
Acute exacerbation of interstitial lung diseases and mortality post-cryobiopsy: a multicenter cohort study
Highlight box
Key findings
• Acute exacerbation of interstitial lung disease (AE-ILD) is rare following bronchoscopic cryobiopsy.
What is known and what is new?
• Cryobiopsy for diagnosis of interstitial lung diseases is safe and effective.
• AE-ILD post cryobiopsy has high mortality rates (50%) and is associated with higher number of samples.
What is the implication, and what should change now?
• AE-ILD following cryobiopsy necessitates further research to optimize patient outcomes and procedural safety.
Introduction
Interstitial lung diseases (ILDs) represent a diverse group of pulmonary disorders affecting the alveoli, interstitial space, and capillary membranes, encompassing over 150 distinct conditions. A significant clinical concern in these diseases is the occurrence of acute exacerbation of ILD (AE-ILD) following medical procedures.
Lung cryoprobe transbronchial biopsy (cryobiopsy) has emerged as a relatively new technique for obtaining tissue diagnoses in ILD cases. Emerging literature has shown that transbronchial cryobiopsy has diagnostic yield that is superior to that of transbronchial biopsy for both fibrotic and non-fibrotic ILDs, and across most diagnoses 78% vs. 48% respectively leading to increasing use of these technique for diagnoses of ILD (1). Despite its increasing use, there are limited data regarding the prevalence and severity of AE-ILD following cryobiopsy (2). Our study addresses this gap by presenting the largest published multicenter, retrospective series of AE-ILD post-cryobiopsy in the U.S. We aim to elucidate the clinical characteristics, potential risk factors, treatment modalities, and outcomes associated with this syndrome. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-270/rc).
Methods
Study design and patient population
This multicenter retrospective cohort study received approval from the institutional review boards at the University of Florida, Tulane University, and the University of Toledo (IRB202201745). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived. We screened patients undergoing cryobiopsy for the diagnosis of ILD from electronic medical records at all three institutions, spanning from January 1, 2014, through August 14, 2022. Eligible participants were patients aged over 18 with confirmed or suspected ILD, based on American Thoracic Society and the European Respiratory Society (ATS/ERS) criteria and/or features consistent with ILD on high-resolution computed tomography (HRCT) of the chest. The study population was divided into two cohorts: those who experienced ILD exacerbation post-cryobiopsy and those who did not, within 30 days following the procedure.
ILD definition
A diagnosis of ILD adhered to guidelines established by the ATS (3), incorporating expert provider diagnosis, imaging, serologies, and multidisciplinary discussions (MDDs). Predominantly, patients had imaging evidence suggestive of ILD but lacked a specific pathologic diagnosis, warranting cryobiopsy for tissue confirmation.
ILD definition
AE-ILD was diagnosed based on the International Working Group Revised Definition and Diagnostic Criteria for Acute Exacerbation of Idiopathic Pulmonary Fibrosis (AE-IPF) from 2016 (4). Considering the lack of a consensus definition for AE-ILD, we applied the AE-IPF criteria, aligning with prior literature (5,6). AE-ILD was characterized as an acute, clinically significant respiratory deterioration, marked by new widespread alveolar abnormality, onset of dyspnea within a month, computed tomography (CT) chest findings of new bilateral ground-glass opacities and/or consolidation superimposed on an ILD background, and symptoms not fully attributable to cardiac failure or fluid overload.
Study variables
We collected baseline clinical characteristics (age, gender, race, medical comorbidities, clinical features, and treatment modalities), diagnostic test variables (pulmonary function testing, histopathological data, HRCT findings), and treatment information, focusing on anticoagulation, steroids, and steroid-sparing disease-modifying agents.
Clinical outcomes
Measured outcomes included procedural complications [bleeding, ILD exacerbation, pneumothorax, infection, venous thromboembolism (VTE), respiratory failure necessitating increased oxygen requirements, and mortality]. All reported outcomes were attributed to the procedure if they occurred within 30 days of the index cryobiopsy.
Procedural details
Patients were reviewed at institutional ILD clinics and were presented at MDD ILD meeting where decision was made to pursue cryobiopsy. Cryobiopsy was pursued only when ILD MDD concluded alternative diagnosis and or usual interstitial pneumonitis (UIP) was possible based on CT scan but not definitive. Majority of the cases were performed under general anesthesia. Nearly half of these patients were intubated with rigid bronchoscope and other half had the procedure done with endotracheal tube in place. Bronchoalveolar lavage (BAL) was first performed in all patients followed by target lobe isolation using a 7-Fr endobronchial blocker for tamponade post biopsy; 1.7 mm non-disposable cryoprobe was used to obtain biopsy. Lung tissue was frozen for 3–4 seconds per proceduralists discretion and probe along with the bronchoscope was removed to retrieve tissue after each pass. As soon as probe was retracted, blocker was inflated for a minute to tamponade target lobe bleeding prior to taking another pass; 3–5 such passes were taken.
Statistical analysis
The majority of analyses conducted were descriptive in nature. For continuous variables, we used Mann-Whitney U test to compare the medians between patients with ILD exacerbation post-procedure and those without. Test was used due to small sample size and the non-normal distribution of data.
Results
Patient demographics and clinical characteristics
In this study, 111 patients underwent cryobiopsy for ILD evaluation. Among them, 4 patients (3.6%) experienced AE-ILD. Baseline clinical characteristics are summarized in Table 1. The cohort was predominantly White (85.6%), with a median age of 69.0 years [interquartile range (IQR), 17.0 years] and a near-equal distribution between male (49.6%) and female (50.4%) patients. The median body mass index (BMI) was 28.9 kg/m2 (IQR, 7.1 kg/m2). A majority had a history of current or former tobacco use (63.1%) and hypertension (56.8%). Other common comorbidities included diabetes mellitus type 2 (25.2%), chronic hypoxic respiratory failure [25.2% with a median requirement of 2.0 liters per minute (LPM)], chronic obstructive lung disease (COPD) (24.3%), and coronary artery disease (22.5%). Patients who experienced AE-ILD post-procedure had histories of tobacco (2/4) and marijuana use (1/4), hypertension (1/4), chronic hypoxic respiratory failure (1/4), COPD (1/4), asthma (1/4), or other immunocompromising conditions (1/4). Of these, two died during hospitalization.
Table 1
| Characteristic | ILD exacerbation post-procedure (n=4) | Patients without ILD exacerbation (n=107) | Total (n=111) |
|---|---|---|---|
| Age (years), median (IQR) | 54.5 (20.8) | 69.0 (15.0) | 69 (17.0) |
| Sex (male), n (%) | 0 | 55 (51.4) | 55 (49.5) |
| Hispanic ethnicity, n (%) | 0 | 1 (0.9) | 1 (0.9) |
| Race, n (%) | |||
| White | 4 (100.0) | 91 (85.0) | 95 (85.6) |
| Black | 0 | 10 (9.3) | 10 (9.0) |
| Hispanic | 0 | 1 (0.9) | 1 (0.9) |
| Asian | 0 | 2 (1.9) | 2 (1.8) |
| BMI (kg/m2), median (IQR) | 33.0 (21.8) | 28.9 (6.5) | 28.9 (7.1) |
| Patients on home oxygen, n (%) | 1 (25.0) | 33 (30.8) | 34 (30.6) |
| Average amount of oxygen (LPM), median (IQR) | 2.0 (4.0) | 2.0 (2.5) | 2.0 (3.0) |
| Prior medical history, n (%) | |||
| Tobacco use (former/current) | 2 (50.0) | 68 (63.6) | 70 (63.1) |
| Marijuana use (former/current) | 1 (25.0) | 11 (10.3) | 12 (10.8) |
| Vaping use (former/current) | 0 | 3 (2.8) | 3 (2.7) |
| Hypertension | 1 (25.0) | 62 (57.9) | 63 (56.8) |
| Type 2 diabetes mellitus | 0 | 28 (26.2) | 28 (25.2) |
| Congestive heart failure | 0 | 13 (12.1) | 13 (11.7) |
| Coronary artery disease | 0 | 25 (23.4) | 25 (22.5) |
| CVA/TIA | 0 | 4 (3.7) | 4 (3.6) |
| Peripheral vascular disease | 0 | 5 (4.7) | 5 (4.5) |
| Chronic hypoxic respiratory failure | 1 (25.0) | 27 (25.2) | 28 (25.2) |
| Chronic obstructive pulmonary disease | 1 (25.0) | 26 (24.3) | 27 (24.3) |
| Asthma | 1 (25.0) | 13 (12.1) | 14 (12.6) |
| Chronic kidney disease | 0 | 9 (8.4) | 9 (8.1) |
| Dementia | 0 | 2 (1.9) | 2 (1.8) |
| Cirrhosis | 0 | 2 (1.9) | 2 (1.8) |
| Immunocompromising condition | 1 (25.0) | 13 (12.1) | 14 (12.6) |
| Common variable immunodeficiency | 0 | 3 (2.8) | 3 (2.7) |
ILD, interstitial lung disease; IQR, interquartile range; BMI, body mass index; LPM, liters per minute; CVA, cerebrovascular accident; TIA, transient ischemic attack.
Medication use prior to procedure
Patients undergoing cryobiopsy were on aspirin (28.8%) or prednisone (22.5%) before the procedure. Anticoagulation was held in 66.7% of patients on such medication, and 25.0% had their antiplatelet agents held. Plavix was held on all patients prior to undergoing procedure and aspirin was held in a few but continued in most patients prior to procedure. Among the patients with AE-ILD, only one was on aspirin before the procedure, and none were on anticoagulants, prednisone, or steroid-sparing disease-modifying medications.
Baseline laboratory results and imaging findings
The median values for hemoglobin were 13.6 gm/dL (IQR, 1.9 gm/dL), platelets 248.0×103/µL (IQR, 110.0×103/µL), international normalized ratio (INR 1.0; IQR, 0.1), and blood urea nitrogen (BUN) 15.0 mg/dL (IQR, 7.0 mg/dL). Baseline pulmonary function test (PFT) and CT findings prior to biopsy are summarized in Table 2. Median forced expiratory volume in one second (FEV1) was 77.0% (IQR, 23.8%), forced vital capacity (FVC) 74.5% (IQR, 24.0%), FEV1/FVC ratio 85.0 (IQR, 76.0), total lung capacity (TLC) 68.0% (IQR, 20.5%), and diffusion capacity of carbon monoxide (DLCO) 51.0 mL/min/mmHg (IQR, 22.5 mL/min/mmHg). Significant CT findings included reticulations (55.9% of patients), traction bronchiectasis (49.5%), and ground-glass opacities (49.5%). Of those with AE-ILD, 50.0% showed traction bronchiectasis, honeycombing, ground-glass opacities, and reticulations. Pre-procedure BALs predominantly showed macrophage cellularity (67.0%, IQR, 59.3%).
Table 2
| Characteristic | ILD exacerbation post-procedure (n=4) | Patients without ILD exacerbation (n=107) | Total (n=111) |
|---|---|---|---|
| FEV1, %, median (IQR) | 81.5 (18.0) | 76.5 (23.0) | 77 (23.8) |
| FVC, %, median (IQR) | 78.0 (22.5) | 74.5 (25.3) | 74.5 (24.0) |
| FEV1/FVC ratio, median (IQR) | 90.0 (83.4) | 85.0 (76.0) | 85.0 (76.0) |
| TLC, %, median (IQR) | 73.0 (15.5) | 68.0 (22.3) | 68.0 (20.5) |
| DLCO, mL/min/mmHg, median (IQR) | 54.7 (28.1) | 50.5 (22.5) | 51.0 (22.5) |
| 6MWT distance, meters, median (IQR) | 381.0 (0.0) | 330.0 (156.2) | 331.5 (149.9) |
| CT findings, n (%) | |||
| Traction bronchiectasis | 2 (50.0) | 53 (49.5) | 55 (49.5) |
| Bronchiolectasis | 1 (25.0) | 21 (19.6) | 22 (19.8) |
| Honeycombing | 2 (50.0) | 23 (21.5) | 25 (22.5) |
| Ground-glass opacities | 2 (50.0) | 53 (49.5) | 55 (49.5) |
| Reticulations | 2 (50.0) | 60 (56.1) | 62 (55.9) |
| Interlobular septal thickening, n (%) | 1 (25.0) | 24 (22.4) | 25 (22.5) |
| Emphysema | 0 | 19 (17.8) | 19 (17.1) |
| Bullae | 0 | 4 (3.7) | 4 (3.6) |
| Cysts | 1 (25.0) | 13 (12.1) | 14 (12.6) |
| Nodules | 1 (25.0) | 23 (21.5) | 24 (21.6) |
| Tree-in-bud | 0 | 5 (4.7) | 5 (4.5) |
| Upper lobe predominant disease | 1 (25.0) | 29 (27.1) | 30 (27.0) |
| Lower lobe predominant disease | 1 (25.0) | 39 (36.4) | 40 (36.0) |
| Diffuse disease | 1 (25.0) | 37 (34.6) | 38 (34.2) |
| TTE findings | |||
| LVEF, %, median (IQR) | 57.5 (8.8) | 60.0 (5.0) | 60.0 (54.0) |
| RVSP, mmHg, median (IQR) | 45.0 (0.0) | 32.0 (18.0) | 33.5 (30.1) |
| Tricuspid regurgitant peak velocity, m/s, median (IQR) | 2.6 (1.5) | 2.4 (0.8) | 2.4 (2.1) |
| Aortic stenosis present, n (%) | 0 | 5 (4.7) | 5 (4.5) |
| Aortic regurgitation present, n (%) | 0 | 22 (20.6) | 22 (19.8) |
| Mitral stenosis present, n (%) | 0 | 2 (1.9) | 2 (1.8) |
| Mitral regurgitation present, n (%) | 2 (50.0) | 47 (43.9) | 49 (44.1) |
| Tricuspid regurgitation present, n (%) | 4 (100.0) | 58 (54.2) | 62 (55.9) |
| Pre-procedure BAL, median (IQR) | |||
| Polys, % | 46.0 (74.3) | 25.0 (45.0) | 25.0 (45.0) |
| Lymphs, % | 9.0 (48.3) | 5.0 (11.5) | 5.0 (12.0) |
| Eosinophils, % | 5.5 (7.0) | 2.0 (5.0) | 2.0 (5.0) |
| Monocytes, % | 2.0 (67.0) | 33.0 (60.3) | 33.0 (62.5) |
| Macrophages, % | 23.0 (0.0) | 67.0 (55.0) | 67.0 (59.3) |
PFT, pulmonary function test; ILD, interstitial lung disease; FEV1, forced expiratory volume in one second; IQR, interquartile range; FVC, forced vital capacity; TLC, total lung capacity; DLCO, diffusion capacity of carbon monoxide; 6MWT, 6-minute walk test; CT, computed tomography; TTE, transthoracic echocardiogram; LVEF, left ventricular ejection fraction; RVSP, right ventricular systolic pressure; BAL, bronchoalveolar lavage.
Procedural characteristics
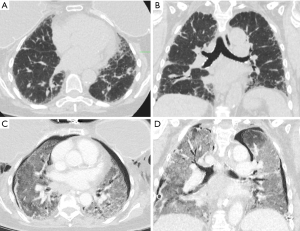
Procedural details are provided in Table 3. Most patients (98.2%) underwent the procedure under general anesthesia, and 49.5% had rigid bronchoscopy for cryobiopsy. The median procedural duration was 49.0 minutes (IQR, 23.3 minutes) with a median of 4.0 biopsies (IQR, 2.0). Overall, 3.6% of patients experienced AE-ILD, 9.9% experienced bleeding (including 2 with AE-ILD), 15.3% had pneumothorax (including one with AE-ILD), and 2 patients developed post-procedural pneumonia. Patients with AE-ILD had a higher median number of biopsies (5.5, IQR, 1.8) and typically experienced AE-ILD within a median of 4.0 days post-procedure (IQR, 6.3 days). Figure 1 shows radiographic evidence of AE-ILD post-cryobiopsy, including associated pneumothorax.
Table 3
| Characteristic | ILD exacerbation post-procedure (n=4) | Patients without ILD exacerbation (n=107) | Total (n=111) |
|---|---|---|---|
| Sedation, n (%) | |||
| General anesthesia | 4 (100.0) | 105 (98.1) | 109 (98.2) |
| Conscious sedation | 0 | 2 (1.9) | 2 (1.8) |
| Rigid bronchoscopy, n (%) | 3 (75.0) | 52 (48.6) | 55 (49.5) |
| Cryobiopsy location, n (%) | |||
| Right upper lobe | 2 (50.0) | 17 (15.9) | 19 (17.1) |
| Right middle lobe | 1 (25.0) | 2 (1.9) | 3 (2.7) |
| Right lower lobe | 2 (50.0) | 61 (57) | 63 (56.8) |
| Left upper lobe | 0 | 7 (6.5) | 7 (6.3) |
| Lingula | 0 | 2 (1.9) | 2 (1.8) |
| Left lower lobe | 0 | 29 (27.1) | 29 (26.1) |
| Procedure duration, minutes, median (IQR) | 40.5 (17.0) | 49.0 (23.0) | 49.0 (23.3) |
| Number of biopsies, median (IQR) | 5.5 (1.8) | 4.0 (1.25) | 4.0 (2.0) |
| Number of lobes sampled, median (IQR) | 1.0 (0.8) | 1.0 (0.0) | 1.0 (0.0) |
| Bilateral biopsies, n (%) | 0 | 0 | 0 |
| Unilateral biopsies, n (%) | 4 (100.0) | 107 (100.0) | 111 (100.0) |
| Complications, n (%) | |||
| Bleeding | 0 | 11 (10.3) | 11 (9.9) |
| Requiring cryotherapy | 0 | 2 (1.9) | 2 (1.8) |
| Requiring endobronchial blocker | 0 | 15 (14) | 15 (13.5) |
| Requiring Fogarty balloon | 1 (25.0) | 11 (10.3) | 12 (10.8) |
| ILD exacerbation | 4 (100.0) | 0 | 4 (3.6) |
| Persistent air leak | 0 | 2 (1.9) | 2 (1.8) |
| Pneumothorax | 0 | 17 (15.9) | 17 (15.3) |
| Pneumonia | 1 (25.0) | 1 (0.9) | 2 (1.8) |
| Sepsis | 0 | 0 | 0 |
| Deep vein thrombosis | 0 | 0 | 0 |
| Pulmonary embolism | 0 | 0 | 0 |
| Mechanical ventilation | 0 | 0 | 0 |
| Time before ILD exacerbation, days, median (IQR) | 4.0 (6.3) | 0 | 0 |
| Post-procedure BAL, median (IQR) | |||
| Polys, % | 11.0 (0.0) | 30.0 (63.5) | 23.0 (62.8) |
| Lymphs, % | 32.0 (0.0) | 8.5 (16.8) | 10.0 (17.5) |
| Eosinophils, % | 0 | 1.0 (8.5) | 0.5 (8.3) |
| Monocytes, % | 0 | 0 (23.3) | 0 (15.5) |
| Macrophages, % | 57 (0.0) | 27.5 (48.0) | 29.0 (53.0) |
| Diagnosis, n (%) | |||
| IPF | 1 (25.0) | 19 (17.8) | 20 (18.0) |
| CTD/ILD | 0 | 10 (9.3) | 10 (9.0) |
| HP | 0 | 23 (21.5) | 23 (20.7) |
| IPAF | 0 | 3 (2.8) | 3 (2.7) |
| Sarcoidosis | 0 | 6 (5.6) | 6 (5.4) |
| Organizing pneumonia | 1 (25.0) | 2 (1.9) | 3 (2.7) |
| Malignancy | 0 | 9 (8.4) | 9 (8.1) |
| Non-diagnostic | 2 (50.0) | 43 (40.2) | 45 (40.5) |
| Normal | 0 | 2 (1.9) | 2 (1.8) |
| Histology, n (%) | |||
| UIP | 0 | 21 (19.6) | 21 (18.9) |
| NSIP | 1 (25.0) | 13 (12.1) | 14 (12.6) |
| Organizing pneumonia | 1 (25.0) | 3 (2.8) | 4 (3.6) |
| Eosinophilia | 0 | 3 (2.8) | 3 (2.7) |
| Fibrosis | 3 (75.0) | 57 (53.3) | 60 (54.1) |
| Acute inflammation | 0 | 7 (6.5) | 7 (6.3) |
| Chronic inflammation | 2 (50.0) | 61 (57.0) | 63 (56.8) |
| Post-procedure radiology, n (%) | |||
| Pneumothorax | 0 | 12 (11.2) | 12 (10.8) |
| Ground-glass opacities (unilateral) | 0 | 1 (0.9) | 1 (0.9) |
| Ground-glass opacities (bilateral) | 1 (25.0) | 24 (22.4) | 25 (22.5) |
| Consolidation (unilateral) | 0 | 23 (21.5) | 23 (20.7) |
| Consolidation (bilateral) | 0 | 6 (5.6) | 6 (5.4) |
| Interlobular septal thickening (unilateral) | 0 | 1 (0.9) | 1 (0.9) |
| Interlobular septal thickening (bilateral) | 0 | 7 (6.5) | 7 (6.3) |
| Findings contralateral to biopsy | 1 (25.0) | 11 (10.3) | 12 (10.8) |
| Findings ipsilateral to biopsy | 1 (25.0) | 30 (28.0) | 31 (27.9) |
ILD, interstitial lung disease; IQR, interquartile range; BAL, bronchoalveolar lavage; IPF, idiopathic pulmonary fibrosis; CTD/ILD, connective tissue disease associated ILD; HP, hypersensitivity pneumonitis; IPAF, interstitial pneumonia with autoimmune features; UIP, usual interstitial pneumonia; NSIP, nonspecific interstitial pneumonitis.
Post-procedure outcomes
Intraoperative BALs were predominantly macrophage (29.0%), followed by polymorphonuclear leukocytes (23.0%). Patients with AE-ILD primarily had macrophage cellularity (57.0%). Diagnostic yield of cryobiopsy in our study was 50% based on pathology alone, however when these cases were discussed at ILD MDD the diagnostic yield increased to 74% which is consistent with what has been previously described in literature (7). Post-procedure CT scans mainly showed changes ipsilateral to the biopsy site.
Out of 111 patients sampled, 4 (approximately 3.6%) experienced AE-ILD. The patients who developed AE-ILD had a median of 5.5 biopsies, compared to a median of 4 biopsies in patients who did not develop AE-ILD. Using a Mann-Whitney U test to compare the median number of biopsies between the two groups, the P value was found to be 0.046, indicating statistically significant association between number of biopsies and exacerbation,
Furthermore, the mortality rate for patients with AE-ILD was observed to be 50%, with 2 out of 4 AE-ILD patients dying. All patients with AE were intubated, treated with high dose steroids and antibiotics and one of them was placed on venovenous (VV) extracorporeal membrane oxygenation (ECMO). A binomial test was conducted to assess whether this mortality rate significantly deviates from an observed general mortality rate for AE-ILD. The test resulted in a P value =0.61, observed mortality was not statistically significant.
Discussion
This multicenter retrospective study sheds light on the AE-ILDs following cryobiopsy, a relatively new technique in pulmonary diagnostics. From our significant cohort, we observed an overall complication rate of 32%, including pneumonia, pneumothorax, AE-ILD, and bleeding requiring intervention. The 30-day mortality rate stood at 1.8% (2 of 111 patients). Notably, the AE-ILD rate was 3.6%, with a significant correlation observed between the number of biopsies taken and the development of AE-ILD; patients developing AE had a median of 5.5 biopsies, compared to 4 in others. The mortality rate for AE-ILD in our series was 50%. Of the AE-ILD cases, two had non-diagnostic pathology, one with findings suggestive of nonspecific interstitial pneumonia (NSIP) and another indicative of idiopathic pulmonary fibrosis.
Transbronchial lung biopsies are considered safe and not been implicated commonly for AE-ILD. Earlier reports of AE-ILD post-cryoprobe lung biopsy emerged around 2014. For instance, Casoni et al. reported a single fatal AE-ILD case in a series of 69 cryoprobe lung biopsies for ILD diagnosis (8). Dhooria et al. reviewed 128 patients undergoing cryoprobe lung biopsies across four centers in India, noting a 2.5% incidence of AE-ILD, all of which were fatal (9). Our study mirrors these incidences and reports a 50% mortality rate for AE-ILD, with an overall mortality of 1.8%.
The incidence of AE-ILDs and mortality following cryobiopsy, as observed in our study, offers a compelling contrast to AE-ILD incidences and mortality following surgical lung biopsy. Fisher et al. (10) reported that of the 3,057 surgical lung biopsies for ILD performed at high volume hospitals; 30-day mortality was 7.1%, 20.2% and 1.9% in overall, nonelective and elective patients, respectively. Other studies have reported incidence of postoperative AE-ILD following surgical lung biopsy for ILD from 9% to 24% (11,12). More recent study by Pastre et al. (13) showed that surgical lung biopsy for ILD when done electively had a 1-month complication rate was 8%, whereas 4% had a severe complication, and there were no deaths with majority of them being discharged the same day. These findings, juxtaposed with our results, suggest that cryobiopsies may be safer in terms of complications, AE and mortality, especially in patients with poor pulmonary function and significant comorbidities who are otherwise not candidates for surgical lung biopsy.
The higher incidence of AE-ILD observed in our study might be attributed to its multicenter nature and diverse patient demographics. It underscores the risk of AE-ILD in patients undergoing invasive diagnostic procedures and emphasizes the need for careful patient selection and pre-procedural assessment. Our data also suggest that certain characteristics, like a history of tobacco use or comorbidities such as hypertension and COPD, may predispose individuals to a higher risk of AE-ILD. However, the small number of AE-ILD cases in our study necessitates further research for definitive conclusions.
The management of AE-ILD in our cohort varied, with all patients getting broad spectrum antibiotics and high dose steroids. One patient received ECMO. The mortality rate observed in our study underscores the severity of AE-ILD and the need for effective management strategies.
Given the study’s retrospective design and limited AE-ILD cases, our findings’ generalizability is restricted. Prospective studies with larger cohorts are needed to validate our observations and to delve deeper into the mechanistic aspects of AE-ILD post-cryobiopsy.
Conclusions
our study contributes to the growing understanding of AE-ILD and mortality following cryobiopsy in ILD patients. Based on existing literature, cryobiopsy may present lower overall mortality compared to surgical lung biopsy, particularly in patients with compromised lung function and significant comorbidities. These results indicate that there may be a notable difference in the median number of biopsies between AE-ILD and non-AE-ILD patients, and the high mortality rate observed in AE-ILD patients is of clinical concern, warranting further investigation. This study not only provides valuable insights, but also underscores the need for ongoing research to enhance patient outcomes and procedural safety in managing ILD.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-270/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-270/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-270/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-270/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review boards at the University of Florida, Tulane University, and the University of Toledo (IRB202201745). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Freund O, Wand O, Schneer S, et al. Transbronchial Cryobiopsy Is Superior to Forceps Biopsy for Diagnosing both Fibrotic and Non-Fibrotic Interstitial Lung Diseases. Respiration 2023;102:852-60. [Crossref] [PubMed]
- Zayed Y, Alzghoul BN, Hyde R, et al. Role of Transbronchial Lung Cryobiopsy in the Diagnosis of Interstitial Lung Disease: A Meta-analysis of 68 Studies and 6300 Patients. J Bronchology Interv Pulmonol 2023;30:99-113. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-68. [Crossref] [PubMed]
- Collard HR, Ryerson CJ, Corte TJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med 2016;194:265-75. [Crossref] [PubMed]
- Amundson WH, Racila E, Allen T, et al. Acute exacerbation of interstitial lung disease after procedures. Respir Med 2019;150:30-7. [Crossref] [PubMed]
- Kolb M, Bondue B, Pesci A, et al. Acute exacerbations of progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018;27:180071. [Crossref] [PubMed]
- Troy LK, Grainge C, Corte TJ, et al. Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): a prospective, comparative study. Lancet Respir Med 2020;8:171-81. [Crossref] [PubMed]
- Casoni GL, Tomassetti S, Cavazza A, et al. Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS One 2014;9:e86716. [Crossref] [PubMed]
- Dhooria S, Mehta RM, Srinivasan A, et al. The safety and efficacy of different methods for obtaining transbronchial lung cryobiopsy in diffuse lung diseases. Clin Respir J 2018;12:1711-20. [Crossref] [PubMed]
- Fisher JH, Shapera S, To T, et al. Procedure volume and mortality after surgical lung biopsy in interstitial lung disease. Eur Respir J 2019;53:1801164. [Crossref] [PubMed]
- Yano T, Koga T, Ninomiya S, et al. A review of Japanese literatures concerning surgery for lung cancer with idiopathic interstitial pneumonia. Kyobu Geka 2002;55:131-3.
- Sato S, Shimizu Y, Goto T, et al. Survival after repeated surgery for lung cancer with idiopathic pulmonary fibrosis: a retrospective study. BMC Pulm Med 2018;18:134. [Crossref] [PubMed]
- Pastre J, Khandhar S, Barnett S, et al. Surgical Lung Biopsy for Interstitial Lung Disease. Safety and Feasibility at a Tertiary Referral Center. Ann Am Thorac Soc 2021;18:460-7. [Crossref] [PubMed]


