
The causal effect of hot beverage temperature preference on esophageal cancer risk: a Mendelian randomization study
Highlight box
Key findings
• The current Mendelian randomization (MR) analysis provides new genetic evidence for a null causal relationship between hot beverage temperature preference and esophageal cancer (EC), both in the European population and the East Asian population.
What is known and what is new?
• Previous research has found a link between the temperature of food and beverages and the risk of EC. But a causal relationship between the two has not been well established.
• In the current study, we used a two-sample MR analysis to estimate the causal effect of hot beverage temperature preference on the risk of EC. No causal effect was detected between hot beverage temperature preference and EC risk, both in the European and East Asian populations.
What is the implication, and what should change now?
• Evidence to prevent EC by reducing the intake of hot beverages is insufficient.
Introduction
Esophageal cancer (EC) is one of the most common digestive tract tumors, and it is the seventh leading cause of cancer-related death (1). Some studies have shown that the development of EC may be related to dietary habits (2). Intaking beverage/food at high temperature is one of the dietary behaviors that raise the risk of EC (3,4). A meta-analysis indicated that consumption of hot beverages and food was associated with increased EC risk (5). The thermal injuries to the esophageal mucosa caused by hot food and beverages may contribute to the development of EC (4). Heat stress has the potential to harm the structure and function of the esophageal epithelium (6).
However, all of the aforementioned findings are from observational research and simply show a correlation between food and beverage temperature and EC. A clear causal relationship between the two has not been well established. Although randomized controlled trials (RCTs) are the gold standard for examining the causal relationship, conducting an RCT to evaluate the correlation between food and beverage temperature and EC is extremely challenging. This is due in part to the difficulties in managing other risk variables as well as the relatively extended follow-up period.
Mendelian randomization (MR) research is a widely used analytic tool for studying causal relationships (7). In the absence of RCTs or when conducting RCTs is difficult, MR research can be used to provide credible information on the causal relationship between exposure factors and disease risks (8). MR uses genetic variants to determine whether an observed correlation between a risk factor and an outcome aligns with a causal relationship. This approach relies on the natural, random allocation of genetic variants during meiosis, resulting in a random distribution of these variants within a population. Individuals are naturally predisposed to inherit or not inherit a genetic variant at birth that impacts a risk factor. Individuals carrying this variant and those without it are followed up for the development of a specific outcome. Since these genetic variants are generally unrelated to confounders, any variations in the outcome among carriers and non-carriers can be attributed to the differences in the risk factor (7,9). These genetic variants are called as instrumental variables (IVs). The genetic variants are randomly allocated during fertilization, which mimics the RCT process. Therefore, the causal relationship is less likely to be biased by confounding factors such as sex and age (10). Furthermore, reverse causality is also less likely because genotypes are formed before disease onset and are normally unaffected by disease development (11). During the process of human gamete formation, the alleles of specific single nucleotide polymorphisms (SNPs) undergo a random distribution among sperm cells. This ensures that the inherited variants are not influenced by potentially confounding environmental exposures. Thus, SNPs are frequently utilized as IVs to proxy the traits of interest in MR research (12).
Because RCTs to evaluate the long-term effect of hot beverage temperature preference on EC are not practicable, MR research offers an alternative strategy. Given the uncertainty about the causality between hot beverage temperature preference and EC risk, we conducted the current MR research to assess the causal effect of hot beverage temperature preference on EC risk using large-scale genome-wide association studies (GWAS) data. Because the frequency of genetic variants and the distribution of EC are different in European and Asian populations, we used GWAS data from the European population and the East Asian population to estimate the causal relationship, respectively. We present this article in accordance with the STROBE-MR reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-361/rc).
Methods
Study design
We performed a two-sample MR analysis to assess the causal effect of temperature preference for hot beverages on EC using GWAS summary data. The MR research fulfills the following three assumptions: (I) the genetic instruments are strongly associated with the exposure; (II) the genetic instruments are not associated with the outcome via a confounding pathway; and (III) the genetic instruments affect the outcome only via the exposure (13). Genetic information for hot beverage temperature preference was obtained from the GWAS datasets. A basic overview of the MR research design is shown in Figure 1. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
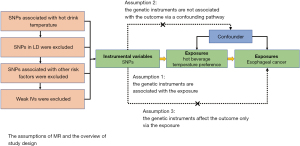
GWAS data for hot beverages temperature
GWAS data for hot beverage temperature preference (ukb-b-14203 for the European population and ukb-e-1518_EAS for the East Asian population) were obtained from the UK biobank (14). There were 457,873 European participants and 2,617 East Asian participants included in the analysis. The participants answered 29 questions about their diet on a touchscreen questionnaire. Regarding the beverage temperature, the participants were questioned about their preferred hot beverage temperature. The possible answers included “Prefer not to answer”, “Do not drink hot beverages”, “Very hot”, “Hot”, and “Warm” (14). Summary statistics of hot beverage temperature preference by GWAS were used to find out the SNPs associated with hot beverage temperature preference. The GWAS data were adjusted for age and sex. To control the quality of the SNPs, we employed the following filtering processes before MR analysis. First, we selected SNPs with P<5×10−8 and SNPs in linkage disequilibrium (with R2≥0.001 and within 10 Mb) were discarded. Considering the limited number of SNPs screened, we relaxed the threshold to P<1×10−5 and linkage disequilibrium with R2<0.01 in the ukb-e-1518_EAS dataset, according to a previous study (15). Second, we used F-statistics to estimate the weak IV bias. An IV with an F value <10 was judged weak. Weak IVs were excluded. Finally, we employed a harmonization procedure to remove ambiguous and indromic SNPs.
GWAS data for EC
GWAS data for EC in the European population (ieu-b-4960 and ebi-a-GCST90018841) and East Asian population (bbj-a-117 and ebi-a-GCST90018621) were obtained from the Integrative Epidemiology Unit (IEU) project database (https://gwas.mrcieu.ac.uk/). For the European population, 1,738 EC cases and 847,324 control subjects were included. For the East Asian population, 2,688 EC cases and 1,202,270 control subjects were included.
MR and statistical analysis
We performed a two-sample MR analysis using the “TwoSampleMR” R package (v 0.5.6). Firstly, we used the “harmonise” function to harmonize the alleles and effects between the exposure and outcome. Then, we applied a random-effect inverse variance weighted (IVW) as the main analytical method to estimate the causal effect and used various sensitivity analyses, including MR Egger, weighted median, simple mode, and weighted mode, to examine the potential violation of the second and third assumptions of MR (16). No pleiotropy of the IVs existed if the Egger-intercept was close to 0 (17). The heterogeneity of the estimates was evaluated using a scatter plot and Cochran’s Q test (18). We also used a leave-one-out sensitivity analysis to further validate the estimates by removing a single variant from the analysis in turn. The result of the leave-one-out analysis reflects the possibility of an outlier variant. To verify whether the independence assumption was satisfied, we searched the PhenoScanner database (http://www.phenoscanner.medschl.cam.ac.uk/). If a SNP was related to an EC risk factor (19) (such as alcohol consumption, tobacco consumption, high body mass index (BMI) (obesity) and gastroesophageal reflux disease) other than hot beverage temperature preference (P<5×10−8), it was removed from the analysis. All analyses were performed using the R software (v4.2.2). The analytic code used for the analysis was included in Appendix 1. P values <0.05 were considered significant.
Meta analysis
To validate the results, we conducted a meta-analysis using the two datasets from the European population and the two datasets from the East Asian population, respectively. If I2>50%, which means high heterogeneity exists, a random effects model was used. Otherwise, a fixed effects model was used.
Results
Selection of IVs
There were 71 and 16 SNPs identified to be related to hot beverage temperature preference for the European and East Asian populations, respectively (Table S1). No SNP was related to other confounding risk factors. For the European population, rs3132487 was removed for incompatibility, and rs12038134 and rs58726064 were removed because they were palindromic with intermediate allele frequencies. For the East Asian population, 4 of the 16 SNPs for hot beverage temperature preference were missed in the EC datasets, and rs6066750 was removed for being palindromic with intermediate allele frequencies. The F values of the SNPs were all larger than 10, indicating that no weak IV was included. Finally, 68 SNPs for the European population and 11 SNPs for the East Asian population were included for MR analysis.
The causal effect of hot beverage temperature preference on EC in the main analysis
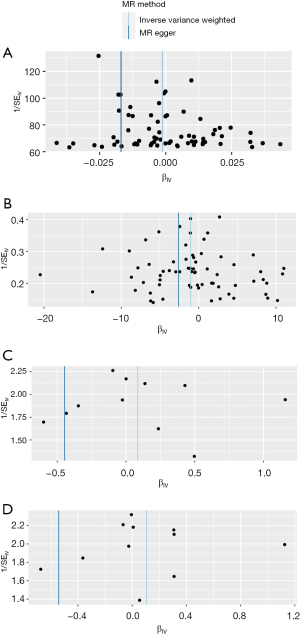
The scatter plot of the SNP effect on EC versus the SNP effect on hot beverage temperature preference is shown in Figure 2. The estimates of the causal effect of hot beverage temperature preference on EC are shown in Figure 3. No significant causal effect was found between hot beverage temperature preference and EC in the European population {for the ieu-b-4960 dataset, inverse variance weighted odd ratio (ORIVW) =1.00 [95% confidence interval (CI): 0.99–1.00], P=0.54; for the ebi-a-GCST90018841 dataset, ORIVW =0.35 (95% CI: 0.10–1.29), P=0.12} or in the East Asian population [for the bbj-a-117 dataset, ORIVW =1.09 (95% CI: 0.80–1.48), P=0.59; for the ebi-a-GCST90018621 dataset, ORIVW =1.11 (95% CI: 0.82–1.50), P=0.49]. Using the MR-Egger, weighted median, weighted mode, and simple mode methods, we also found no association between hot beverage temperature preference and EC both in the European and East Asian populations (Figure 3). Heterogeneity was detected between hot beverage temperature preference and EC by Cochran Q test in the IVW and MR-Egger analysis for the European population (P<0.05), but no heterogeneity was detected for the East Asian population (P>0.05). Funnel plots of the IVW and MR-Egger analyses are shown in Figure 4. No horizontal pleiotropy was observed in the relationship between hot beverage temperature preference and EC in the two populations (P>0.05).
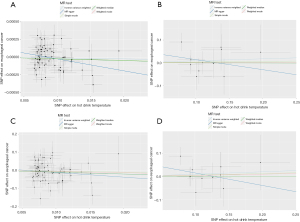

The causal effect of hot beverage temperature preference on EC in the sensitivity analysis
Because heterogeneity was detected for the European population, we used MR-Pleiotropy Residual Sum and Outlier (MR-PRESSO) to exclude the outlier SNPs. In the ieu-b-4960 dataset, rs2472297 was excluded. Estimates before and after excluding the outlier differed (P=0.03 for the MR-PRESSO distortion test). We repeated the analyses after removing the outlier. The results revealed that hot beverage temperature preference remained unrelated to EC [ORIVW =1.00 (95% CI: 0.99–1.00), P=0.93]. No causal relationship was found using the MR-Egger, weighted median, weighted mode, and simple mode methods (Figure S1). In the ebi-a-GCST90018841 dataset, rs1260326 and rs210600 were excluded. Estimates before and after excluding the outlier did not differ (P=0.19 for the MR-PRESSO distortion test). A leave-one-out analysis showed that removing any of the SNPs did not change the result of the main analysis both in the European and East Asian populations (Figure 5).

Meta-analysis confirmed the null causal relationship between hot beverage temperature preference and EC
A meta-analysis was conducted to further validate the results. In the European population, high heterogeneity existed, and thus a random effect model was used. In the East Asian population, the heterogeneity was low, and a fixed effect model was used. The combined analysis showed that there was no causal relationship between hot beverage preference and risk of EC in the two populations [for the European population, OR =0.73 (95% CI: 0.29–1.87), P=0.12; for the East Asian population, OR =1.10 (95% CI: 0.89–1.36), P=0.93] (Figure 6). The results were consistent with the previous results.
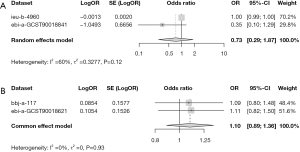
Discussion
In the current study, we used a two-sample MR analysis to estimate the causal effect of hot beverage temperature preference on the risk of EC. No causal effect was detected between hot beverage temperature preference and EC risk, both in the European and East Asian populations.
Some studies indicate that hot food and beverage consumption may increase the risk of EC. A meta-analysis including 23 observational studies showed that those who drank hot tea more often were more likely to develop esophageal squamous cell carcinoma (ESCC) (20). Another meta-analysis, including 12 case-control studies, found that increased consumption of hot tea beverages was associated with an increased risk of EC, with an OR of 2.04 (95% CI: 1.78–2.31) (21). A case-control study, which included 849 cases and 906 controls, also showed that increased consumption of very hot beverage /food was associated with an increased risk of ESCC (OR =1.92, 95% CI: 1.50–2.46) (22). However, the above findings are from observational or case-control studies, which indicate only a correlation between the temperature of food/beverages and EC, not a causal relationship. Though RCTs provide the highest level of evidence for causal relationships, conducting an RCT to explore the correlation between food and beverage temperature and EC is extremely difficult. The MR study mimics the process of RCTs and is widely used to study the causal relationship in cases where prospective RCTs are difficult to conduct. Thus, we used the MR research in the current study to investigate the causal effect between the hot beverage temperature preference and EC. Because ESCC is more common in Asia and esophageal adenocarcinoma (EAC) is more common in Western countries, and because the risk factors for ESCC and EAC are different, we analyzed the East Asian population and the European population separately.
Whether the IVs satisfy the three assumptions has a significant impact on the reliability of the MR analysis results. The three assumptions are: (I) the IVs are strongly associated with the exposure; (II) the IVs are not associated with the outcome via a confounding pathway; and (III) the IVs do not affect the outcome directly, but only possibly indirectly via the exposure (13). In the current study, we selected the SNPs with P<5×10−8 for the European population and P<1×10−5 for the East Asian population, which means that the selected SNPs are associated with exposure factors at a genome-wide level of significance (15,23,24). Furthermore, we performed the F-statistic and found that the F values of all the SNPs were larger than 10. The F-statistic result indicated that there was no weak instrument bias in this study (25). Thus, the IVs in the current study fulfill the first assumption. Pleiotropy refers to an IV being associated with multiple risk factors on different pathways. If an IV has pleiotropy, then the second or third assumption is violated (26). In our research, no horizontal pleiotropy was observed. Alcohol consumption, tobacco consumption, high BMI, and gastroesophageal reflux disease are considered to be risk factors associated with EC (3,19). We searched the PhenoScanner database to check whether the SNPs were associated with alcohol consumption/tobacco consumption/high BMI/gastroesophageal reflux disease one by one and found that no SNP in this analysis was related to the above confounding risk factors. Therefore, we can assume that the IVs satisfy the second and third assumptions. The population effect can also lead to violations of the IV assumptions. Considering the risk factors for the European population and Asian population are different, and to decrease the population effect, we conducted the MR analysis using GWAS data from the European population and East Asian population for hot beverage preference separately.
Because heterogeneity was detected for the European population, we used a random-effects model to address heterogeneity by decreasing the precision of estimates. To further explore the effect of heterogeneity on the estimates, we conducted the MR-PRESSO analysis. Although a difference was observed, no causal relationship was detected before or after excluding the outlier.
In cases where analyses of exposures, like proteins, using variants in a coding gene region for the protein, the variants are referred as “cis-variants”. However, in many cases, it is not possible to find a cis-variant, like the case in our study, and a more agnostic polygenic analysis is necessary. In a polygenic analysis where more genetic variants are included, a null finding is more convincing evidence of a true null relationship (27). In this study, we used a polygenic analysis including more than 60 SNPs for the European population and more than 10 SNPs for the East Asian population, and we found no causal relationship between hot beverage temperature preference and EC in both populations. This is new evidence on the causal relationship between the temperature preference of hot beverages and EC risk, especially for the East Asian population, because hot beverage/food preference has been for a long time considered a risk factor for EC in this population.
However, some limitations of our research should be noticed. Firstly, although the exposure data and outcome data were from the European and East Asian populations, the populations include people from different countries, and there may be some differences in genetic background between the exposure and outcome data. Secondly, the study did not specify the temperature of “hot beverages”, but simply classified them as “very hot”, “hot” or “warm”. The definition of “very hot”, “hot” and “warm” may vary from person to person. Thirdly, the study only took into account the temperature of hot beverages, not the frequency of drinking them. Although temperature preference does not indicate exposure level, preference is positively correlated with drinking amount in general, with higher preference leading to greater consumption. However, the impact of the hot beverages drinking frequency on EC risk needs further verification. Fourth, all cases of EC from the UK Biobank were diagnosed as EC, using the International Classification of Diseases code (ICD) of C15, without further differentiation of pathological type. So as the cases from other datasets. Finally, the MR results only reflect the long-term effect of the hot beverage temperature preference on EC risk. The short-term effect of hot beverage temperature preference needs to be further studied.
Conclusions
In conclusion, the current MR analysis provides new genetic evidence for a null causal relationship between the hot beverage temperature preference and EC in the European and East Asian populations. Evidence to prevent EC by reducing the intake of hot beverages is insufficient. However, more experimental studies are required to further confirm our results.
Acknowledgments
We are grateful to the individuals and institutions that have helped in the current study.
Funding: The study received funding from
Footnote
Reporting Checklist: The authors have completed the STROBE-MR reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-361/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-361/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-361/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Yu X, Bao Z, Zou J, et al. Coffee consumption and risk of cancers: a meta-analysis of cohort studies. BMC Cancer 2011;11:96. [Crossref] [PubMed]
- Sharp L, Chilvers CE, Cheng KK, et al. Risk factors for squamous cell carcinoma of the oesophagus in women: a case-control study. Br J Cancer 2001;85:1667-70. [Crossref] [PubMed]
- Islami F, Boffetta P, Ren JS, et al. High-temperature beverages and foods and esophageal cancer risk--a systematic review. Int J Cancer 2009;125:491-524. [Crossref] [PubMed]
- Andrici J, Eslick GD. Hot Food and Beverage Consumption and the Risk of Esophageal Cancer: A Meta-Analysis. Am J Prev Med 2015;49:952-60. [Crossref] [PubMed]
- Tobey NA, Sikka D, Marten E, et al. Effect of heat stress on rabbit esophageal epithelium. Am J Physiol 1999;276:G1322-30. [Crossref] [PubMed]
- Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003;32:1-22. [Crossref] [PubMed]
- Zuccolo L, Holmes MV. Commentary: Mendelian randomization-inspired causal inference in the absence of genetic data. Int J Epidemiol 2017;46:962-5. [Crossref] [PubMed]
- Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. JAMA 2017;318:1925-6. [Crossref] [PubMed]
- Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 2013;37:658-65. [Crossref] [PubMed]
- Cai J, Li X, Wu S, et al. Assessing the causal association between human blood metabolites and the risk of epilepsy. J Transl Med 2022;20:437. [Crossref] [PubMed]
- Sekula P, Del Greco M F, Pattaro C, et al. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J Am Soc Nephrol 2016;27:3253-65. [Crossref] [PubMed]
- Boef AG, Dekkers OM, le Cessie S. Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int J Epidemiol 2015;44:496-511. [Crossref] [PubMed]
- Bradbury KE, Young HJ, Guo W, et al. Dietary assessment in UK Biobank: an evaluation of the performance of the touchscreen dietary questionnaire. J Nutr Sci 2018;7:e6. [Crossref] [PubMed]
- Yang J, Yan B, Zhao B, et al. Assessing the Causal Effects of Human Serum Metabolites on 5 Major Psychiatric Disorders. Schizophr Bull 2020;46:804-13. [Crossref] [PubMed]
- Deng Y, Ge W, Xu H, et al. A Mendelian randomization study of the effect of tea intake on breast cancer. Front Nutr 2022;9:956969. [Crossref] [PubMed]
- Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44:512-25. [Crossref] [PubMed]
- Burgess S, Bowden J, Fall T, et al. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology 2017;28:30-42. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Bentrem DJ, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2023;21:393-422. [Crossref] [PubMed]
- Luo H, Ge H. Hot Tea Consumption and Esophageal Cancer Risk: A Meta-Analysis of Observational Studies. Front Nutr 2022;9:831567. [Crossref] [PubMed]
- Zhong Y, Yang C, Wang N, et al. Hot Tea Drinking and the Risk of Esophageal Cancer: A Systematic Review and Meta-Analysis. Nutr Cancer 2022;74:2384-91. [Crossref] [PubMed]
- Masukume G, Mmbaga BT, Dzamalala CP, et al. A very-hot food and beverage thermal exposure index and esophageal cancer risk in Malawi and Tanzania: findings from the ESCCAPE case-control studies. Br J Cancer 2022;127:1106-15. [Crossref] [PubMed]
- Martens EP, Pestman WR, de Boer A, et al. Instrumental variables: application and limitations. Epidemiology 2006;17:260-7. [Crossref] [PubMed]
- Dashti HS, Chen A, Daghlas I, et al. Morning diurnal preference and food intake: a Mendelian randomization study. Am J Clin Nutr 2020;112:1348-57. [Crossref] [PubMed]
- Burgess S, Thompson SG. Bias in causal estimates from Mendelian randomization studies with weak instruments. Stat Med 2011;30:1312-23. [Crossref] [PubMed]
- Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol 2015;181:251-60. [Crossref] [PubMed]
- Burgess S, Scott RA, Timpson NJ, et al. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol 2015;30:543-52. [Crossref] [PubMed]


