AME evidence series 001—The Society for Translational Medicine: clinical practice guidelines for diagnosis and early identification of sepsis in the hospital
Definition of sepsis
The Surviving Sepsis Campaign (SSC) has the goal to improve the outcome of patients with sepsis and therefore it is important to define criteria for an early identification and treatment of these patients (1). Sepsis is most commonly defined as a systemic inflammatory response syndrome (SIRS) plus documented or suspected infection (2). Historically, several “versions” of this definition have been proposed, all aiming to reflect the common underlying mechanisms of the inflammatory response induced by infection (Table 1). The first version of the ACCP/SCCM [1992] definition is easily incorporated for bedside clinical use, but its specificity is vigorously debated (3). The second version adopted by both the 2001 SCCM/ESICM/ACCP/ATS/SIS and 2012 SSC guidelines is more complex and included some novel biomarkers such as procalcitonin (PCT) (2,4). Diagnosis of sepsis is based on five broad categories: general parameters, inflammatory markers, hemodynamic variables, organ dysfunction and indicators of tissue perfusion. This complex definition reflects the heterogeneous clinical presentations of sepsis, which is also shown in meta-analyses of diagnostic criteria through its tests for heterogeneity (5,6). The definitions do not specify how many items should be met before sepsis is considered to be present. Also, clinicians need to memorize too many items, which limits the applicability of the new definition for clinical use and research purposes. This sepsis definition also is not “clear-cut” but merely helps clinicians identifying a patient who “looks septic”. As a result, clinical research still relies on the “old” ACCP/SCCM definition to screen patients with sepsis.
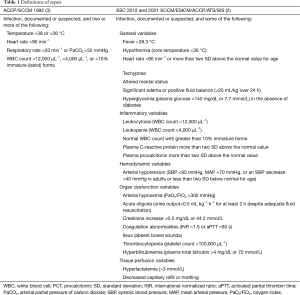
Full table
More recently, defining sepsis on the basis of organ dysfunction has been found to be helpful for the identification of patients requiring intensive and secondary-line treatments (7,8), leading to a new definition of severe sepsis and septic shock (Sepsis-3). In this perspective, sepsis has been defined as a “life-threatening organ dysfunction caused by dysregulated host response to infection”, identifying, as we will see below, the condition-sine-qua-non for its diagnosis in the presence of an acute and sepsis-related organ failure. Organ dysfunction is identified as an acute change in sequential organ failure assessment (SOFA) score of two points or more following infection (9). The concept of the quick SOFA (qSOFA) clinical score based on mental status, respiratory rate and systolic blood pressure (SBP) was introduced in order to provide rapid and repeated assessments of patients without laboratory tests. The major difference of these definitions compared to the previous ones was that they were not only based on expert opinion; instead a broad analysis of clinical and laboratory parameters of patients from five large independent cohorts was performed to develop these definitions (5,9). Sepsis-3 definitions are not universally accepted and many controversies have surfaced (10-12). Clinical data utilized for the development of the Sepsis-3 definitions were mainly recorded in patients hospitalized in US intensive care units (ICU). Analysis was driven by mortality as the main outcome measure. However, the presence of organ failure at infection onset or the development of an infection-associated organ failure during the patient physical course appears to be a more attractive outcome for analysis. Furthermore, the sepsis-3 appears to focus on a more restrictive definition rather than on therapeutic interventions at earlier stages of sepsis where SIRS is detectable. The justification of sepsis-3 requires further clinical investigation to prove that delayed intervention is not implicated in this cohort of sepsis-3 patients.
Classification and staging of sepsis
According to the 2012 SSC guidelines, sepsis can be categorized by ascending severity into sepsis, severe sepsis and septic shock (4). Severe sepsis is defined as sepsis complicated by acute and sepsis-induced organ dysfunction. The method to evaluate for organ dysfunction is adapted from the SOFA score, which includes a scoring system to evaluate, on a daily basis, the function of the main six organs or systems (cardiovascular, renal, liver, coagulation, respiratory and neurological) (Table 2) (13,14). In general, organ dysfunction within severe sepsis criteria does correspond normally with organ failure according to the SOFA score, i.e., to a SOFA score equal to or greater than two points for each subcomponent. Central nervous system dysfunction was not incorporated into the assessment of sepsis severity, because of the use of sedative agents as major confounders on the neurological status of the most severe patients. The new definitions of sepsis (Sepsis-3) had removed the term “severe sepsis” considering that sepsis is by definition a severe life-threatening disease. Sepsis-3 explicitly includes the SOFA score to identify infected patients at risk of having sepsis, and at increased risk of mortality (9). Septic shock, according to the original ACCP/SCCM definition, is defined as “a state of acute circulatory failure characterized by persistent arterial hypotension unexplained by other causes” and associated with an infection (2). It can be identified in a septic patient with hypotension requiring vasopressors to maintain MAP >65 mmHg and a serum lactate level >2 mmol/L (18 mg/dL) despite adequate volume resuscitation. Interestingly, in addition to clinical criteria, the Delphi consensus process by the task force included a serum lactate level of >2 mmol/L as part of the definition of septic shock. The inclusion of hyperlactatemia highlights the role of lactate in the understanding of this syndrome (15).
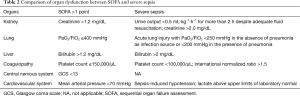
Full table
Analogous to the TNM classification used for staging malignant tumors, some authors have proposed the PIRO (predisposition, infection, response and organ dysfunction) system to better evaluate sepsis and its severity. It was originally formulated as IRO in the Fifth Toronto Sepsis Roundtable Talk, with P (predisposition) added thereafter (16). Although some investigators argued that PIRO was only attractive in its conceptual framework (17), recent evidence supported it as an accurate predictor of mortality. The PIRO system indeed outperformed the SOFA score in predicting mortality (AUC: 0.86; 95% CI: 0.80 to 0.92 vs. 0.78; 95% CI: 0.71 to 0.87) of patients with severe sepsis and of septic shock patients in the emergency department (18). However, inferior results were obtained from other studies with AUCs ranging between 0.68 and 0.744, from various emergency department cohorts (18-21). To date, no randomized controlled trials (RCT) have explored how patient-important outcomes (e.g., mortality, long term physical and cognitive behavior, return to previously normal function etc.) are influenced by applying the PIRO system. More studies are needed to determine its clinical utility.
Early identification of sepsis
Sepsis may benefit from early identification (1), thus many biomarkers and screening strategies to identify patients with sepsis have been investigated (22,23). The reference standard used in these studies was defined in the ACCP/SCCM, 2001 SCCM/ESICM/ACCP/ATS/SIS and 2012 SSC guidelines. The index test (i.e., potential diagnostic biomarkers) included, among others, presepsin (sCD14-ST) (24), PCT, Neutrophil CD64 (25), sTREM-1 (26), lipopolysaccharide-binding protein, pro-adrenomedullin, pro-vasopressin and a variety of inflammatory cytokines (27-29). Furthermore, the efficacy of many scoring systems and screening tools for detecting early sepsis has been evaluated (30,31). These include BioScore system (32), computer-weighted bedside scoring system (33), three-step sepsis screening tool (34), and spot check tissue oxygen saturation (StO2) (35). However, the clinical usefulness of these screening tools has not been established. In the next sections, benefits and pitfalls of early identification of sepsis within the Grades of Recommendation Assessment, Development and Evaluation (GRADE) framework will be discussed (36).
Assessment of diagnostic testing in the GRADE framework
Clinical usefulness of screening tools for early identification of sepsis can be investigated by RCTs and cohort studies (Figure 1). RCTs also allow evaluating potential pitfalls in the early diagnosis of sepsis. For example, a three-step sepsis screening tool can be assessed with one arm assigned to the control and the other to the screening. Benefits may include the early use of antibiotics or early initiation of a “resuscitation bundle”, whereas potential drawbacks may involve, as an example, anxiety of being diagnosed with sepsis, pulmonary edema due to fluid overload and more expensive treatment. Furthermore, inherent false positives and negatives will stir up the debate on benefits and harms. Such problems should be tackled by well-designed RCTs which directly evaluate patient important outcomes including mortality, ICU and hospital length of stay and organ-failure free days. This is the so-called “one step reference”. Inferences become more complicated when “two step” diagnostic performance studies are used. The first step assesses the accuracy of the new biomarker (or the new diagnostic strategy) and related quality of evidence. The second step involves subjective judgment on the impact of test results on clinically relevant outcomes, which may range from survival, to other clinical end-points (such as reduction or prevention of organ failures, reduction of length of stay, and reduction of antibiotic therapy).
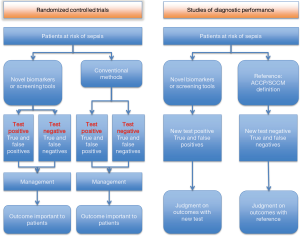
Evidence from RCTs
A PubMed search from inception to May 2016 looked for RCTs investigating the effect of early identification of sepsis by using biomarkers and screening tools on patient important outcomes. The search strategy included: ((((((early diagnosis[Title/Abstract]) OR screening[Title/Abstract]) OR screen[Title/Abstract]) OR early identification[Title/Abstract])) AND ((randomized[Title/Abstract]) OR randomization[Title/Abstract])) AND ((sepsis[Title/Abstract]) OR septic[Title/Abstract]). The initial search identified 62 studies. However, we found no RCTs that fulfilled the inclusion criteria.
PCT has been investigated in RCTs for its usefulness in patients with established sepsis (37,38). Although PCT was employed mainly as a biomarker to guide subsequent treatment in target populations with established diagnosis of sepsis or severe sepsis, it was also shown to be valuable for differentiating sepsis from SIRS due to non-infectious etiologies (39-41). Thus, it is discussed here within the scope of diagnosis of sepsis. A systematic review and meta-analysis published in 2013 showed that PCT-guided therapy significantly reduced the duration of antimicrobial therapy with no effect on mortality, or length of ICU and hospital stay. The risks of bias in included studies were mostly low or unclear (42-48). Only one study by Jensen and coworkers was considered to have high risk of bias in terms of selective reporting (46). A recent RCT, not included in Prkno’s systematic review, showed that PCT-guided therapy in patients with undifferentiated infection or suspected sepsis did not achieve a clinically significant 25% reduction in duration of antibiotic treatment (49). Taken together, PCT-guided therapy has no significant adverse consequences. It may shorten the duration of antibiotic exposure and therefore could reduce financial cost and development of antibiotic resistance.
Evidence from observational cohort studies
Many biomarkers and screening tools have been used for diagnostic purpose in observational cohort studies for their diagnostic performance. This performance should be evaluated by weighting their benefits and risks in the GRADE framework. Benefits and potential harms were evaluated in the context of each possible outcome of the diagnostic test: true positives, false positives, true negatives and false negatives (Table 3). A major issue is whether early recognition of sepsis may reduce mortality or other negative outcomes (true positives). One large observational study reported a decrease in mortality risk (from 37% to 30.8%) when complying with a sepsis resuscitation bundle. Although still robust after adjustment for known confounding factors, this study included historical controls and therefore some unmeasured confounders cannot be excluded (50). The study served as the primary evidence for the effect of early use of a sepsis bundle (4). However, it actually compared patients resuscitated according to bundle target with those without bundle target. Thus, it remains to be proven whether early initiation of therapy is of significant benefit. True negatives allow clinicians and patients to reduce uncertainty or anxiety related to diagnosis, cost and ICU admission. The potential benefits of true negative outcomes are debated. False positive (e.g., patients testing positive for sepsis but actually not septic) will result in unnecessary resuscitation procedures, more antibiotic exposure, and higher cost. Extensive evidence shows that a persisting positive fluid balance is associated with adverse outcomes (51-56). Therefore, inappropriate fluid management based on false positive results may create an unwarranted positive fluid balance with a corresponding higher mortality risk. Finally, initiation of resuscitation may be delayed in false negatives but it is not known whether this has any impact on patient-important outcome parameters. The potential clinical benefit and cost-effectiveness of early resuscitation (i.e., before diagnosis of sepsis is confirmed) needs further evaluation.

Full table
Biomarkers and screening tools for early recognition of sepsis
To be clinically useful, the diagnostic performance of a test is of vital importance. Sensitivity, specificity, likelihood ratios that are based on pre- and post-test odds/probabilities of sepsis in individual patients should be investigated in target populations. In this section, the diagnostic performance of each tool is extracted from the literature. In case of meta-analyses, pooled data have been employed (Table 4).
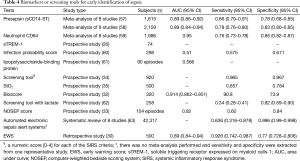
Full table
The diagnostic performance of biomarkers and screening tools varied widely in these studies. Screening by assigning numeric scores for each of the SIRS criteria appears to be most accurate for determination of sepsis (34). However, such screening scores actually employ the diagnostic criteria of sepsis and thus cannot guarantee early recognition of sepsis (sepsis prediction). Other screening strategies have moderate to good diagnostic accuracy but a substantial number of patients may be misclassified. More recently, the qSOFA score has been recommended to use for early recognition of patients with sepsis who require urgent monitoring or interventions (9). Since the impact of false positives and negatives on patient-important outcomes is still largely unexplored, these screening strategies cannot be fully recommended for clinical use until further prospective studies are done to address the abovementioned outcomes.
Use of automated electronic sepsis alert system (AeSAS) to improve sepsis management represents an area of active research (63). Advances in electronic medical system technology and sophisticated machine learning techniques will “upgrade” prediction models making them more accurate and individualized (64-66). AeSAS employs two or more SIRS criteria as alert threshold (67-70). Other studies use additional threshold such as SBP (71), and a lactic acid concentration >2 mmol/L (72,73). Two studies employed recursive partitioning tree analysis involving a variety of variables such as shock index, mean arterial blood pressure, international normalized ratio (INR), white blood cell (WBC) count, absolute neutrophil count, bilirubin, albumin, hemoglobin and sodium (74,75). One high-quality RCT, however, failed to identify any beneficial effect of this alerting system on patient-important outcomes (67). Up to now, AeSAS have only poor to moderate diagnostic performance and no beneficial effect on mortality risk and length of ICU stay.
Special considerations in low-income and middle-income countries (LIMC)
Because most of the literature focuses on identification of sepsis in developed countries, some screening tools and strategies may not be applicable to LIMC (76). For example, it has been reported that approximately 37% hospitals in African and Sub-Saharan African countries have no access to lactate measurement (77). As a result, the diagnosis of septic shock involving lactate criterion cannot be readily made in substantial number of hospitals in LIMC. Instead, there are other non-invasive, cheap and easy methods for screening inadequate tissue perfusion. Capillary refilling time can be a good alternative to blood lactate in measuring peripheral perfusion. In addition, pulse oximetry is also sensitive to poor perfusion with arterial oxygen saturation below 90% indicating hypoxemia and hypoxia (78).
With respect to the causes of sepsis, the Sepsis-3 was based on a large in-hospital cohort in the USA and respiratory and postoperative infections were the primary causes of sepsis. However, the community-acquired infections are more common in LIMC, with higher prevalence of gastroenteritis, septic abortion, skin and soft tissue infections. Sepsis and septic shock caused by these infections usually have different pathogens as compared with those used for the development of Sepsis-3. For example, salmonella was found to be the most prevalent isolate in a meta-analysis of 19 bacteremia studies in Africa (79). Some reports from LIMC show that dengue is an important cause of septic shock requiring ICU admission (80,81). Mycobacteria and HIV are prevalent in some areas of LIMC and their coexisting with sepsis and septic shock may impose great challenges to the treatment of this syndrome. Collectively, early recognition of sepsis in LIMC cannot be performed with screening tools as those being used in developed countries. Of note, there are some specific pathogens that can cause the form of sepsis and septic shock. Identification of such pathogens should take priority.
Conclusions
Sepsis is a heterogeneous syndrome characterized by a complex immune-inflammatory response to presumed or proven infection. However, due to some common features of this disorder, sepsis is treated and researched in one paradigm. In the absence of a gold standard, the diagnosis of sepsis remains challenging and subject to change. As a result, the clinical diagnosis of sepsis is ever changing to meet the clinical and research requirements. The original diagnosis criteria that were developed two decades ago were criticized for their lack of specificity. The later definition was deemed too complex and unsuitable for clinical purpose. The Sepsis-3 definitions better captures increased mortality risk of sepsis with organ dysfunction in response to infection, but the late progression or a highly time-dependent definition of septic conditions might result in delay of effective therapeutic intervention.
Early recognition of sepsis is an important research target. There are many novel biomarkers and screening tools for predicting the risk of sepsis. However, their diagnostic performance and effectiveness are poorly documented and thus cannot be recommended for clinical use. In the future, electronic medical record systems may allow better prediction of sepsis by using sophisticated machine learning techniques. Due to its heterogeneity and clinical impact, sepsis represents an exceptional example of the necessity of applying precision medicine, both for its early diagnosis and individualized treatment. The years to come encompass such important challenge.
Acknowledgements
We appreciate the help and comments on this guideline from our consultant Dr. Kenneth Nugent and the Secretary Grace Li (Science Editor, The Society for Translational Medicine. Email: lsl@amegroups.com).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Herrán-Monge R, Muriel-Bombín A, García-García MM, et al. Mortality Reduction and Long-Term Compliance with Surviving Sepsis Campaign: A Nationwide Multicenter Study. Shock 2016;45:598-606. [Crossref] [PubMed]
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003;29:530-8. [Crossref] [PubMed]
- Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644-55. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165-228. [Crossref] [PubMed]
- Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:775-87. [Crossref] [PubMed]
- Yu H, Chi D, Wang S, et al. Effect of early goal-directed therapy on mortality in patients with severe sepsis or septic shock: a meta-analysis of randomised controlled trials. BMJ Open 2016;6:e008330. [Crossref] [PubMed]
- Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med 2015;372:1629-38. [Crossref] [PubMed]
- Drewry AM, Hotchkiss RS. Sepsis: Revising definitions of sepsis. Nat Rev Nephrol 2015;11:326-8. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Angus DC. Opening the Debate on the New Sepsis Definition Defining Sepsis: A Case of Bounded Rationality and Fuzzy Thinking? Am J Respir Crit Care Med 2016;194:14-5. [Crossref] [PubMed]
- Simpson SQ. New Sepsis Criteria: A Change We Should Not Make. Chest 2016;149:1117-8. [Crossref] [PubMed]
- Angus DC, Seymour CW, Coopersmith CM, et al. A Framework for the Development and Interpretation of Different Sepsis Definitions and Clinical Criteria. Crit Care Med 2016;44:e113-21. [Crossref] [PubMed]
- Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001;286:1754-8. [Crossref] [PubMed]
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. [Crossref] [PubMed]
- Liu VX, Morehouse JW, Marelich GP, et al. Multicenter Implementation of a Treatment Bundle for Patients with Sepsis and Intermediate Lactate Values. Am J Respir Crit Care Med 2016;193:1264-70. [Crossref] [PubMed]
- Marshall JC, Vincent JL, Fink MP, et al. Measures, markers, and mediators: toward a staging system for clinical sepsis. A report of the Fifth Toronto Sepsis Roundtable, Toronto, Ontario, Canada, October 25-26, 2000. Crit Care Med 2003;31:1560-7. [Crossref] [PubMed]
- Granja C, Póvoa P. PIRO and sepsis stratification: reality or a mirage? Rev Bras Ter Intensiva 2015;27:196-8. [Crossref] [PubMed]
- Macdonald SP, Arendts G, Fatovich DM, et al. Comparison of PIRO, SOFA, and MEDS scores for predicting mortality in emergency department patients with severe sepsis and septic shock. Acad Emerg Med 2014;21:1257-63. [Crossref] [PubMed]
- Nguyen HB, Van Ginkel C, Batech M, et al. Comparison of Predisposition, Insult/Infection, Response, and Organ dysfunction, Acute Physiology And Chronic Health Evaluation II, and Mortality in Emergency Department Sepsis in patients meeting criteria for early goal-directed therapy and the severe sepsis resuscitation bundle. J Crit Care 2012;27:362-9. [Crossref] [PubMed]
- de Groot B, Lameijer J, de Deckere ER, et al. The prognostic performance of the predisposition, infection, response and organ failure (PIRO) classification in high-risk and low-risk emergency department sepsis populations: comparison with clinical judgement and sepsis category. Emerg Med J 2014;31:292-300. [Crossref] [PubMed]
- Chen YX, Li CS. Risk stratification and prognostic performance of the predisposition, infection, response, and organ dysfunction (PIRO) scoring system in septic patients in the emergency department: a cohort study. Crit Care 2014;18:R74. [Crossref] [PubMed]
- Surani S, Varon J. Biomarkers in the early diagnosis of sepsis: the quest continues. Am J Emerg Med 2015;33:1671. [Crossref] [PubMed]
- Stoppelkamp S, Veseli K, Stang K, et al. Identification of Predictive Early Biomarkers for Sterile-SIRS after Cardiovascular Surgery. PLoS One 2015;10:e0135527. [Crossref] [PubMed]
- Mussap M, Puxeddu E, Puddu M, et al. Soluble CD14 subtype (sCD14-ST) presepsin in premature and full term critically ill newborns with sepsis and SIRS. Clin Chim Acta 2015;451:65-70. [Crossref] [PubMed]
- Yang AP, Liu J, Yue LH, et al. Neutrophil CD64 combined with PCT, CRP and WBC improves the sensitivity for the early diagnosis of neonatal sepsis. Clin Chem Lab Med 2016;54:345-51. [Crossref] [PubMed]
- Halim B, Özlem T, Melek Ç, et al. Diagnostic and prognostic value of procalcitonin and sTREM-1 levels in sepsis. Turk J Med Sci 2015;45:578-86. [Crossref] [PubMed]
- Arabestani MR, Rastiany S, Kazemi S, et al. Conventional, molecular methods and biomarkers molecules in detection of septicemia. Adv Biomed Res 2015;4:120. [Crossref] [PubMed]
- Mickiewicz B, Tam P, Jenne CN, et al. Integration of metabolic and inflammatory mediator profiles as a potential prognostic approach for septic shock in the intensive care unit. Crit Care 2015;19:11. [Crossref] [PubMed]
- Sandquist M, Wong HR. Biomarkers of sepsis and their potential value in diagnosis, prognosis and treatment. Expert Rev Clin Immunol 2014;10:1349-56. [Crossref] [PubMed]
- Keep JW, Messmer AS, Sladden R, et al. National early warning score at Emergency Department triage may allow earlier identification of patients with severe sepsis and septic shock: a retrospective observational study. Emerg Med J 2016;33:37-41. [Crossref] [PubMed]
- Gibot S, Béné MC, Noel R, et al. Combination biomarkers to diagnose sepsis in the critically ill patient. Am J Respir Crit Care Med 2012;186:65-71. [Crossref] [PubMed]
- Liu Z, Chen J, Liu Y, et al. A simple bioscore improves diagnostic accuracy of sepsis after surgery. J Surg Res 2016;200:290-7. [Crossref] [PubMed]
- Mahieu LM, De Muynck AO, De Dooy JJ, et al. Prediction of nosocomial sepsis in neonates by means of a computer-weighted bedside scoring system (NOSEP score). Crit Care Med 2000;28:2026-33. [Crossref] [PubMed]
- Moore LJ, Jones SL, Kreiner LA, et al. Validation of a screening tool for the early identification of sepsis. J Trauma 2009;66:1539-46; discussion 1546-7. [Crossref] [PubMed]
- Goerlich CE, Wade CE, McCarthy JJ, et al. Validation of sepsis screening tool using StO2 in emergency department patients. J Surg Res 2014;190:270-5. [Crossref] [PubMed]
- Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 2013;66:719-25. [Crossref] [PubMed]
- Prkno A, Wacker C, Brunkhorst FM, et al. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock--a systematic review and meta-analysis. Crit Care 2013;17:R291. [Crossref] [PubMed]
- de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016;16:819-27. [Crossref] [PubMed]
- Mat-Nor MB, Md Ralib A, Abdulah NZ, et al. The diagnostic ability of procalcitonin and interleukin-6 to differentiate infectious from noninfectious systemic inflammatory response syndrome and to predict mortality. J Crit Care 2016;33:245-51. [Crossref] [PubMed]
- Anand D, Das S, Bhargava S, et al. Procalcitonin as a rapid diagnostic biomarker to differentiate between culture-negative bacterial sepsis and systemic inflammatory response syndrome: a prospective, observational, cohort study. J Crit Care 2015;30:218.e7-12. [Crossref] [PubMed]
- Gattas DJ, Cook DJ. Procalcitonin as a diagnostic test for sepsis: health technology assessment in the ICU. J Crit Care 2003;18:52-8. [Crossref] [PubMed]
- Annane D, Maxime V, Faller JP, et al. Procalcitonin levels to guide antibiotic therapy in adults with non-microbiologically proven apparent severe sepsis: a randomised controlled trial. BMJ Open 2013.3. [PubMed]
- Nobre V, Harbarth S, Graf JD, et al. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med 2008;177:498-505. [Crossref] [PubMed]
- Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010;375:463-74. [Crossref] [PubMed]
- Hochreiter M, Köhler T, Schweiger AM, et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care 2009;13:R83. [Crossref] [PubMed]
- Jensen JU, Hein L, Lundgren B, et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med 2011;39:2048-58. [Crossref] [PubMed]
- Schroeder S, Hochreiter M, Koehler T, et al. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg 2009;394:221-6. [Crossref] [PubMed]
- Svoboda P, Kantorová I, Scheer P, et al. Can procalcitonin help us in timing of re-intervention in septic patients after multiple trauma or major surgery? Hepatogastroenterology 2007;54:359-63. [PubMed]
- Shehabi Y, Sterba M, Garrett PM, et al. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. Am J Respir Crit Care Med 2014;190:1102-10. [Crossref] [PubMed]
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010;36:222-31. [Crossref] [PubMed]
- Acheampong A, Vincent JL. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care 2015;19:251. [Crossref] [PubMed]
- Sirvent JM, Ferri C, Baró A, et al. Fluid balance in sepsis and septic shock as a determining factor of mortality. Am J Emerg Med 2015;33:186-9. [Crossref] [PubMed]
- de Oliveira FS, Freitas FG, Ferreira EM, et al. Positive fluid balance as a prognostic factor for mortality and acute kidney injury in severe sepsis and septic shock. J Crit Care 2015;30:97-101. [Crossref] [PubMed]
- Kelm DJ, Perrin JT, Cartin-Ceba R, et al. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock 2015;43:68-73. [Crossref] [PubMed]
- Zhang Z, Zhang Z, Xue Y, et al. Prognostic value of B-type natriuretic peptide (BNP) and its potential role in guiding fluid therapy in critically ill septic patients. Scand J Trauma Resusc Emerg Med 2012;20:86. [Crossref] [PubMed]
- Sadaka F, Juarez M, Naydenov S, et al. Fluid resuscitation in septic shock: the effect of increasing fluid balance on mortality. J Intensive Care Med 2014;29:213-7. [Crossref] [PubMed]
- Zhang X, Liu D, Liu YN, et al. The accuracy of presepsin (sCD14-ST) for the diagnosis of sepsis in adults: a meta-analysis. Crit Care 2015;19:323. [Crossref] [PubMed]
- Wu J, Hu L, Zhang G, et al. Accuracy of Presepsin in Sepsis Diagnosis: A Systematic Review and Meta-Analysis. PLoS One 2015;10:e0133057. [Crossref] [PubMed]
- Wang X, Li ZY, Zeng L, et al. Neutrophil CD64 expression as a diagnostic marker for sepsis in adult patients: a meta-analysis. Crit Care 2015;19:245. [Crossref] [PubMed]
- Ratzinger F, Schuardt M, Eichbichler K, et al. Utility of sepsis biomarkers and the infection probability score to discriminate sepsis and systemic inflammatory response syndrome in standard care patients. PLoS One 2013;8:e82946. [Crossref] [PubMed]
- Kitanovski L, Jazbec J, Hojker S, et al. Diagnostic accuracy of lipopolysaccharide-binding protein for predicting bacteremia/clinical sepsis in children with febrile neutropenia: comparison with interleukin-6, procalcitonin, and C-reactive protein. Support Care Cancer 2014;22:269-77. [Crossref] [PubMed]
- Singer AJ, Taylor M, Domingo A, et al. Diagnostic characteristics of a clinical screening tool in combination with measuring bedside lactate level in emergency department patients with suspected sepsis. Acad Emerg Med 2014;21:853-7. [Crossref] [PubMed]
- Makam AN, Nguyen OK, Auerbach AD. Diagnostic accuracy and effectiveness of automated electronic sepsis alert systems: A systematic review. J Hosp Med 2015;10:396-402. [Crossref] [PubMed]
- Zhang Z. Big data and clinical research: focusing on the area of critical care medicine in mainland China. Quant Imaging Med Surg 2014;4:426-9. [PubMed]
- Zhang Z. Big data and clinical research: perspective from a clinician. J Thorac Dis 2014;6:1659-64. [PubMed]
- Angus DC. Fusing Randomized Trials With Big Data: The Key to Self-learning Health Care Systems? JAMA 2015;314:767-8. [Crossref] [PubMed]
- Hooper MH, Weavind L, Wheeler AP, et al. Randomized trial of automated, electronic monitoring to facilitate early detection of sepsis in the intensive care unit*. Crit Care Med 2012;40:2096-101. [Crossref] [PubMed]
- Meurer WJ, Smith BL, Losman ED, et al. Real-time identification of serious infection in geriatric patients using clinical information system surveillance. J Am Geriatr Soc 2009;57:40-5. [Crossref] [PubMed]
- Berger T, Birnbaum A, Bijur P, et al. A Computerized Alert Screening for Severe Sepsis in Emergency Department Patients Increases Lactate Testing but does not Improve Inpatient Mortality. Appl Clin Inform 2010;1:394-407. [Crossref] [PubMed]
- McRee L, Thanavaro JL, Moore K, et al. The impact of an electronic medical record surveillance program on outcomes for patients with sepsis. Heart Lung 2014;43:546-9. [Crossref] [PubMed]
- Nelson JL, Smith BL, Jared JD, et al. Prospective trial of real-time electronic surveillance to expedite early care of severe sepsis. Ann Emerg Med 2011;57:500-4. [Crossref] [PubMed]
- Umscheid CA, Betesh J, VanZandbergen C, et al. Development, implementation, and impact of an automated early warning and response system for sepsis. J Hosp Med 2015;10:26-31. [Crossref] [PubMed]
- Nguyen SQ, Mwakalindile E, Booth JS, et al. Automated electronic medical record sepsis detection in the emergency department. Peer J 2014;2:e343. [Crossref] [PubMed]
- Thiel SW, Rosini JM, Shannon W, et al. Early prediction of septic shock in hospitalized patients. J Hosp Med 2010;5:19-25. [Crossref] [PubMed]
- Sawyer AM, Deal EN, Labelle AJ, et al. Implementation of a real-time computerized sepsis alert in nonintensive care unit patients. Crit Care Med 2011;39:469-73. [Crossref] [PubMed]
- Rello J, Leblebicioglu H. members of ESGCIP. Sepsis and septic shock in low-income and middle-income countries: need for a different paradigm. Int J Infect Dis 2016;48:120-2. [Crossref] [PubMed]
- Baelani I, Jochberger S, Laimer T, et al. Availability of critical care resources to treat patients with severe sepsis or septic shock in Africa: a self-reported, continent-wide survey of anaesthesia providers. Crit Care 2011;15:R10. [Crossref] [PubMed]
- Neustein SM. The use of pulse oximetry in patients with poor peripheral perfusion. Acta Anaesthesiol Scand 2009;53:415-6. [Crossref] [PubMed]
- Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis 2010;10:417-32. [Crossref] [PubMed]
- Chandralekha Gupta P, Trikha A. The north Indian dengue outbreak 2006: a retrospective analysis of intensive care unit admissions in a tertiary care hospital. Trans R Soc Trop Med Hyg 2008;102:143-7. [Crossref] [PubMed]
- Singhi S, Kissoon N, Bansal A. Dengue and dengue hemorrhagic fever: management issues in an intensive care unit. J Pediatr (Rio J) 2007;83:S22-35. [Crossref] [PubMed]


