
Novel techniques and future developments in minimally invasive pectus repair
Introduction
The minimally invasive repair of pectus excavatum (MIRPE) described by Dr. Donald Nuss more than 20 years ago was a paradigm shift in the treatment of chest wall excavated deformities. Ever since, several modifications have been described in the search for improvement in technical, procedural, and clinical outcomes. In this article, we provide an in-depth discussion of the most relevant innovative concepts related to the treatment of chest wall malformations; including three-dimensional (3D) printing and implant customization, compulsive sternal elevation, complete thoracic remodeling as a goal of repair, and implant fixation with bridges.
3D printing and implant customization
Although the use of 3D technology in medicine is a very current topic, publications on its use for the resolution of pectus excavatum are scarce, limited to scattered reports mainly related to simulation and educational purposes (1,2).
Other authors, such as Lai et al. and Lin et al. (3,4), have reported the implementation of 3D technology for implant customization. In their study, Lai et al. compared the resultant shape of the implant using the traditional method of adding 2.5 cm laterally to the mid-axillary line and a computed tomography (CT) model in 75 patients and found that the CT model was more precise and accurate. Lin et al. used 3D-printed templates designed using software on CT reconstruction to model implants in 10 patients. In 2014, Vilaça et al. (5) reported one of the first large experiences with automatic pre-bent customized implants for 41 patients showing that automatic-made implants were more precise than manually bent implants when calculating the skin-to-costal margin distance. Using customized implants based on real-size chest wall models of 15 patients, Huang et al. reported a propensity score matching using 342 cases undergoing repair with manually bent implants. They found that patients with customized implants had a shorter surgical time, a decreased number of bar placements, and improvement in postoperative Haller indexes measured with X-rays in the postoperative period (6).
In 2020, we reported our experience using 3D technology in several steps of the MIRPE (7). In this report, we used 3D technology for the preoperative planning process, using 3D reconstructions of end-expiration chest CT as an adjunct to determine the number, direction, and position of the implants in each case. Next, the exact shape and size of each implant were designed virtually by a software program (Erkom Pro 3.0, Buenos Aires, Argentina) (Figure 1A) and 3D printed templates created from standard tessellation language (STL) files were measured on the patients’ chest wall at a fitting session at an outpatient setting (Figure 1B). If the templates were correct, we subsequently approved the pre-bent customized manufacture of the metallic bars with a proven implant/deformity match over 90% (Figure 1C).
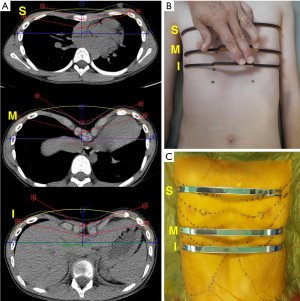
This approach might save time in the operation room, facilitating the process, especially for surgeons performing less than 20 procedures per year, and avoiding the creation of scar-forming scratches and notches on the implants’ surface. In 2017, Alvarez-Garcia et al. published a survey to Chest Wall International Group members on bar removal complications (8). Severe bleeding from the bar tunnel due to adhesions was experienced by 26.9% of the respondents. Thus, we might infer that any strategies that decrease the formation of ossification and fibrous adhesions to the bars due to scratches and notches might decrease the possibility of bleeding during bar removal.
However, studies with objective, measurable endpoints and larger cohorts are still necessary to ascertain the benefits of the implementation of 3D technology in chest wall malformations.
Sternal elevation
One of the most feared complications of the Nuss procedure is cardiac and pulmonary perforation during “blind” retrosternal tunneling. This was clearly evidenced in a case report by Beati et al. (9) about a 13-year-old patient with a deep, asymmetric pectus excavatum undergoing MIRPE without sternal elevation who suffered one lung and two cardiac perforations when the introducer was passed from right to left under thoracoscopic guidance.
Various strategies have been proposed to overcome this potentially ominous complication. In 2001, Miller et al. acknowledged this issue for the first time and reported no cardiac injury after implementing blunt retrosternal dissection through a subxiphoid incision (10). In 2005, Schier et al. reported the use of a vacuum bell for intraoperative sternal elevation in 14 patients (11). They reported a clear elevation of the sternum confirmed by thoracoscopy in all patients, although it sunk back after a few minutes.
In 2008, Park et al. described several modifications to the original Nuss procedure (12), being one of these the successful elevation of the sternum in 122 adults using trans sternal percutaneous wire sutures and a crane (“the crane-technique”) before precardiac dissection to decrease intercostal muscle tearing and the force required to rotate the bars.
In the following years, several sternal elevation techniques emerged including the use of wire stitches and a Kent retractor (13), a manual sternum elevator (14), two Langenbeck retractors (15), a Lewin Spinal Perforating Forceps (V Mueller NL6960; CareFusion, Inc., San Diego, CA, USA) and a table-mounted retractor (7,16), a vacuum bell (17), a sternal lift and an anchor through a subxiphoid incision (18), a Wolkmann bone hook (19), and a T-fastener suture technique (20). Also, other approaches aimed at avoiding injury to the mediastinum include dissection of the retrosternal tunnel from the left to the right side to avoid the use of the thoracic introducer (21), among others. Since cardiac perforation is infrequent, the comparison between these techniques is limited to the size and number of additional incisions and sternal retraction stability.
Nowadays, the two most widely used strategies for sternal elevation are the intraoperative use of a vacuum bell, later popularized by Haecker and Sesia (17,22,23), and the application of a crane or retractor. Adult chest wall surgeons such as Jaroszewski et al. who described the use of a Rultract Retractor (Rultract Inc., Cleveland, OH, USA) for forced sternal elevation in 63 patients between 2010 and 2013 (24), recommended the use of a retractor in all cases with rigid chest walls (Figure 2). In 2021, de Loos et al. reported a quantitative analysis of the degree of superficial correction achieved with a crane attached to the sternum by a wire suture in 30 patients undergoing MIRPE (25). They used an optical scanner to compare external pectus depth before and after sternal lifting and found that it decreased by 78% [interquartile range (IQR), 63%, 100%]. Although they concluded that the technique might be effective in providing a safer retrosternal passage, they found less degree of correction in more severe cases. Further studies are warranted to determine whether a different attachment element such as the Lewin clamp or the new Screw developed by Dr. Park or the duplication of the attachment elements are effective in improving these findings.

In our own practice, the crane is applied in all procedures regardless of the patient’s rigidity or age, as we reported in 2020 in 130 patients (7). Alternatively, we believe that the vacuum bell might only be used as a second-best complementary choice since, in our experience, it can provoke subcutaneous emphysema and petechiae, and retraction lasts for a few minutes before it is necessary to apply suction again.
According to a recent review by Notrica, “This modification [sternal elevation with a crane] has been the single most important, breakthrough modification in improving the safety of the technique.” (26).
Complete chest wall remodeling
Total chest wall remodeling is a novel concept amongst the pectus surgeon community. It refers to a new goal when repairing chest wall deformities that originated in recognizing that this ailment is diverse and that a single technique cannot possibly resolve all the phenotypic variations. In pectus excavatum, it consists of remodeling not only the deepest point of the excavation but the whole anterior chest wall, including the upper chest, asymmetries, and deformed rib-flares.
One of the pioneers of this concept is Dr. Park who, in 2010, introduced the multiple target approach, the terrain contour matching, and the multiple momentum theory (27). In keeping with this, in 2016, Park et al. reported the sandwich technique, consisting of the utilization of intra and extrathoracic implants to press-mold asymmetries and complex excavatum-carinatum cases (28).
In parallel, more adult patients visited the chest wall surgeon clinics requesting a MIRPE. However, adult chest wall is more rigid than that of teenagers requiring more implants to achieve appropriate remodeling as stated by Jaroszewski et al. in 2016 (29) in a retrospective report including 207 patients older than 30 years.
However, this concept’s implementation requires an extensive review of the usual practice and the disposition for change. In our experience, after understanding this postulation, we were able to recognize that patients from our previous cohorts were under-corrected (Figure 3).

This new conception led us to the introduction of multiple implants as reported in 2020 (7) (2.6±0.5 implants per patient vs. 1.7±0.6 in a previous cohort) (Figure 4). We recently reported the use of a CT index, the Titanic Index, to determine the number of implants necessary to achieve complete thoracic remodeling (30). This index measures the cephalocaudal extent of sternal sinking behind the anterior costal line and the cut point between two and three bars was 66.5%, or in other words, two-thirds of the sternum.

Aspects to be considered for complete chest wall remodeling include:
- Sternal depression: the degree of depression of the sternum has been categorized using classifications such as the one described by Lawson et al. in 2006 (31), the “titanic index” by Bellía-Munzón et al. (30), or the “vertebral-level-specific-pectus indices” by Moon et al. (32). These classifications aim to provide a practical tool to determine the number of bars required.
- Symmetry: the asymmetric pectus excavatum deformities are difficult to repair, and the implants need to be shaped accordingly to reduce the possibility of over or under-correction (31,33).
- Sternal rotation: commonly detected in asymmetrical deformities. In significantly rotated sternums, it might be necessary to consider osteotomies.
- Mixed deformities: includes excavated/carinated deformities that may require strategies such as “the sandwich technique” (28).
- Banana-like sternum (7): which may be approached with the crossed bar technique using two implants to avoid rotation. If the upper sternum also needs correction, the “XI” technique may be employed.
- Spine deformities: although the exact interaction between chest wall and spine deformities still needs to be elucidated (34), there seems to be a relationship between them as postural changes become evident after chest wall repair with correction of shoulder and head ante pulsion, and thoracic kyphosis.
All this being said, there is still debate in the community of chest wall specialists. As Dr. Nuss himself commented in a letter to the editor recently, further studies showing the benefits and safety of the use of multiple bars are warranted for this concept to gain wider popularity (25).
Implant fixation with bridges
As a natural consequence of using multiple implants, the stabilization system had to be reappraised, given that lateral stabilizers were big and bulky when fixating more than one implant on each side. This situation concurred with the need to reduce the rates of rotation and dislocation of the implants, frequently observed in cases of banana sternum and heavy, rigid chest walls. This aspect was approached by medial fixation including medially placed stabilizers (35), sternal wire interspace reinforcement (the hammock technique) (13,29,36), a hinge reinforcement plate (37), and sternal fixation (38).
In 2015, Park et al. (39) reported “the bridge technique”, the implementation of a new device to fixate implants between them and with no need to fixate to the chest wall itself. He reported its use in 80 cases with parallel bars during 1 year with no evidence of implant dislocation or reoperations in any case. In 2020, we also reported the use of implants with self-blocking lateral bridges, and our experience was similar with a zero rate of implant rotation in that series (Figure 5). In 2019, a large study by Vinh et al. (40) compared 560 cases undergoing bridge stabilization to 1,200 patients with other stabilization techniques, being bilateral stabilization the best approach in terms of bar dislocations.

In 2023, Dr. Kim et al. reported another large cohort of cases with pectus excavatum and carinatum focusing on their stabilization system (41). They included 497 cases with the bridge technique, having adopted this approach over the hinge plate technique in all patients since 2022 in search of complete chest wall remodeling with zero rate of bar displacement.
These last three experiences might support the introduction of an implant as high as the second intercostal space without fear of bar rotation and compression of the great vessels or the airway in that area, improving the extent of remodeling in a cephalocaudal direction. However, further studies are warranted showing evidence that this technical aspect is safe and useful.
Conclusions
Twenty-five years after the original description of MIRPE, many modifications and ideas have been proposed and tested in the search for excellence. Conceptual and technical innovations include the use of sternal elevation, complete thoracic remodeling as a therapeutic goal, lateral bridge fixation, and the use of 3D technology for preoperative planning and implant customization amongst other uses. All these strategies and concepts allow the chest wall surgeon to improve efficacy and safety in a procedure meant to improve quality of life and their pursuit and widespread application will likely mark the future of pectus excavatum surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Erik R. de Loos, Jean H. T. Daemen & Frank-Martin Haecker) for the series “Minimally Invasive Treatment of Pectus Deformities” published in Journal of Thoracic Disease. The article has undergone external peer review.
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1676/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1676/coif). The series “Minimally Invasive Treatment of Pectus Deformities” was commissioned by the editorial office without any funding or sponsorship. M.M.F. has ownership interest in Pampamed SRL. G.B.M. has pending patents with Pampamed SRL. L.T. was a speaker for Novelty Importaciones. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Matsuo N, Matsumoto K, Taura Y, et al. Initial experience with a 3D printed model for preoperative simulation of the Nuss procedure for pectus excavatum. J Thorac Dis 2018;10:E120-4. [Crossref] [PubMed]
- Shan Y, Yu G, Lu Y, et al. Application of three-dimensional reconstruction technology combined with three-dimensional printing in the treatment of pectus excavatum. Ann Thorac Med 2022;17:173-9. [Crossref] [PubMed]
- Lai JY, Wang CJ, Chang PY. The measurement and designation of the pectus bar by computed tomography. J Pediatr Surg 2009;44:2287-90. [Crossref] [PubMed]
- Lin KH, Huang YJ, Hsu HH, et al. The Role of Three-Dimensional Printing in the Nuss Procedure: Three-Dimensional Printed Model-Assisted Nuss Procedure. Ann Thorac Surg 2018;105:413-7. [Crossref] [PubMed]
- Vilaça JL, Rodrigues PL, Soares TR, et al. Automatic prebent customized prosthesis for pectus excavatum minimally invasive surgery correction. Surg Innov 2014;21:290-6. [Crossref] [PubMed]
- Huang YJ, Lin KH, Chen YY, et al. Feasibility and Clinical Effectiveness of Three-Dimensional Printed Model-Assisted Nuss Procedure. Ann Thorac Surg 2019;107:1089-96. [Crossref] [PubMed]
- Bellia-Munzon G, Martinez J, Toselli L, et al. From bench to bedside: 3D reconstruction and printing as a valuable tool for the chest wall surgeon. J Pediatr Surg 2020;55:2703-9. [Crossref] [PubMed]
- Alvarez-Garcia N, Ardigo L, Bellia-Munzon G, et al. Close Examination of the Bar Removal Procedure: The Surgeons' Voice. Eur J Pediatr Surg 2018;28:406-12. [Crossref] [PubMed]
- Beati F, Frediani S, Pardi V, et al. Case report-Every thoracic surgeon's nightmare: cardiac and lung perforation during placement of Nuss bar for pectus excavatum. Front Pediatr 2023;11:1241273. [Crossref] [PubMed]
- Miller KA, Woods RK, Sharp RJ, et al. Minimally invasive repair of pectus excavatum: a single institution's experience. Surgery 2001;130:652-7; discussion 657-9. [Crossref] [PubMed]
- Schier F, Bahr M, Klobe E. The vacuum chest wall lifter: an innovative, nonsurgical addition to the management of pectus excavatum. J Pediatr Surg 2005;40:496-500. [Crossref] [PubMed]
- Park HJ, Chung WJ, Lee IS, et al. Mechanism of bar displacement and corresponding bar fixation techniques in minimally invasive repair of pectus excavatum. J Pediatr Surg 2008;43:74-8. [Crossref] [PubMed]
- Yoon YS, Kim HK, Choi YS, et al. A modified Nuss procedure for late adolescent and adult pectus excavatum. World J Surg 2010;34:1475-80. [Crossref] [PubMed]
- Takagi S, Oyama T, Tomokazu N, et al. A new sternum elevator reduces severe complications during minimally invasive repair of the pectus excavatum. Pediatr Surg Int 2012;28:623-6. [Crossref] [PubMed]
- Tedde ML, de Campos JR, Wihlm JM, et al. The Nuss procedure made safer: an effective and simple sternal elevation manoeuvre. Eur J Cardiothorac Surg 2012;42:890-1. [Crossref] [PubMed]
- Jaroszewski DE. Forced mechanical sternal elevation for Nuss repair. Ann Thorac Surg 2013;96:1914. [Crossref] [PubMed]
- Haecker FM, Sesia SB. Intraoperative use of the vacuum bell for elevating the sternum during the Nuss procedure. J Laparoendosc Adv Surg Tech A 2012;22:934-6. [Crossref] [PubMed]
- Johnson WR, Fedor D, Singhal S. A novel approach to eliminate cardiac perforation in the nuss procedure. Ann Thorac Surg 2013;95:1109-11. [Crossref] [PubMed]
- Rygl M, Vyhnanek M, Kucera A, et al. Technical innovation in minimally invasive repair of pectus excavatum. Pediatr Surg Int 2014;30:113-7. [Crossref] [PubMed]
- Kim D, Idowu O, Palmer B, et al. Anterior chest wall elevation using a T-fastener suture technique during a Nuss procedure. Ann Thorac Surg 2014;98:734-6. [Crossref] [PubMed]
- Tedde ML, Togoro SY, Eisinger RS, et al. Back to the future: a case series of minimally invasive repair of pectus excavatum with regular instruments. J Bras Pneumol 2019;45:e20170373. [Crossref] [PubMed]
- Haecker FM, Sesia S. Vacuum bell therapy. Ann Cardiothorac Surg 2016;5:440-9. [Crossref] [PubMed]
- Haecker FM, Krebs T, Kocher GJ, et al. Sternal elevation techniques during the minimally invasive repair of pectus excavatum. Interact Cardiovasc Thorac Surg 2019;29:497-502. [Crossref] [PubMed]
- Jaroszewski DE, Johnson K, McMahon L, et al. Sternal elevation before passing bars: a technique for improving visualization and facilitating minimally invasive pectus excavatum repair in adult patients. J Thorac Cardiovasc Surg 2014;147:1093-5. [Crossref] [PubMed]
- de Loos ER, Daemen JHT, Coorens NA, et al. Sternal elevation by the crane technique during pectus excavatum repair: A quantitative analysis. JTCVS Tech 2021;9:167-75. [Crossref] [PubMed]
- Notrica DM. Modifications to the Nuss procedure for pectus excavatum repair: A 20-year review. Semin Pediatr Surg 2018;27:133-50. [Crossref] [PubMed]
- Park HJ, Jeong JY, Jo WM, et al. Minimally invasive repair of pectus excavatum: a novel morphology-tailored, patient-specific approach. J Thorac Cardiovasc Surg 2010;139:379-86. [Crossref] [PubMed]
- Park HJ, Kim KS. The sandwich technique for repair of pectus carinatum and excavatum/carinatum complex. Ann Cardiothorac Surg 2016;5:434-9. [Crossref] [PubMed]
- Jaroszewski DE, Ewais MM, Chao CJ, et al. Success of Minimally Invasive Pectus Excavatum Procedures (Modified Nuss) in Adult Patients (≥30 Years). Ann Thorac Surg 2016;102:993-1003. [Crossref] [PubMed]
- Bellía-Munzón G, Sanjurjo D, Toselli L, et al. Novel index to estimate the cephalocaudal extent of the excavation in pectus excavatum: The Titanic index. J Pediatr Surg 2023;58:605-7. [Crossref] [PubMed]
- Lawson ML, Barnes-Eley M, Burke BL, et al. Reliability of a standardized protocol to calculate cross-sectional chest area and severity indices to evaluate pectus excavatum. J Pediatr Surg 2006;41:1219-25. [Crossref] [PubMed]
- Moon DH, Park CH, Moon MH, et al. The effectiveness of double-bar correction for pectus excavatum: A comparison between the parallel bar and cross-bar techniques. PLoS One 2020;15:e0238539. [Crossref] [PubMed]
- Park HJ, Lee SY, Lee CS, et al. The Nuss procedure for pectus excavatum: evolution of techniques and early results on 322 patients. Ann Thorac Surg 2004;77:289-95. [Crossref] [PubMed]
- Zhong W, Ye J, Feng J, et al. Effects of Pectus Excavatum on the Spine of Pectus Excavatum Patients with Scoliosis. J Healthc Eng 2017;2017:5048625. [Crossref] [PubMed]
- Pilegaard HK. Nuss technique in pectus excavatum: a mono-institutional experience. J Thorac Dis 2015;7:S172-6. [PubMed]
- Ashfaq A, Beamer S, Ewais MM, et al. Revision of Failed Prior Nuss in Adult Patients With Pectus Excavatum. Ann Thorac Surg 2018;105:371-8. [Crossref] [PubMed]
- Park HJ, Jeong JY, Kim KT, et al. Hinge reinforcement plate for adult pectus excavatum repair: a novel tool for the prevention of intercostal muscle strip. Interact Cardiovasc Thorac Surg 2011;12:687-91. [Crossref] [PubMed]
- Jaroszewski DE, Velazco CS. Minimally invasive pectus excavatum repair (MIRPE). Oper Tech Thorac Cardiovasc Surg 2018;23:198-215. [Crossref]
- Park HJ, Kim KS, Moon YK, et al. The bridge technique for pectus bar fixation: a method to make the bar un-rotatable. J Pediatr Surg 2015;50:1320-2. [Crossref] [PubMed]
- Vinh VH, Khanh HQ, Binh NH, et al. Pectus excavatum repair using bridge fixation system. Asian Cardiovasc Thorac Ann 2019;27:374-80. [Crossref] [PubMed]
- Kim H, Rim G, Park HJ. Technical Advances in Pectus Bar Stabilization in Chest Wall Deformity Surgery: 10-Year Trends and an Appraisal with 1,500 Patients. J Chest Surg 2023;56:229-37. [Crossref] [PubMed]


