
Increased lung cancer recurrence following transthoracic needle biopsy
Highlight box
Key findings
• For patients with stage IA non-small cell lung cancer, pre-operative transthoracic needle biopsy (TNB) was a negative prognostic factor for recurrence.
What is known and what is new?
• Computed tomography-guided TNB could damage lung structures and may disseminate tumor cells into the airway, blood vessels, and pleural cavity, affecting post-operative outcomes.
• This study makes a novel contribution to the body of literature through the assessment of the effect of TNB on cancer recurrence.
What is the implication, and what should change now?
• Surgical diagnosis and treatment without pre-operative tissue diagnosis may be considered first in patients with clinically early lung cancer.
Introduction
Background
Lung cancer reigns as the world’s most common cancer, striking both men and women, and it remains the leading cause of cancer death (1,2). Recently, advances in medical technology, such as computed tomography (CT), have led to an increased detection of small pulmonary nodules. The National Comprehensive Cancer Network (NCCN) guidelines recommend that follow-up strategies for the incidental finding of nodules should be established depending on the characteristics of the nodules, such as the size of the nodule and the solid part as well as the patient’s risk factors. Further, the NCCN recommends that interventional radiologists, thoracic surgery experts, and interventional pulmonologists should discuss the safest and most effective method for biopsy if a biopsy is necessary (3).
Unlike surgical biopsy that requires general anesthesia, CT-guided transthoracic needle biopsy (TNB) that requires local anesthesia has been the first choice for conventional histologic diagnosis (4). However, TNB could cause severe complications (i.e., pneumothorax, hemorrhage, hemothorax, and air embolism), leading to neurologic sequelae or death (5,6). In theory, TNB could damage the lung structures and may disseminate tumor cells into the airway, blood vessels, and pleural cavity. Consequently, these methods may influence post-operative outcomes.
Rationale and knowledge gap
In terms of oncological outcomes, several studies have been conducted to investigate the effects of TNB procedures on the prognosis of patients, but the effects remain unclear (7-12). The degree of visceral pleural invasion was an important factor mentioned numerous times in previous studies that compared oncologic outcomes due to TNB (10-12). According to the modified Hammar classification of visceral pleural invasion, a PL0 tumor is one within the subpleural lung parenchyma or invading superficially into the pleural connective tissue beneath the elastic layer. PL1, PL2, and PL3 indicate invasion beyond the elastic layer, to the pleural surface, and into any component of the parietal pleura, respectively (13).
Objective
This retrospective study aimed to analyze the effects of TNB on the prognosis of patients with stage IA non-small cell lung cancer (NSCLC) with confirmed PL0 that excluded visceral pleural invasion. This study makes a novel contribution to the body of literature through the assessment of the effect of TNB on cancer recurrence. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-419/rc).
Methods
Patients
In this retrospective study, we enrolled 1,077 patients with pathologic stage IA NSCLC who underwent surgery at a single tertiary hospital from 2010 to 2020. The pathologic stage was decided based on the guidelines of the 8th edition of the International Association for the Study of Lung Cancer and the American Joint Committee on Cancer Stage Classification of NSCLC (14,15). Follow-up data were collected by additionally reviewing the thoracic surgery department’s database in September 2022.
Patients who received neoadjuvant therapy, underwent bilateral lung surgery for other reasons, or those without mediastinal lymph node (LN) dissection were excluded.
Post-operative follow-ups varied according to the surgeons. The first post-operation follow-up CT was performed at 1–3 months post-operatively, and regular follow-ups were performed every 3–6 months thereafter until the fifth year after surgery. Then, after the fifth year, CT follow-ups were performed at 1-year intervals. If a recurrent tumor was suspected on CT, a follow-up was performed more frequently to confirm tumor recurrence through additional CT workup or another modality, such as biopsy or positron emission tomography (PET)/CT.
The primary outcomes of this study were the locoregional recurrence and the overall recurrence, and the secondary outcome was TNB procedure-related complications.
TNB protocol
In our institution, TNB is considered for small peripheral nodules with a solid portion of less than 8 mm after consultation with the thoracic radiologist if there is no significant risk of pneumothorax and access is available. TNB is also performed when the patient wants tissue confirmation prior to surgical treatment.
For all TNBs, a multi-detector CT scanner (Siemens Definition AS Plus, Siemens Healthcare, Erlangen, Germany) with no CT fluoroscopy mode was used. All procedures with the coaxial technique were performed by the thoracic radiologist. Pre-procedural CT was performed before the biopsy to determine the most appropriate needle path. Then, the 18-gauge coaxial introducer was inserted under intermittent CT guidance. After checking that the needle tip was appropriately inserted into the target lesion, tissue biopsy was performed using a 20-gauge cutting needle (Stericut, TSK Laboratory, Tochigi, Japan). After an outer cannula of the coaxial needle was fixed in the lesion, multiple tissue samples were obtained from different locations of the tumor by rotating the angle of the biopsy needle as much as possible. After removing the coaxial introducer, immediate procedure-related complications were confirmed by performing post-procedural CT. Further, erect chest radiography was performed after approximately 2 h to check for the development of pneumothorax (16,17).
Definition of variables
Locoregional recurrence was defined as recurrence at the ipsilateral lung, ipsilateral pleural seeding, malignant pleural effusion, or regional and mediastinal LN on the same side as the surgical site. Overall recurrence included distant recurrence, which was defined as the recurrence in the contralateral lung and outside the thoracic cavity, and locoregional recurrence. Recurrence was confirmed by cytological examination of pleural fluid or histological examination of biopsy material and when malignancy was suspected based on the readings of at least two radiologists owing to the increased number and size on the follow-up CT scan. Patients who did not experience a recurrence at the most recent workup were considered to be recurrence-free. Recurrence-free survival (RFS) was defined as the period between the date of surgery and the date of recurrence.
Statistical analysis
The baseline characteristics of each group were analyzed using the independent t-test for continuous variables and the Chi-squared and Fisher’s exact test for categorical variables. Continuous variables are presented as means and standard deviations, and categorical variables are presented as frequencies and percentages. The normality of individual parameter distributions was assessed using the Shapiro-Wilk test. The propensity scores, which were calculated from the logistic regression models, included the following variables: age, sex, history of smoking, total tumor size, invasive tumor size, histology, extent of resection, histologic differentiation, microscopic lymphatic invasion, and microscopic vascular invasion. Through the matching procedure for propensity scores, the no-TNB and TNB groups showed similar distributions of propensity scores, indicating that the differences in covariates between the two groups were minimized. We matched the scores using optimal methods. For comparison between the matched groups, Student’s t-test or the Wilcoxon rank-sum test was used to compare continuous variables, depending on the normality of distribution. The Kaplan-Meier method was used to estimate the locoregional RFS, overall RFS, and distant RFS, and the log-rank test was used to evaluate them. For the visualization of the locoregional recurrence distribution, a kernel smoothing method was used to estimate recurrence hazard rate. In both the univariable and multivariable analyses, the Cox proportional hazards model was used to evaluate the prognostic factors for recurrence in matched patients. Following elimination of correlated factors, independent variables with P<0.20 in univariable analysis were included in the initial multivariate Cox model. The final multivariable model (Model 1) was chosen using a backward stepwise selection procedure (P≤0.10 for entering and P≤0.05 for staying in the model).
All statistical analyses were performed using R Statistical Software (v4.2.2; R Core Team 2022, Vienna, Austria) using the MatchIt (v4.5.0; Ho et al. 2011), ggplot2 (v.3.4.0; Wickham 2016), and moonBook (v.0.3.1; Moon 2015) packages. Statistical significance was set at P<0.05.
Ethical statement
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Catholic University College of Medicine (No. KC22RISI0761), and individual consent for this retrospective analysis was waived.
Results
Patient characteristics
According to our exclusion criteria, patients who received neoadjuvant treatment (n=32); patients who underwent bilateral lung surgery due to previous lung double primary lung cancer (n=13); and patients who did not undergo node dissection (n=19) were excluded from the study. A total of 1,013 patients were included; they were assigned to the group that underwent TNB (n=190) (TNB group) or the group that did not undergo TNB (n=823) (no-TNB group) in a 1:2 ratio using the propensity score matching (PSM) method (Figure 1).
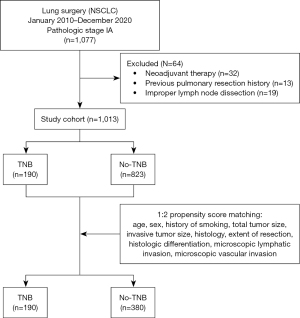
Table 1 shows the baseline demographics of the two groups before matching. In the TNB group, the mean tumor size was significantly larger (P<0.001), and more patients underwent lobectomy than those who underwent sublobar resection (P<0.001). The two groups that were matched showed good matches (Table 2). The mean follow-up durations in the no-TNB and TNB groups were 51.1±27.4 and 61.5±36.3 months, respectively.
Table 1
| Characteristics | No-TNB (N=823) | TNB (N=190) | P value |
|---|---|---|---|
| Age, years | 64.0±9.9 | 64.0±10.8 | 0.92 |
| Sex, male | 365 (44.3) | 88 (46.3) | 0.68 |
| Non-smoker | 525 (63.8) | 112 (58.9) | 0.25 |
| Extent of resection | <0.001 | ||
| Sublobar resection | 261 (31.7) | 22 (11.6) | |
| Lobectomy | 562 (68.3) | 168 (88.4) | |
| Cell type | 0.01 | ||
| Adenocarcinoma | 746 (90.6) | 160 (84.2) | |
| Non-adenocarcinoma | 77 (9.4) | 30 (15.8) | |
| Number of harvested LNs | 11.7±6.9 | 13.7±7.5 | 0.001 |
| Total tumor size, mm | 17.7±6.7 | 20.7±5.7 | <0.001 |
| Invasive tumor size, mm | 15.4±6.7 | 20.4±5.5 | <0.001 |
| Histologic differentiation | 0.003 | ||
| Well or moderately | 779 (94.7) | 168 (88.4) | |
| Poorly | 44 (5.3) | 22 (11.6) | |
| Microscopic vascular invasion | 37 (4.5) | 19 (10.0) | 0.005 |
| Microscopic lymphatic invasion | 187 (22.7) | 54 (28.4) | 0.12 |
Values are presented as numbers (%) or means ± standard deviations. NSCLC, non-small cell lung cancer; TNB, transthoracic needle biopsy; LN, lymph node.
Table 2
| Characteristics | No-TNB (N=380) | TNB (N=190) | P value | SMD |
|---|---|---|---|---|
| Age, years | 63.7±9.7 | 64.0±10.8 | 0.72 | 0.030 |
| Sex, male | 175 (46.1) | 88 (46.3) | >0.99 | 0.005 |
| Non-smoker | 230 (60.5) | 112 (58.9) | 0.79 | 0.032 |
| Extent of resection | 0.74 | 0.041 | ||
| Sublobar resection | 39 (10.3) | 22 (11.6) | ||
| Lobectomy | 341 (89.7) | 168 (88.4) | ||
| Cell type | 0.64 | 0.050 | ||
| Adenocarcinoma | 327 (86.1) | 160 (84.2) | ||
| Non-adenocarcinoma | 53 (13.9) | 30 (15.8) | ||
| Total tumor size, mm | 20.3±5.2 | 20.7±5.7 | 0.40 | 0.070 |
| Invasive tumor size, mm | 19.9±5.1 | 20.4±5.5 | 0.31 | 0.086 |
| Histologic differentiation | 0.46 | 0.050 | ||
| Well or moderately | 345 (90.8) | 168 (88.4) | ||
| Poorly | 35 (9.2) | 22 (11.6) | ||
| Microscopic vascular invasion | 28 (7.4) | 19 (10.0) | 0.36 | 0.088 |
| Microscopic lymphatic invasion | 101 (26.6) | 54 (28.4) | 0.71 | 0.041 |
| Follow-up (months) | 51.1±27.4 | 61.5±36.3 | 0.001 | – |
Values are presented as numbers (%) or means ± standard deviations. NSCLC, non-small cell lung cancer; TNB, transthoracic needle biopsy; PSM, propensity score matching; SMD, standardized mean difference.
Recurrence pattern
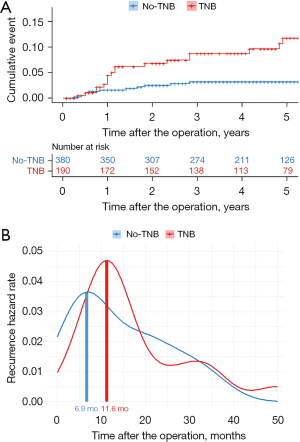
Locoregional recurrence was compared, and the 5-year locoregional RFS rates were 96.8% and 88.3% in the no-TNB and TNB groups, respectively (P=0.001) (Figure 2A). In addition, the 5-year overall RFS rates were 93.7% and 84.2% in the no-TNB and TNB groups, respectively (P=0.02) (Figure 2B). However, no significant difference was noted in the distant RFS between the two groups (P=0.15) (Figure 2C).

In addition, based on the multivariable analysis, history of TNB [hazard ratio (HR), 3.15; 95% confidence interval (CI): 1.49–6.67; P=0.003] and cell types (HR, 3.62; 95% CI: 1.68–7.76; P<0.001) were risk factors for locoregional recurrence (Table 3). Multivariable analysis of overall RFS demonstrated that the history of TNB was a risk factor (Table 4).
Table 3
| Characteristics | Locoregional recurrence-free survival | ||||
|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age, years | 1.01 (0.97–1.04) | 0.73 | |||
| Sex | |||||
| Female | 1 | Ref. | 1 | Ref. | |
| Male | 1.73 (0.83–3.62) | 0.15 | 1.03 (0.42–2.54) | 0.96 | |
| History of smoking | 1.47 (0.71–3.05) | 0.30 | |||
| Total tumor size, mm | 1.01 (0.94–1.08) | 0.77 | |||
| Invasive tumor size, mm | 1.02 (0.95–1.09) | 0.62 | |||
| Extent of resection | |||||
| Lobectomy | 1 | Ref. | |||
| Sublobar resection | 1.84 (0.70–4.83) | 0.22 | |||
| Cell type | |||||
| Adenocarcinoma | 1 | Ref. | 1 | Ref. | |
| Non-adenocarcinoma | 4.16 (1.96–8.80) | <0.001 | 3.62 (1.68–7.76) | <0.001 | |
| Histologic differentiation | |||||
| Well or moderately | 1 | Ref. | 1 | Ref. | |
| Poorly | 2.23 (0.85–5.85) | 0.10 | 0.87 (0.29–2.55) | 0.79 | |
| Microscopic lymphatic invasion | 1.55 (0.72–3.33) | 0.27 | |||
| Microscopic vascular invasion | 3.13 (1.27–7.69) | 0.01 | 2.27 (0.91–5.69) | 0.08 | |
| Number of harvested LNs | 1.03 (0.99–1.08) | 0.14 | 1.04 (0.99–1.08) | 0.12 | |
| Pre-operative TNB | 3.26 (1.54–6.90) | 0.002 | 3.15 (1.49–6.67) | 0.003 | |
NSCLC, non-small cell lung cancer; PSM, propensity score matching; HR, hazard ratio; CI, confidence interval; Ref., reference; LN, lymph node; TNB, transthoracic needle biopsy.
Table 4
| Characteristics | Recurrence-free survival | ||||
|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age, years | 1.02 (0.99–1.05) | 0.27 | |||
| Sex | |||||
| Female | 1 | Ref. | 1 | Ref. | |
| Male | 1.95 (1.10–3.46) | 0.02 | 1.22 (0.48–3.11) | 0.67 | |
| History of smoking | 1.96 (1.12–3.45) | 0.02 | 1.60 (0.86–2.99) | 0.14 | |
| Total tumor size, mm | 1.04 (0.98–1.10) | 0.16 | |||
| Invasive tumor size, mm | 1.05 (0.99–1.11) | 0.10 | |||
| Extent of resection | |||||
| Lobectomy | 1 | Ref. | |||
| Sublobar resection | 1.32 (0.56–3.11) | 0.53 | |||
| Cell type | |||||
| Adenocarcinoma | 1 | Ref. | 1 | Ref. | |
| Non-adenocarcinoma | 2.75 (1.48–5.12) | <0.001 | 1.91 (0.96–3.82) | 0.07 | |
| Histologic differentiation | |||||
| Well or moderately | 1 | Ref. | 1 | Ref. | |
| Poorly | 1.52 (0.65–3.57) | 0.34 | |||
| Microscopic lymphatic invasion | 2.09 (1.18–3.70) | 0.01 | 1.54 (0.83–2.88) | 0.17 | |
| Microscopic vascular invasion | 3.12 (1.56–6.26) | 0.001 | 2.70 (1.34–5.48) | 0.006 | |
| Number of harvested LNs | 1.03 (0.99–1.06) | 0.10 | 1.03 (1.00–1.07) | 0.08 | |
| Pre-operative TNB | 1.98 (1.02–3.48) | 0.02 | 1.93 (1.10–3.39) | 0.02 | |
NSCLC, non-small cell lung cancer; PSM, propensity score matching; HR, hazard ratio; CI, confidence interval; Ref., reference; LN, lymph node; TNB, transthoracic needle biopsy.
Regarding the time of locoregional recurrence, the mean peak points were 6.9 and 11.6 months in the no-TNB and TNB groups, respectively, showing a significant difference (Figure 3).
TNB-related complications
The proportion of patients who developed pneumothorax in the TNB group was 21.6% (41/190), of which 22% (9/41) required air drainage and others required conservative care, such as oxygen supplementation. Hemoptysis was observed in 3.2% (6/190) of patients, and hemothorax that did not require any further intervention was observed in 1.6% of patients (3/190). The overall incidence of complications observed during the study period was 26.3% (50/190) (Table 5).
Table 5
| Complications | Number | Rate (%) |
|---|---|---|
| Pneumothorax [requiring drainage] | 41 [9] | 21.6 [4.7] |
| Hemoptysis | 6 | 3.2 |
| Hemothorax | 3 | 1.6 |
| Air embolism | 0 | 0 |
| Respiratory arrest | 0 | 0 |
| Death | 0 | 0 |
| Total | 50 | 26.3 |
TNB, transthoracic needle biopsy.
Discussion
Key findings
This study demonstrated that pre-operative TNB increased the risk of locoregional recurrence, leading to an increased overall recurrence in patients with stage IA NSCLC. Unlike previous studies that included patients with stage I, this study is significant in that the clinical effects of TNB were investigated by including patients with stage IA NSCLC with no visceral pleural invasion. Furthermore, this study compared the oncological outcomes by minimizing heterogeneity between the TNB and no-TNB groups through PSM and demonstrated different frequencies and onsets of recurrence between the groups.
Strengths and limitations
This study has some limitations. First, this was a retrospective study that was conducted at a single center, which might have introduced a bias within the groups, although it was minimized using PSM. Second, there might have been heterogeneity (number of tissue samples and needle passage method) within the TNB group that could have occurred in connection with the TNB procedures. However, we believe that this bias has been minimized, given that only a limited number of radiologists at a single center performed the protocol. Third, this study lacks a controlled comparison of postoperative complication rates for pulmonary resection surgery and TNB procedures. We believe follow-up studies are necessary to address this. Finally, it is difficult to determine the time to recurrence since the number of events was limited. However, the present study showed that the incidence of recurrence was different between the TNB and no-TNB groups, suggesting that the time to recurrence differs between the two groups.
Explanation of findings and comparison with similar research
Many studies that investigated the association between TNB and oncological outcomes have reported different results (7-12). Compared to the present study, previous studies have demonstrated that TNB is not related to the prognosis of patients, in which adverse effects of TNB might have been masked because the heterogeneity was not properly considered, even in a relatively large-scale study (8). Moreover, although pleural invasion is a variable that must be controlled when considering tumor seeding of the biopsy tract and structure disruption owing to external force, it was not appropriately controlled in previous studies (7-9).
Kashiwabara et al. suggested pre-operative TNB as an oncologic negative risk factor, but it was confined to the sub-pleural pure solid nodules (11). In addition, this study had a limitation in that it used a relatively small sample size that was unmatched (11). Inoue et al. also compared patients by matching, using a method similar to the one used in the present study, to reduce selection bias, even though they included patients with pathologic stage IB with visceral pleural invasion (10). A recent patient-level meta-analysis of 2,394 patients also revealed that TNB in patients with stage I early lung cancer was a risk factor for isolated ipsilateral recurrence and concomitant ipsilateral recurrence. A sub-group analysis that was conducted in that study also revealed that TNB in stage IA is a negative prognostic factor, similar to the results of our study. This was not the primary endpoint of the study but was the result of the sub-group analysis, and it resulted from a bias within groups that was not fully controlled, which is a significant limitation (12).
According to a recent meta-analysis encompassing 32 studies, the incidence of complications in 8,133 patients who underwent cutting needle biopsy was 38.8%, of which the incidence of severe complications (pneumothorax requiring intervention, hemothorax, air embolism, needle tract seeding, and death) was 5.7% (18). A Japanese multicenter study used data from 9,783 biopsies and reported that the incidence of pneumothorax, the most common complication, was 35% (N=2,412) among 6,881 patients. Further, it reported that the incidence of needle tract seeding, one of the severe complications, was 0.06% (N=6) (6). Most complication events in this study were relatively less frequent than those of previous studies. These findings suggest that surgical and TNB complication rates might be comparable, based on existing studies (19-21). Moreover, according to a meta-analysis that included 12 studies, the sensitivity and specificity of TNB were 90% (95% CI: 85–94%) and 99% (95% CI: 92–100%), respectively. TNB has a limitation in that it is not a surgical biopsy that identifies malignancy by removing the whole tumor. Accordingly, owing to this limitation, TNB cannot assist clinicians in regard to future management when a negative TNB result is observed in patients with a clinically high suspicion of malignancy (22). Additional limitations, such as an increased medical cost, shed additional light on the necessity of TNB in patients with stage IA NSCLC (23).
Implications and actions needed
Future studies with a large sample size are warranted to derive clinically significant results in the post-operative follow-up protocol of the TNB and no-TNB groups.
Conclusions
For patients with stage IA NSCLC, the pre-operative TNB was a negative prognostic factor for recurrence. Surgical diagnosis and treatment without pre-operative tissue diagnosis may be considered first in patients with clinically early lung cancer.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-419/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-419/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-419/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-419/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Catholic University College of Medicine (No. KC22RISI0761), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med 2020;41:1-24. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:497-530. [Crossref] [PubMed]
- Kim TJ, Lee JH, Lee CT, et al. Diagnostic accuracy of CT-guided core biopsy of ground-glass opacity pulmonary lesions. AJR Am J Roentgenol 2008;190:234-9. [Crossref] [PubMed]
- Winokur RS, Pua BB, Sullivan BW, et al. Percutaneous lung biopsy: technique, efficacy, and complications. Semin Intervent Radiol 2013;30:121-7. [Crossref] [PubMed]
- Tomiyama N, Yasuhara Y, Nakajima Y, et al. CT-guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol 2006;59:60-4. [Crossref] [PubMed]
- Wisnivesky JP, Henschke CI, Yankelevitz DF. Diagnostic percutaneous transthoracic needle biopsy does not affect survival in stage I lung cancer. Am J Respir Crit Care Med 2006;174:684-8. [Crossref] [PubMed]
- Lee GY, Chung JH, Cho S, et al. Impact of Preoperative Diagnostic Biopsy Procedure on Spread Through Airspaces and Related Outcomes in Resected Stage I Non-Small Cell Lung Cancer. Chest 2022;162:1199-212. [Crossref] [PubMed]
- Ahn SY, Yoon SH, Yang BR, et al. Risk of pleural recurrence after percutaneous transthoracic needle biopsy in stage I non-small-cell lung cancer. Eur Radiol 2019;29:270-8. [Crossref] [PubMed]
- Inoue M, Honda O, Tomiyama N, et al. Risk of pleural recurrence after computed tomographic-guided percutaneous needle biopsy in stage I lung cancer patients. Ann Thorac Surg 2011;91:1066-71. [Crossref] [PubMed]
- Kashiwabara K, Semba H, Fujii S, et al. Preoperative Percutaneous Transthoracic Needle Biopsy Increased the Risk of Pleural Recurrence in Pathological Stage I Lung Cancer Patients With Sub-pleural Pure Solid Nodules. Cancer Invest 2016;34:373-7. [Crossref] [PubMed]
- Hong H, Hahn S, Matsuguma H, et al. Pleural recurrence after transthoracic needle lung biopsy in stage I lung cancer: a systematic review and individual patient-level meta-analysis. Thorax 2021;76:582-90. [Crossref] [PubMed]
- Travis WD, Brambilla E, Rami-Porta R, et al. Visceral pleural invasion: pathologic criteria and use of elastic stains: proposal for the 7th edition of the TNM classification for lung cancer. J Thorac Oncol 2008;3:1384-90.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Beck KS, Kim SJ, Kang JH, et al. CT-guided transthoracic needle biopsy for evaluation of PD-L1 expression: Comparison of 22C3 and SP263 assays. Thorac Cancer 2019;10:1612-8. [Crossref] [PubMed]
- Beck KS, Chang S, Han DH, et al. The effectiveness and safety of local pleural anesthesia for pain control in patients undergoing CT-guided transthoracic needle biopsy. Eur Radiol 2021;31:8282-90. [Crossref] [PubMed]
- Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017;27:138-48. [Crossref] [PubMed]
- Bertolaccini L, Prisciandaro E, Bardoni C, et al. Minimally Invasive Anatomical Segmentectomy versus Lobectomy in Stage IA Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Cancers (Basel) 2022;14:6157. [Crossref] [PubMed]
- Ma J, Li X, Zhao S, et al. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or segmentectomy in patients with non-small cell lung cancer: a meta-analysis. BMC Cancer 2021;21:498. [Crossref] [PubMed]
- Li R, Wang K, Qu C, et al. The effect of the enhanced recovery after surgery program on lung cancer surgery: a systematic review and meta-analysis. J Thorac Dis 2021;13:3566-86. [Crossref] [PubMed]
- Kim J, Chee CG, Cho J, et al. Diagnostic accuracy and complication rate of image-guided percutaneous transthoracic needle lung biopsy for subsolid pulmonary nodules: a systematic review and meta-analysis. Br J Radiol 2021;94:20210065. [Crossref] [PubMed]
- Na KJ, Park IK, Park S, et al. Efficacy and Cost-effectiveness of Surgical Biopsy for Histologic Diagnosis of Indeterminate Nodules Suspected for Early Stage Lung Cancer: Comparison with Percutaneous Needle Biopsy. J Korean Med Sci 2020;35:e261. [Crossref] [PubMed]


