
Long-term outcomes of the BalMedic bovine pericardial bioprosthetic valve in female patients ≤50 years: a multicenter retrospective study
Highlight box
Key findings
• The long-term results of the BalMedic bovine pericardial bioprosthetic valve in female patients under the age of 50 years were found to be comparable to those of similar competitor valves across different age groups.
What is known and what is new?
• There is considerable variability in the reporting of bioprosthetic valve long-term outcomes.
• The bovine pericardial bioprosthetic valve has been identified as a viable long-term solution for younger individuals.
What is the implication, and what should change now?
• Guideline recommendations for bioprosthetic valve replacement by age remain controversial. Our insights may provide data needed to revise the current guidelines for younger individuals.
Introduction
The etiology of heart valvular disease in China is multifaceted, and there is a high prevalence of rheumatic valvular disease (1). In contrast to developed countries where degenerative valvular disease is more prevalent, the occurrence of valvular disease in China is a relatively recent development, which has historically led to a lower utilization rate of biological valves (2). Nevertheless, as knowledge regarding complications associated with mechanical valves deepens, clinical experience with biological valves accumulates, and the emphasis on patients’ quality of life increases, there is a growing trend toward greater utilization of bioprosthetic valves, particularly in women planning for pregnancy (3-5).
The determination of the most suitable prosthetic heart valve for women of childbearing age remains equivocal in the literature, as both biologic and mechanical valve prostheses present significant drawbacks in terms of fetal and maternal morbidity (6). Nonetheless, studies have shown that biologic valve replacement yields superior clinical outcomes compared to mechanical valve replacement during pregnancy, as evidenced by lower rates of pregnancy loss, hemorrhage, and severe maternal morbidity (4,6). A systematic review and meta-analysis provided supporting evidence for bioprosthetic valves being the most favorable choice for women of reproductive age who express a desire for future pregnancy subsequent to undergoing mitral valve replacement (MVR) (7). Due to the potential drawbacks of bioprostheses for young women, the process of selecting prosthetic valves for women of childbearing age necessitates comprehensive pregnancy counseling and careful long-term planning.
The findings from our previous studies on medium long-term follow-up outcomes indicate that the BalMedic bovine pericardial bioprosthetic valve (Beijing Balance Medical Tech Co., Ltd., Beijing, China) is a dependable option for both aortic valve replacement (AVR) and MVR (8,9). Furthermore, the postoperative outcomes demonstrated noninferiority when compared to similar valves from other manufacturers (8,9). Balance Medical conducted a comprehensive long-term follow-up study on a large cohort of patients (8,9) who underwent bioprosthetic valve replacement in three medical centers, with a total of over 700 patients and more than 800 implanted valves. Specifically, the Affiliated Hospital of Qingdao University followed up with 264 patients from 2007 to 2015, during which 347 BalMedic bovine pericardial bioprosthetic valves were implanted. The First People’s Hospital of Yulin monitored 299 patients from 2005 to 2014, with a total of 336 valve implants. In the present study, we collected follow-up clinical data from three cardiac centers (The First People’s Hospital of Yulin, The Affiliated Hospital of Qingdao University, and Teda International Cardiovascular Hospital) to evaluate the postoperative survival time, incidence of structural valve deterioration (SVD), and other clinical outcomes in female patients aged 50 years and younger who underwent BalMedic bovine pericardial bioprosthetic valve replacement. The findings of this research will serve as a basis for the clinical validation of bioprosthetic valve selection in women of aged ≤50 years. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-441/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Our research was approved by the ethics committees of Teda International Cardiovascular Hospital (No. [2020]-1029-6). The Affiliated Hospital of Qingdao University and The First People’s Hospital of Yulin were informed and agreed. Individual consent was waived due to the retrospective nature of this study. BalMedic bovine pericardial bioprosthetic valve (Beijing Balance Medical Tech Co., Ltd.) obtained premarket approval from the China Food and Drug Administration in 2003.
We conducted a comprehensive search of hospital management systems and medical records to gather clinical data from a cohort of 86 female patients, all of whom were 50 years old or younger. These patients underwent the BalMedic bovine pericardial bioprosthetic valve replacement procedure, which included a total of 97 valves, including AVR, double valve replacement (DVR), MVR, and tricuspid valve replacement (TVR) (Figure 1) at the three different medical centers between the years 2004 and 2015. The study collected demographic data and clinical characteristics, including age, preoperative cardiac New York Heart Association (NYHA) classification, etiology, overall survival (OS), reoperation, SVD incidence, complications, and EuroQol five-dimension questionnaire (EQ-5D) score, in order to obtain clinical outcomes. Throughout the follow-up period, we made efforts to communicate with the patients or their family members to gather information regarding survival and subsequent reoperation. After the reoperation, the surviving patients who still had at least one BalMedic valve underwent electrocardiogram (ECG) and echocardiography. The majority of patients scheduled follow-up visits by phone to return to the First People’s Hospital of Yulin (Yulin, China), the Affiliated Hospital of Qingdao University (Qingdao, China), and the Teda International Cardiovascular Hospital (Tianjin, China) where the valve replacement surgery was performed, while a portion of patients sought follow-up care at local hospitals with accompanying hospital reports. For patients who were unable to attend hospital visits due to factors such as advanced age, economic constraints, limited mobility, or personal preference, our study staff conducted home visits to administer ECG and echocardiography. Two patients were lost to follow-up as a result of communication breakdown, while 97.7% of the patients (84/86) completed the follow-up.

Study endpoints
The primary endpoint of this study was the OS of female patients aged 50 years old and younger who underwent BalMedic bovine pericardial bioprosthetic valve replacement. Additionally, secondary endpoints included the occurrence of reoperation, SVD, and complications associated with bioprosthetic valve replacement, such as nonstructural valve deterioration (NSVD), bleeding, thrombus formation, and endocarditis. SVD, in the context of this research, was defined as dysfunction of the valve due to calcification, tearing, or degenerative changes, and was assessed using echocardiography throughout the follow-up period.
Statistical analysis
Statistical analysis was conducted using SAS v. 9.4 (SAS Institute) according to our previous description (8,9).
Results
A total of 86 female patients, with a mean age of 39.79±9.38 years, underwent the BalMedic bovine pericardial bioprosthetic valve replacement procedure. Within our cohort (Table 1), 76.74% (66/86) of patients underwent MVR, 6.98% (6/86) underwent AVR, 12.79% (11/86) underwent DVR, and 3.49% (3/86) underwent TVR. According to the NYHA classification, 70.93% (61/86) of patients were classified as class III–IV. The etiological investigation revealed that in 83.72% (72/86) of patients, valvulopathy was induced by rheumatic lesions, while in 4.65% (4/86) of patients, it was induced by degenerative injury. A total of 97 valves were used, including 19 mm (1/97, 1.03%), 21 mm (11/97, 11.34%), 23 mm (4/97, 4.12%), 25 mm (6/97, 6.19%), 27 mm (63/97, 64.95%), 29 mm (10/97, 10.31%), and 31 mm (2/97, 2.06%) sizes. The EQ-5D scores for patients who underwent MVR, AVR, and DVR were 0.799, 0.848, and 0.813, respectively. These results indicate that individuals who received a bioprosthetic valve replacement experienced a favorable quality of life (Table 1).
Table 1
| Variables | MVR (n=66) | AVR (n=6) | DVR (n=11) | TVR (n=3) | Total (n=86) |
|---|---|---|---|---|---|
| Age (years) | 40 [12, 50] | 42 [21, 50] | 40.6 [26, 50] | 27.3 [11, 49] | 37.5 [11, 50] |
| NYHA classification | |||||
| I | 0 (0.00) | 1 (16.67) | 0 (0.00) | 0 (0.00) | 1 (1.16) |
| I–II | 3 (4.55) | 0 (0.00) | 0 (0.00) | 1 (33.33) | 4 (4.65) |
| II | 12 (18.18) | 1 (16.67) | 1 (9.09) | 0 (0.00) | 14 (16.28) |
| II–III | 2 (3.03) | 1 (16.67) | 2 (18.18) | 1 (33.33) | 6 (6.98) |
| III | 35 (53.03) | 3 (50.00) | 7 (63.64) | 1 (33.33) | 46 (53.49) |
| IV | 14 (21.21) | 0 (0.00) | 1 (9.09) | 0 (0.00) | 15 (17.44) |
| Etiology | |||||
| Rheumatic | 59 (89.39) | 3 (50.00) | 9 (81.82) | 1 (33.33) | 72 (83.72) |
| Degenerative | 2 (3.03) | 2 (33.33) | 0 (0.00) | 0 (0.00) | 4 (4.65) |
| Infective endocarditis | 1 (1.52) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (1.16) |
| Others | 4 (6.06) | 1 (16.67) | 2 (18.18) | 2 (66.67) | 9 (10.47) |
| Valve size (mm) | |||||
| 19 | 0 (0.00) | 0 (0.00) | 1† (4.55) | 0 (0.00) | 1‡ (1.03) |
| 21 | 0 (0.00) | 4 (66.67) | 7† (31.82) | 0 (0.00) | 11‡ (11.34) |
| 23 | 0 (0.00) | 2 (33.33) | 2† (9.09) | 0 (0.00) | 4‡ (4.12) |
| 25 | 4 (6.06) | 0 (0.00) | 2† (9.09) | 0 (0.00) | 6‡ (6.19) |
| 27 | 55 (83.33) | 0 (0.00) | 7† (31.82) | 1 (33.33) | 63‡ (64.95) |
| 29 | 7 (10.61) | 0 (0.00) | 2† (9.09) | 1 (33.33) | 10‡ (10.31) |
| 31 | 0 (0.00) | 0 (0.00) | 1† (4.55) | 1 (33.33) | 2‡ (2.06) |
| EQ-5D | 0.799 [0.052, 0.0848] | 0.848 [0.848, 0.848] | 0.813 [0.675, 0.848] | – | – |
Data are presented as mean [minimum, maximum] or n (%). †, each of these patients underwent replacement of two valves, so 11 patients received a total of 22 valve replacements; ‡, each of 11 DVR patients underwent replacement of two valves, so 86 patients received a total of 97 valve replacements. MVR, mitral valve replacement; AVR, aortic valve replacement; DVR, double-valve replacement; TVR, tricuspid valve replacement; NYHA, New York Heart Association; EQ-5D, EuroQol five-dimension questionnaire.
Postoperative mortality
There were no bleeding events, infective endocarditis, or perioperative mortality observed within our cohort. Over the course of the follow-up period, a total of 21 patients (21/86, 24.4%) experienced mortality, with the primary cause being heart failure (4/86, 4.7%) as indicated in Table 2. The survival rates at 5 and 10 years postoperation are depicted in Figure 2, with the Kaplan-Meier curve illustrating rates of 97.56% and 71.93%, respectively. Because there were only seven patients in the 15-year statistics, the survival rate is not presented.
Table 2
| Variables | MVR (n=66) | AVR (n=6) | DVR (n=11) | TVR (n=3) | Total (N=86) |
|---|---|---|---|---|---|
| Perioperative death | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Follow-up | 66 | 6 | 11 | 1 | 84 |
| Loss to follow-up | 0 | 0 | 0 | 2 | 2 |
| Reoperation | 32/86 (37.2) | 0/86 (0.0) | 5/86 (5.8) | 0/86 (0.0) | 37 (43.0) |
| Postoperative death | 16/86 (18.6) | 2/86 (2.3) | 3/86 (3.5) | 0/86 (0.0) | 21 (24.4) |
| Cause of death | |||||
| Exclusion of cardiopathy | 2 | 1 | 0 | 0 | 3 |
| Heart failure | 3 | 0 | 1 | 0 | 4 |
| Unknown | 11 | 1 | 2 | 0 | 14 |
Data are presented as n (%), n, or n/N (%). MVR, mitral valve replacement; AVR, aortic valve replacement; DVR, double-valve replacement; TVR, tricuspid valve replacement.

Second surgery
A total of 37 patients (37/86, 43.0%) underwent reoperation, with 32 patients (32/86, 37.2%) undergoing MVR and 5 patients (5/86, 5.8%) undergoing DVR. As shown in Figure 3, the reoperation-free rates at 5 and 10 years were found to be 92.83% and 80.68%, respectively. These findings suggest that the patients included in our study exhibited a notably low reoperation rate within the first decade following bioprosthetic valve replacement. In addition, reoperation had no obvious effect on the OS of younger female patients of childbearing age who underwent BalMedic bovine pericardial bioprosthetic valve replacement.

SVD
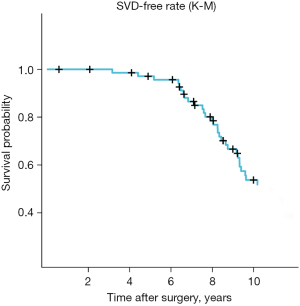
SVD has been employed as a significant determinant for subsequent surgical intervention; however, its precise delineation remains elusive. Consequently, certain patients afflicted with SVD may not require a secondary surgical procedure. SVD serves as an encompassing measure of the cumulative effects of postoperative occurrences that may precipitate valve malfunction. In this study, the diagnostic criteria for SVD were established in accordance with our previously delineated specifications (9). Figure 4 illustrates the comprehensive rates of SVD-free outcomes. Following surgical intervention, the implanted valves exhibited a notable level of stability over a period of 10 years (with SVD-free rates of 95.65% at 5 years and 51.82% at 10 years). In our study, the average duration of bioprosthetic valve replacement was 9.34±3.31 years. In addition, SVD did not exert any discernible impact on the OS outcomes among female patients of childbearing age who underwent BalMedic bovine pericardial bioprosthetic valve replacement (Figure 4).
Discussion
Although mechanical prosthesis remains favorable among younger individuals, there has been a significant lowering in the suggested age for the replacement of bioprosthetic valves in recent years (10,11). This shift is evidenced by a notable increase, from 14% to 47%, in the utilization of AVR in patients below the age of 50 years between the years 1997 and 2014 (12-14). Meanwhile, age was not found to be a significant independent predictor for SVD in the multivariable model (12-14), and the incidence and timing of SVD occurrence were found to be similar to previous study conducted on older age cohorts (15). According to recent research, bovine pericardial MVR may be a viable long-term solution for younger individuals, with results indicating that the overall occurrence of SVD leading to MVR to be 6.2% after 10 years and 9.0% after 12 years, with no significant variance observed among patients aged 40 to 70 years (16). The findings of another study indicate that the survival rates after 10 years for individuals under the age of 50 years who undergo bioprosthetic AVR are comparable to those who receive mechanical valves (13), suggesting that younger patients should be considered in the choice of bioprosthetic valves. This is due to the development of tissue treatment technology in anticalcification and antithrombosis, as well as the introduction of transcatheter valve applications. One of the most important extensions of placement of a bioprosthetic valve is the ability to place a transcatheter heart “valve in valve”, which involves putting another new tissue valve into the previous dysfunctional tissue valve. The stent of the destroyed valve can be used as a reliable anchor site of the new transcatheter valve. Keeping this in mind, surgical implantation of the largest possible bioprosthetic valve should be performed at the initial operation. With no thoracotomy surgery, the reimplantation of the artificial valve largely extends the overall lifetime of tissue valves.
Clinical studies have demonstrated that the reoperation rate subsequent to the initial valve replacement significantly impacts patient outcomes and quality of life (14,17). Moreover, delivery has been shown to be a significant risk factor in reoperation for both biologic (hazard ratio 2.5, 95% confidence interval 1.6–3.8 after time-dependent propensity matching) and mechanical (hazard ratio 2.3, 95% confidence interval 1.3–4.1 after time-dependent propensity matching) prostheses. Half of reoperations in women with mechanical valves who experience pregnancy occur within 1 year after delivery, and most are associated with mitral valve thrombosis. Despite the fact that pregnancy expedites the need for reoperation in both biologic and mechanical prostheses, it is worth noting that mechanical valves exhibit higher incidences of hemorrhage and severe maternal morbidity (6). Furthermore, it has been observed that younger patients and those who have received bioprosthetic valve implants are more prone to reoperation (18,19). In a study involving 288 patients who underwent MVR who had a mean age of 54.5±10.8 years (8), the reoperation-free rate at 10 years was 76.3%. In contrast, our study observed reoperation-free rates of 92.83% and 80.68% at 5 and 10 years, respectively. Despite the relatively younger age of patients in our cohort, the reoperation-free rate demonstrated comparable outcomes to those observed in older populations.
Corona et al. conducted a retrospective analysis on a cohort of 73 consecutive patients [only 21.9% (16/73) were female] aged ≤50 years who underwent bioprosthetic AVR. The findings revealed that the rates of freedom from SVD at 10 and 12.5 years were 73.5% and 41.9%, respectively (15). In our study, the rates of SVD-free at 10 years were 51.82%, which is consistent with previously reported research outcomes (15). Our findings suggest that BalMedic bovine pericardial bioprosthetic valves exhibit favorable durability even in younger individuals within the first decade.
Previous study has demonstrated that accelerated SVD occurs approximately 5 years post-implantation of biological valves, potentially contributing to decreased OS rates (20). In their investigation of 2,659 patients who underwent surgical AVR with the Carpentier-Edwards Perimount valve, Bourguignon et al. (21) revealed age-specific rates of freedom from reoperation due to SVD at 15 and 20 years. Specifically, the aged 60 years or less group exhibited rates of 70.8%±4.1% and 38.1%±5.6%, respectively, while those aged 60 to 70 years demonstrated rates of 82.7%±2.9% and 59.6%±7.6%. David and colleagues (22) demonstrated a 12-year freedom from SVD rate of 69%±4% for the entire study population, which decreased to 52%±8% for patients under the age of 65 years. In our study, the rates of freedom from SVD at 5 and 10 years were 95.65% and 51.82%, respectively. At first glance, the observed SVD rate in our study appears notably lower than that of rival products; however, it is important to note that our study recruiter has a relatively young average age of 37.5 years. Given the well-established inverse relationship between SVD rate and subject age, a direct comparison with competitors may not be entirely appropriate without a clinical head-to-head evaluation. We maintain confidence in the favorable clinical outcomes achieved with our BalMedic valve.
In our previous study, we conducted a single-center 14-year follow-up analysis of the BalMedic bovine pericardial bioprosthetic valve in a cohort of 299 patients (mean age 53.5 years, 59.86% female). The results revealed 5- and 10-year OS rates of 89.95% and 72.53%, respectively (9). In the present study that specifically consisted of women of childbearing age (age ≤50 years; mean age 39.79±9.38 years), we observed 5- and 10-year OS rates of 97.56% and 71.93%, respectively. The results of this study indicate that the use of BalMedic bovine pericardial bioprosthetic valve implantation may lead to a favorable survival outcome in younger female patients.
Despite these conclusions, most younger patients are concerned with the inevitable second replacement after 10 years’ implantation. As mentioned above, a valve-in-valve procedure via the transcatheter approach can address this issue, but this still involves several necessary preparations, such as using an expandable stent for the first surgical valve to avoid prosthesis-patient mismatch when the second transcatheter valve is implanted, or intentionally disrupted the valve in the catheterization laboratory in order to facilitate the implantation of the largest transcatheter valve available. On the other hand, this is attainable if two valves from the same or different manufacturers are used, meaning these valves are a comprehensive solution for the treatment for the younger patients.
Limitations
Our study encountered several limitations. First, based on a comprehensive analysis of studies published within the past two decades, it is evident that a significant degree of heterogeneity exists in the reporting of long-term outcomes related to bioprosthetic MVR (23). Firstly, our study only considered the BalMedic bovine pericardial bioprosthetic valve, and also restricted the gender and age, thus the number of patients recruited was smaller, potentially impacting the accuracy of the study results. Secondly, our data did not allow for the analysis of the influence of pregnancy on OS, reoperation rates, and SVD in women of childbearing age. Thirdly, it is important to note that the reported rate of reoperation attributed to SVD varies across different studies (24,25), and our study did not investigate the correlation between reoperation and SVD. Fourthly, although our study includes heterogeneous replacement locations, it does not affect the clinical outcomes. This further demonstrates the strong clinical adaptability of BalMedic products. Finally, the hemodynamics has not been investigated in our research. Evaluating the hemodynamics of BalMedic valve poses challenges due to variations in design, material composition, and anti-calcification treatments. Hemodynamics, which can be influenced by the implantation process and exhibit dynamic fluctuations, is intricately linked to clinical outcomes, particularly SVD. Given the complexity of studying hemodynamics, this study focuses on reviewing three key clinical outcomes in patients with BalMedic valve implants.
Conclusions
Female patients younger than 50 years of age who had undergone BalMedic bovine pericardial bioprosthetic valve implantation demonstrated satisfactory OS, reoperation-free survival, and SVD-free rates when compared to those treated with similar valves from other manufacturers and those examined in our previous studies. The utilization of BalMedic bovine pericardial bioprosthetic valves may result in a favorable survival prognosis for younger patients. These findings indicate that bioprosthetic aortic valves offer a viable alternative to mechanical valve replacement for women of childbearing age.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-441/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-441/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-441/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-441/coif). A.M.Q. is a consultant for W. L. Gore and Associates, Medtronic Inc. and B. Braun, and has received consulting fees and support for attending meetings from them, not related to the submitted article. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was also reviewed and approved by the ethics committees of Teda International Cardiovascular Hospital (No. [2020]-1029-6). The Affiliated Hospital of Qingdao University and The First People’s Hospital of Yulin were informed and agreed. Individual consent was waived due to the retrospective nature of this study. The tissue valves implanted are all products that obtained premarket approval from the China Food and Drug Administration.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yang Y, Wang Z, Chen Z, et al. Current status and etiology of valvular heart disease in China: a population-based survey. BMC Cardiovasc Disord 2021;21:339. [Crossref] [PubMed]
- Coffey S, Roberts-Thomson R, Brown A, et al. Global epidemiology of valvular heart disease. Nat Rev Cardiol 2021;18:853-64. [Crossref] [PubMed]
- Bouhout I, Poirier N, Mazine A, et al. Cardiac, obstetric, and fetal outcomes during pregnancy after biological or mechanical aortic valve replacement. Can J Cardiol 2014;30:801-7. [Crossref] [PubMed]
- Kataoka G, Asano R, Sato A, et al. Outcomes of prosthetic valve replacement in women of child-bearing age. Surg Today 2017;47:755-61. [Crossref] [PubMed]
- Siu SC, Lam M, Le B, et al. Morbidity in Pregnant Women with a Prosthetic Heart Valve. Am J Obstet Gynecol MFM 2020;2:100105. [Crossref] [PubMed]
- Batra J, Itagaki S, Egorova NN, et al. Outcomes and Long-term Effects of Pregnancy in Women With Biologic and Mechanical Valve Prostheses. Am J Cardiol 2018;122:1738-44. [Crossref] [PubMed]
- Grashuis P, Khargi SDM, Veen K, et al. Pregnancy outcomes in women with a mitral valve prosthesis: A systematic review and meta-analysis. JTCVS Open 2023;14:102-22. [Crossref] [PubMed]
- Yang S, Hu H, Lin M, et al. Medium long-term follow-up outcomes of BalMedic(®) bovine pericardial bioprosthetic valve in the mitral position. J Thorac Dis 2021;13:3652-9. [Crossref] [PubMed]
- Lin M, Gan N, Chen J, et al. A single-center 14-year follow-up study of the BalMedic(®) bovine pericardial bioprosthetic valve. Ann Transl Med 2020;8:692. [Crossref] [PubMed]
- Head SJ, Çelik M, Kappetein AP. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J 2017;38:2183-91. [Crossref] [PubMed]
- David T. How to Decide Between a Bioprosthetic and Mechanical Valve. Can J Cardiol 2021;37:1121-3. [Crossref] [PubMed]
- Hirji SA, Kolkailah AA, Ramirez-Del Val F, et al. Mechanical Versus Bioprosthetic Aortic Valve Replacement in Patients Aged 50 Years and Younger. Ann Thorac Surg 2018;106:1113-20. [Crossref] [PubMed]
- Schnittman SR, Adams DH, Itagaki S, et al. Bioprosthetic aortic valve replacement: Revisiting prosthesis choice in patients younger than 50 years old. J Thorac Cardiovasc Surg 2018;155:539-547.e9. [Crossref] [PubMed]
- Harky A, Suen MMY, Wong CHM, et al. Bioprosthetic Aortic Valve Replacement in <50 Years Old Patients - Where is the Evidence? Braz J Cardiovasc Surg 2019;34:729-38. [Crossref] [PubMed]
- Corona S, Manganiello S, Pepi M, et al. Bioprosthetic aortic valve replacement in patients aged 50 years old and younger: Structural valve deterioration at long-term follow-up. Retrospective study. Ann Med Surg (Lond) 2022;77:103624. [Crossref] [PubMed]
- Romano M, McCarthy PM, Baldridge AS, et al. Should mitral valve replacement age guidelines be lowered due to better bioprosthetic mitral valve durability? J Thorac Cardiovasc Surg 2023;S0022-5223(23)00968-6.
- Fuller SM, Borisuk MJ, Sleeper LA, et al. Mortality and Reoperation Risk After Bioprosthetic Aortic Valve Replacement in Young Adults With Congenital Heart Disease. Semin Thorac Cardiovasc Surg 2021;33:1081-92. [Crossref] [PubMed]
- Forcillo J, El Hamamsy I, Stevens LM, et al. The perimount valve in the aortic position: twenty-year experience with patients under 60 years old. Ann Thorac Surg 2014;97:1526-32. [Crossref] [PubMed]
- Bourguignon T, Lhommet P, El Khoury R, et al. Very long-term outcomes of the Carpentier-Edwards Perimount aortic valve in patients aged 50-65 years. Eur J Cardiothorac Surg 2016;49:1462-8. [Crossref] [PubMed]
- Montero Cruces L, Carnero Alcázar M, Pérez Camargo D, et al. 5-Year haemodynamic performance of three aortic bioprostheses. A randomized clinical trial. Eur J Cardiothorac Surg 2023;64:ezad261. [Crossref] [PubMed]
- Bourguignon T, Bouquiaux-Stablo AL, Candolfi P, et al. Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann Thorac Surg 2015;99:831-7. [Crossref] [PubMed]
- David TE, Feindel CM, Bos J, et al. Aortic valve replacement with Toronto SPV bioprosthesis: optimal patient survival but suboptimal valve durability. J Thorac Cardiovasc Surg 2008;135:19-24. [Crossref] [PubMed]
- Koulouroudias M, Di Mauro M, Lorusso R. Long-term outcomes of bioprosthetic valves in the mitral position a systematic review of studies published over the last 20 years. Eur J Cardiothorac Surg 2023;64:ezad384. [Crossref] [PubMed]
- Johnston DR, Soltesz EG, Vakil N, et al. Long-term durability of bioprosthetic aortic valves: implications from 12,569 implants. Ann Thorac Surg 2015;99:1239-47. [Crossref] [PubMed]
- Bourguignon T, El Khoury R, Candolfi P, et al. Very Long-Term Outcomes of the Carpentier-Edwards Perimount Aortic Valve in Patients Aged 60 or Younger. Ann Thorac Surg 2015;100:853-9. [Crossref] [PubMed]


