancer undergoing bronchoscopy: a randomised controlled trial. Eur Clin Respir J 2016;3:33472. [Crossref] [PubMed]
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013;68:i1-i44. [Crossref] [PubMed]




