
Prevalence and management of pulmonary nodules: a systematic review and meta-analysis
Highlight box
Key findings
• Overall prevalence of pulmonary nodules detected via computed tomography (CT) scans is 30% with no significant difference between Chinese and non-Chinese populations.
• Age and smoking are the primary risk factors for pulmonary nodules, and recent studies advocate for personalized management strategies based on individual risk factors and nodule characteristics.
What is known and what is new?
• Although most pulmonary nodules are benign, a small percentage can develop into lung cancer, making their detection and management crucial. The detection rate of pulmonary nodules varies widely; previous studies have reported detection rates of 10–60%.
• This study provides a comprehensive analysis of the prevalence of pulmonary nodules, showing an overall prevalence rate of 30% among participants. This study underscores the significance of age and smoking as the primary risk factors for pulmonary nodules, reinforcing the need for targeted screening and personalized management strategies.
What is the implication, and what should change now?
• Given the high prevalence of pulmonary nodules and the significant risk factors of age and smoking, public health policies should focus more on targeted screening programs among the high-risk population.
• That personalized management strategies have been shown to be more effective underscores the need for clinicians to adopt a more individualized approach in the management of pulmonary nodules.
Introduction
Pulmonary nodules, are small focal lesions in the lungs, typically round or oval, ≤30 mm, and have increased density compared to the surrounding lung tissue (1,2). They are commonly identified through computed tomography (CT) scans as localized, rounded areas of opacity. These nodules can be classified as solid or subsolid, based on appearance on CT scans (2,3). A systematic review encompassing 8 randomized controlled trials and 13 cohort studies revealed that the average rate of nodule detection per screening round stands at 20% (1). A large-scale study conducted in the United States between 2006 and 2012 estimated that approximately 1.57 million nodules were identified in 2010, with a notable increase in the rate of nodule identification, rising from 3.9 to 6.6 per 1,000 person-years during that period (4). Similarly, a screening study in China comprising 22,351 participants between 2015 and 2018 reported a detection rate of 31% for pulmonary nodules (5).
Although pulmonary nodules are predominantly benign and pose minimal health risk, they can occasionally serve as early indicators of lung cancer, even considering the clinical possibility of slow-growth of pulmonary tumor (4,6). In a 2023 study of 4,181 patients with pulmonary nodules, 6% of cases developed into lung cancer during the 3-year follow-up period (7). Another study conducted in Shanghai observed that among 6,925 participants with pulmonary nodules during baseline screening, 0.7% developed lung cancer during a 35-month follow-up period (5). Furthermore, this study indicated that the incidence of lung cancers can vary based on factors such as age, sex, and nodule classification. The malignancy rate was notably high at 4.5% for participants with solid nodules larger than 5 mm (5).
Lung cancer is the second and third most commonly diagnosed cancer in China and globally, respectively (8). Unfortunately, it remains the leading cause of cancer-related mortality both on a global scale and in China, accounting for a significant proportion of deaths each year (8). Despite advancements in therapies, the prognosis of lung cancer is largely dependent on the stage at which the disease is detected. Many large-scale lung cancer and pulmonary nodule screening programs and trials have been initiated globally in many countries including China. Multiple studies such as National Lung Screening Trial (NLST), International Association for the Study of Lung Cancer (IASLC) and Dutch-Belgian Randomized Lung Cancer Screening Trial (NELSON) have shown that lung cancer screening improves survival due to detection of early-stage disease (9-12). Early and accurate diagnosis, coupled with high quality follow-up management, is essential for the effective care of these nodules, particularly in the high-risk population.
Few studies have comprehensively reviewed the overall prevalence of pulmonary nodules in Chinese and non-Chinese populations. In this study, we aimed to review and compare the results of previous studies conducted in these populations to evaluate differences and similarities in prevalence of pulmonary nodules, and lung cancer detection rates. Through a comprehensive analysis, we also aimed to provide a clearer understanding of the epidemiology, risk factors, and management strategies pertaining to pulmonary nodules. The overarching goal of our project was to establish a foundational risk assessment for the actuarial calculations to support medical insurance coverage for lung cancer screening and pulmonary nodule management in China. We present this article in accordance with the MOOSE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-874/rc) (13).
Methods
This is a systematic review encompassing both cross-sectional and longitudinal studies from 1990 to 2023 that investigated lung cancer screening and pulmonary nodule risk assessment and management. In conducting our meta-analysis, the protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) on 7 December 2023 (registration number: CRD42023485546).
Search strategy
We conducted the search on 2 platforms: PubMed for publications in English language and China National Knowledge Infrastructure (CNKI) for Chinese language publications. For PubMed, we employed the search terms “pulmonary nodules” AND (“incidence” OR “prevalence”) to identify relevant studies. For the CNKI database, we used the Chinese-translated term “pulmonary nodules” AND (“Detection rate” OR “Incidence”) with the period from 1990 to November 2023. Detailed searching strategies are reported in Table S1.
Study selection process
In the initial phase of our study selection, 2 reviewers independently examined the title and abstract of each identified article. This step ensured an unbiased and thorough screening process. Following the first round of assessment, articles that met our inclusion criteria were then subjected to a detailed full-text review by the same reviewers focusing on the study population and study designs. The flow diagram for study selection process is shown in Figure 1.
The inclusion criteria were as follows: (I) the study evaluated data on the prevalence of pulmonary nodules; (II) CT imaging was employed for the detection of pulmonary nodules; (III) publication in peer-reviewed journals. Exclusion criteria included studies with non-original studies such as meta-analysis and reviews.
Data collection process
We collected the following data from each of the included studies: geographic locations, study design, screening time frames, sample size, data collection institutions, average or median age of participants, percentage of male participants, the definition of pulmonary nodules, detection rate of pulmonary nodules, and the smoking status of the sample. A pre-designed table was used by 2 reviewers (J.S. and L.Y.) to extract data from all selected articles.
Risk of bias assessment
Based on a previous study (14), we utilized a modified version of the Newcastle-Ottawa Scale (NOS) to assess the risk of bias in each included study to align better with the characteristics of the studies included in our review. We also modified the NOS to omit components not applicable to our analysis. The scoring methods are shown in Appendix 1. Each study was evaluated by 2 reviewers independently (L.Y. and W.Z.), focusing on domains such as representativeness of the sample, sample size, ascertainment methods, and completeness of the descriptive statistics provided. The overall risk of bias for each study was categorized as ‘low’, ‘moderate’, ‘high’, or ‘no information’. Specifically, a study was classified as having a high risk of bias if one or more of the assessed domains were deemed high risk. Additionally, any study classified as having a risk of bias other than low in one or more of the assessed domains was counted as ’some concern’.
Statistical analysis
We employed a quantitative data synthesis approach to consolidate the detection data from the included studies. For longitudinal studies, only the detection data from the baseline would be used since we intended to analyze the prevalence of pulmonary nodules.
Based on the suggestions of a previous article (15), we utilized the random effect model to estimate the overall prevalence, employing the ‘meta’ package in R for data synthesis. To evaluate the heterogeneity among the included studies, we calculated the I2 statistic.
A previous study suggest that incidental pulmonary nodules (IPNs) are often detected in populations that are different than the population that undergo lung cancer screening (16). To minimize the heterogeneity of the meta-analysis, we only included pulmonary nodules that were detected through screening programs in meta-analysis. A ’screen-detected’ nodule is defined as one identified during a routine LDCT scan, conducted as part of an annual health examination or specific lung cancer screening program. We also excluded the study with overly rigorous criteria for pulmonary nodules that have requirements other than size and characteristics. For two studies used the same data source, the smaller study was excluded.
We used subgroup analysis to compare the difference of detection rate between male and female patients, and Chinese and non-Chinese populations. However, due to disparities and complexities in reporting age-associated detection rates across the studies, a quantitative synthesis of age-associated observations was not feasible. Consequently, we provided a descriptive summary of the age-associated risk of pulmonary nodules. Additionally, we descriptively synthesized other reported risk factors and the recommendations for the future management of pulmonary nodules based on included studies.
Results
Study characteristics and patient population
A total of 32 studies, including 21 cross-sectional (5,6,17-35) and 11 longitudinal studies (4,36-45) reporting on the prevalence and characteristics of pulmonary nodules, were identified and included in the analysis. These studies were performed in the years between 1993 and 2020, involving a total of 699,944 individuals (Table 1). The sample size for each study ranged from 243 to 415,581 with diverse age ranges and gender distributions. The data collection institutions were varied across the globe. The characteristics of the included studies are presented in detail in Table 1.
Table 1
| Authors | Country/region | Screening years | Sample size | Age, years (mean) | Male, n (%) | Definition of pulmonary nodules | Current smokers, n (%) |
|---|---|---|---|---|---|---|---|
| Gould et al. (4)* | United States | 2006–2012 | 415,581 | 45.6 | NR | Any nodules 4–30 mm | NR |
| Zhao et al. (5) | China | 2015–2018 | 22,351 | 50 (median) | 9,149 (40.93) | Any nodules | NR |
| Hammerschlag et al. (6)* | Australia | 2021 | 248 | 41 (median) | NR | Any nodules | NR |
| Ouyang et al. (17) | China | 2018 | 1,372 | 58.97 | 503 (36.67) | Any nodules | NR |
| Xu et al. (18) | China | 2018–2019 | 920 | 20–69 (range) | 588 (63.91) | NR | NR |
| Pan et al. (19) | China | 2016–2018 | 63,521 | 21–61 (range) | 36,842 (57.99) | (I) A single round or oval dense shadow is found within the parenchyma of both lungs; (II) not accompanied by enlargement of the hilar or mediastinal lymph nodes, nor signs of lung atelectasis or pneumonia; (III) the longest diameter does not exceed 3 cm; (IV) the lesion has sufficiently measurable diameter and defined, sharp edges; (V) calcification or cavitation may be present within the lesion (excluding those where the cavity nearly occupies the entire lesion) | 20,767 (32.69) |
| Wei et al. (20) | China | 2020 | 2,311 | 35.76 | 537 (23.24) | 5–14 mm solid/partially solid nodules and 8–14 mm non-solid nodules; ≥15 mm nodules (including solid nodules, partially solid nodules, and non-solid nodules) | 176 (7.62) |
| Ji et al. (21) | China | 2018–2020 | 3,068 | 50.29 | 1,484 (48.37) | Any nodules | 679 (22.13) |
| Wu et al. (22) | China | 2019 | 9,776 | 47 | 6,061 (62.00) | Any non-calcified nodule ≤30 mm | 3,117 (31.88) |
| Wu et al. (23) | China | 2021–2022 | 5,597 | 55.19 | 3,162 (58.88) | NR/any nodules | 2,148 (38.38) |
| Zhang et al. (24) | China | 2022 | 3,631 | NR | 1,379 (37.98) | NR | 1,364 (37.57) |
| Tapio Vehmas (25) | Finland | 2008 | 526 | 63 | 517 (98.29) | NR | 144 (27.37) |
| Hall et al. (26)* | United States | 2002–2005 | 589 | 53 | 218 (37.00) | Any new nodules | NR |
| Sigel et al. (27)* | United States | 2003–2012 | 1,617 | 49.74 | 260 (16.08) | NR | 317 (19.60) |
| Rinaldi et al. (28)* | Italy | 2007 | 243 | 62.6 | 113 (46.50) | Any nodules | NR |
| Lin et al. (29) | China | 2016–2019 | 2,082 | 56.35 (median) | 1,556 (74.74) | NR | NR |
| Xu et al. (30) | China | 2018–2019 | 23,695 | 50.52 | 16,142 (68.12) | Any non-calcified nodule ≤30 mm | 15,281 (64.49) |
| Li et al. (31) | China | 2020–2021 | 10,277 | 51.15 | 5,267 (51.25) | Any nodule ≤30 mm | NR |
| Zhang et al. (32) | China | 2021 | 19,923 | NR | 15,696 (78.78) | Any nodules | NR |
| He et al. (33) | China | 2014–2016 | 7,752 | 40–75 (range) | 4,025 (51.92) | Any nodules | NR |
| Yorgun et al. (34)* | Turkey | 2007–2008 | 1,206 | 58.75 | 701 (58.1) | NR | NR |
| Infante et al. (35) | Italy | 2001–2006 | 1,276 | 60–74 (range) | 1,276 (100.0) | Any dubious nodules | NR |
| The International Early Lung Cancer Action Program (36) | Worldwide | 1993–2005 | 31,567 | 61 | NR | Any solid or partly solid non-calcified pulmonary nodules ≥5 mm | 23,052 (73.03) |
| The National Lung Screening Trial (37) | United States | 2002–2004 | 26,715 | 55–74 (range) | 15,765 (59.01) | Any noncalcified nodules ≥4 mm | 12,643 (47.33) |
| Wilson et al. (38) | United States | 2002–2005 | 3,642 | 59 | 1,872 (51.40) | Any suspicious nodules | 2,192 (60.2) |
| Henschke et al. (39) | United States | 1993 | 1,000 | 67 (median) | 540 (54.0) | Any non-calcified nodules | NR |
| Pedersen et al. (40) | Denmark | 2004–2006 | 2,052 | 49–74 (range) | 1,120 (54.58) | Any suspicious nodules | 1,052 (76.9) |
| Field et al. (41) | United Kingdom | 2010–2014 | 2,028 | 67.1 | 1,529 (75.39) | Nodules greater than 15 mm3 or 3 mm in maximum diameter | 777 (38.3) |
| Hendrix et al. (42) | United States | 2018–2019 | 13,286 | 58.1 | NR | Any suspicious nodules | NR |
| Becker et al. (43) | German | 2007–2011 | 2,029 | 50–69 (range) | 1,315 (64.81) | Any nodules ≥5 mm | NR |
| Klaveren et al. (44) | Netherland | 2006–2009 | 7,557 | 58 | 6,303 (83.41) | NR | 7,557 (100.0) |
| Pegna et al. (45) | Italy | 2004–2013 | 1,406 | 60.92 | 1,035 (64.17) | Any non-calcified nodule ≥5 mm or a non-solid nodule ≥10 mm or the presence of a part-solid nodule | 1,060 (65.7) |
*, studies reported incidental pulmonary nodules. NR, not reported by the study.
Risk of bias assessment
The summarized findings of our bias assessment are presented in Table S2. Notably, most studies exhibited low concerns across most categories. However, areas such as representation and descriptive statistics frequently demonstrated higher levels of concern. The main concern in the representation domain was the selective inclusion of participants at high risk for lung cancer, predominantly those with a significant history of smoking in some studies (44,46), while others allowed for individuals with perceived lower risk (19-22,27,32). Several studies also showed a lack of comprehensive descriptive data, particularly regarding the percentage of male participants and combustible tobacco users which brings concerns in descriptive statistics domain (4,6,42). These concerns suggest that although our results provide valuable insights, they may not be fully generalizable to all populations at risk for lung cancer.
Definition
The pulmonary nodule definitions varied significantly in nodule size and property (4-6,17,19-23,26,27,30-33,35-43,45). Nodule size ranged from 4 to 30 mm (4,23,31,37). A total of 6 studies specifically mentioned non-calcified nodules in their definition (20,23,36,37,39,45). Nodule solidity is another property addressed in several studies; 1 study necessitated nodules to be either solid or partially solid (36), whereas another, conversely, excluded solid nodules in their definition (45). One study imposed stringent criteria for pulmonary nodules, including overall shape, absence of associated lymph node enlargement, distinctness of boundary, size, and calcification status (19), which may result in a lower positive rate when reporting the prevalence of pulmonary nodules. Meanwhile, 12 studies had broader definitions of pulmonary nodules, merely mentioning “any nodules”, “any suspicious nodules”, or “any new nodules” without specific size or property requirements (5,6,17,21,24-26,28,35,38,40,42).
Prevalence of screen detected pulmonary nodules
The reported prevalence of pulmonary nodules varied distinctly across the 32 studies ranging from 1% [95% confidence interval (CI): 0.011–0.013] (19) to 80% (95% CI: 0.79–0.81) (22). The notably low prevalence in the study by Pan et al. may be attributed to their stringent criteria for defining pulmonary nodules (19). We carried out a pooled meta-analysis of the prevalence data to reduce heterogeneity and excluded 1 study with overly rigorous criteria for pulmonary nodules (19), 4 studies that reported IPNs (26-28,34), 1 study with an overlapped dataset (22), and 2 studies with sample size lower than 300 (6,28). Consequently, only data from 24 studies were included for the quantitative analysis (Figure 2). The pooled estimated prevalence of pulmonary nodules for all 24 studies was 0.30 (95% CI: 0.24–0.38). However, this estimated prevalence was highly unreliable due to considerable statistical heterogeneity with an I2 value of 100%. We then utilized the outliner removal function provided by the R package ‘dmetar’ to minimize the heterogeneity. After the exclusion of 14 outlier studies, the heterogeneity was slightly reduced (I2=95%). The remain 10 studies accounted for 19,259 cases of pulmonary nodules among a total of 67,296 participants (5,17,18,24,25,33,37,39,43,45). The pooled estimated prevalence of pulmonary nodules for the remain 10 studies was 0.27 (95% CI: 0.25–0.29) (Figure 3).
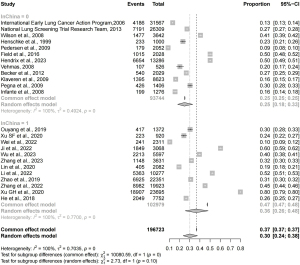

The studies included both Chinese and non-Chinese populations, with nearly half focusing on the Chinese population. To account for potential heterogeneity caused by the ethnic differences, we then conducted a subgroup analysis for Chinese and non-Chinese populations separately (Figure 3). Among 31,270 non-Chinese participants, the overall prevalence of pulmonary nodules was 0.26 (95% CI: 0.23–0.29). In contrast, among 36,026 Chinese participants, the prevalence was slightly higher at 0.29 (95% CI: 0.26–0.31), though this difference was not statistically significant (P=0.11). Heterogeneity was higher among Chinese populations (I2=95%) compared to non-Chinese populations (I2=85%).
Age
A total of 19 of 32 included studies analyzed the relationship between age and the prevalence of pulmonary nodules (4,5,17-25,27,29-32,37,41,42). These studies either stratified participants by age or included age as a covariate in regression analyses to predict pulmonary nodule detection risks. In general, there was a noticeable correlation between advancing age and the incidence of pulmonary nodules. A total of 11 of the 20 studies reported an overall positive association between advanced age and the risk of pulmonary nodules (4,5,19,20,22,25,27,29-31,37). In contrast, only 3 studies reported an inverse association that advanced age is associated with lower risk of pulmonary nodules (17,18,21). Interestingly, several studies identified a U-shaped relationship between age and prevalence (5,41,42). For instance, Hendrix et al. identified a peak prevalence of 47% in the 65–69-year age group, which subsequently decreased in older cohorts (42). Likewise, Field et al. noted a similar trend with a peak in the 74-year age group (41). These observed declines in the older age groups might be attributed to smaller sample sizes which caused unreliable results.
Gender
Of the 32 included studies, 12 reported on the prevalence difference of pulmonary nodules between males and females (5,17,18,20,21,24,29-33,37). Notable variations were observed in these data. For instance, several studies, observed significantly higher prevalence rates in males (5,21,30,42). Conversely, three studies documented slightly higher prevalence rates in females (32,33,37). Meanwhile, three studies revealed nearly identical rates between the genders (20,24,31). To synthesize these findings, we conducted a pooled meta-analysis of these studies. Adopting consistent criteria, 1 study with stringent pulmonary nodule criteria was excluded to minimize heterogeneity. As depicted in Figure 4, the overall prevalence of pulmonary nodules in males (0.38, 95% CI: 0.27–0.50) was slightly higher than in females (0.36, 95% CI: 0.25–0.49), but not statistically significant (P=0.88). This discrepancy might be attributable to the higher smoking rates and alcohol consumption observed in the male population, potentially increasing their risk for pulmonary nodules.

Other risk factors
Among the 32 included studies, 11 reported the risk factor of pulmonary nodules they identified (20-24,27,28,30,31,33,37). Smoking was shown as the predominant risk factor mentioned in 8 studies (21-24,27,28,30,37). For instance, both Sigel et al. and the NLST Research Team emphasized the significant influence of current smoking habits and the cumulative effect of pack-years (27,37).
The history of chronic diseases is commonly mentioned as risk factors. A total of 6 studies identified hypertension as the risk factor, 2 reported hyperlipidemias, and 3 pointed out diabetes. Zhang et al. revealed a prevalence of 52.7% for pulmonary nodules in participants with hypertension history, which was significantly higher than the 46.3% in those without (24).
Several studies highlighted unique risk factors. Xu et al. emphasized alcohol consumption, less physical activity, and a history of infectious diseases as contributory factors (30). Wu et al. pointed out environmental exposures, lung disease history, and lung cancer family history as risk factors (23). Wei et al. introduced dietary patterns into the analysis, identifying a high intake of vegetables, limited meat consumption, and regular meal times as protective against the development of pulmonary nodules (20). He et al. reported that dust and pesticide exposure, history of lung disease, family history of lung cancer, infrequent vegetable and fruit consumption, and a high intake of pickled foods all elevated the risk (33). Surprisingly, they also observed that single individuals were at a heightened risk for pulmonary nodules, suggesting potential risk induced by lack of social support.
Management of pulmonary nodules
A total of 11 of 32 studies give suggestions for future management of the detected pulmonary nodules cases (5,6,30,32,35,36,38,39,41,42,45). The recommendations varied from broad follow-up intervals of 3–6 months to detailed recommendations based on nodule size, including options for annual scans for nodules smaller than 5 mm or more frequent monitoring for larger nodules.
For studies outside China, the management recommendations were relatively consistent. Typically, these studies divided patients into several categories based on nodule size and morphology and offered specific suggestions for each. For example, Henschke et al. advised that patients with nodules ≤5 mm undergo follow-up CT scans at intervals of 3, 6, 12, and 24 months (39). For nodules between 6 to 10 mm, an optional biopsy and further monitoring scans were recommended, whereas for nodules >11 mm, a biopsy was strongly advised. Notably, Hammerschlag et al. referenced the 2017 Fleischner Society Guidelines (6). Fleischner Society Guidelines, which are designed for incidentally detected nodules, not only consider nodule size and morphology but also the patient’s individual risk factors (47). This approach is paralleled in the British Thoracic Society (BTS) guidelines, which is for the screening detected pulmonary nodules (48). The application of fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is critical in further diagnosis and management of high-risk nodules (47,48). PET/CT can further investigate the nodules by identifying nodules that are metabolically active and helps in identification and characterization of potentially malignant nodules with high accuracy, particularly in nodules that are inappropriate for biopsy (49,50). Although there is variation in the criteria for pulmonary nodules categorization and the corresponding management recommendation, most were based on the size and morphology associated with the patient and recommend routine CT scans. The integration of individual risk factors into the decision-making process, highlighting the evolving nature of clinical guidance, emphasizes the need for continual research and importance of personalized plan in pulmonary nodule management.
Discussion
In this study, we reveal a significant variability in the prevalence of pulmonary nodules across different studies, with a pooled estimated prevalence of 27%. The reported prevalence of pulmonary nodules varied from 9% to 80% after the removal of 1 study with stringent criteria for pulmonary nodules. This is still much wider compared to a previous study which only included 8 large trials of lung cancer screening which were all in English language literature (51). The high differences reflected the variability in the inclusion criteria of pulmonary nodules across the included studies and the heterogeneity of the study population which cause challenges for comparison across studies and generate a reliable estimate through meta-analysis. The various requirements for nodule size and morphology such as calcification and solidity added complexity to the meta-analysis. These 32 studies were carried out in populations with diverse demographics, including age, ethnicity, gender, and smoking status. Additionally, differences in sample size and the time in which these studies were performed can introduce variations in the prevalence rates. Studies conducted over longer durations or with larger sample sizes may provide a more accurate estimation of pulmonary nodule prevalence.
When comparing prevalence between Chinese and non-Chinese populations, our results indicated a slightly higher prevalence among Chinese participants compared to non-Chinese participants. This may potentially cause by the higher burden of tuberculosis cases in China which is a risk factor for pulmonary nodules (52,53). However, this difference was not statistically significant (P=0.09), suggesting that ethnicity alone may not be a major contributor to the prevalence of pulmonary nodules.
In this study, we saw an overall consistent trend of the relationship between advancing age and the increased prevalence of pulmonary nodules. In most studies, the detection rate of pulmonary nodules increased as age advanced (4,5,19,20,22,25,27,29-31,37). However, there were also several different observations. For instance, the U-shaped relationship in studies by Field et al. and Hendrix et al. emphasize the nonlinear relationship between age and pulmonary nodules prevalence (41,42). However, the decline in older age groups could also indeed be due to smaller sample sizes in those cohorts and lower life expectancy for population with worse lifestyle, leading to less reliable results, but further research is needed to explore if biological or other underlying factors might be responsible.
The overall higher prevalence of pulmonary nodules in male participants was also observed. Although there were several studies showing slightly higher rates in females, the overall trend from the meta-analysis supports the higher prevalence in males. The reasons behind the difference may be multi-factorial. Lifestyle factors such as smoking and alcohol consumption, which are statistically higher in males, are potential contributors (54,55).
Smoking is the predominant risk factor for pulmonary nodules. The association between smoking and pulmonary nodules was shown in 8 out of 11 studies (21-24,27,28,30,37). The effect of smoking could even be cumulative in that a higher smoking pack-year can also cause higher risk of pulmonary nodules. These results emphasize the importance of smoking cessation as a key prevention method in pulmonary nodules. Interestingly, a previous study showed that individuals with abnormal results in lung cancer screening are more likely to quit smoking (56). This highlights the potential positive impact of such screenings not only in detecting cancer at an early stage but also in motivating individuals to adopt healthier lifestyles.
Additionally, other risk factors such as chronic diseases, environmental exposures, dietary habits, and psychological stress, were also identified in various studies (20,23,24,30,33). It remains ambiguous whether the observed associations between the chronic diseases and pulmonary nodules is attributable. These chronic diseases, such as hypertension, are highly correlated with detrimental lifestyle factors such as smoking and alcohol consumption (57), which are recognized risk factors for pulmonary nodules (27,30). These results highlight the complexity of assessing the individual risk of developing pulmonary nodules. Combining smoking cessation with regular lung cancer screenings and lifestyle modifications can collectively contribute to reducing the incidence of pulmonary nodules.
The management strategies for the pulmonary nodules patients across different studies all focused on stratifying recommendations based on nodule size and morphology. However, the standards for stratifying and the follow-up protocols were slightly different across the studies. The BTS guidelines and Fleischner Society Guideline are both widely accepted guideline for the management of pulmonary nodules which provides an integrated approach that accounts not only for the nodule characteristics but also individual patient risk factors (47,48). Fleischner Society Guideline is applied to IPNs and BTS guidelines can be applied to broader range of pulmonary nodules (47,48). For high-risk individuals, especially those with a history of smoking, more progressive monitoring might be necessary. Guidelines recommend the use of PET/CT to assess the characteristics and potential malignancy of large pulmonary nodules which is essential in identifying nodules that are metabolically active (50). These guidelines underscores the need for personalized treatment plans, adapting the monitoring frequency and methods to each patient’s personal risk. Incorporating advanced imaging data analysis into the management of pulmonary nodules provides a significant opportunity to enhance personalized management plans. With further advancement of imaging analysis techniques and machine learning algorithms, it becomes possible to characterize the morphology and potential malignancy risk of pulmonary nodules more accurately (58,59).
Although our review included a diverse range of studies, few studies have reported the patient’s adherence to the follow-up management. Due to many barriers such as inadequate health care resources and financial problems, many nodules do not receive proper management based on well-established guidelines (60). Patient adherence is a critical issue in the management of pulmonary nodules. Previous studies have reported that only half of pulmonary nodule patients received guideline-concordant follow-up management (47,61) and patients with small size of nodules are less likely to adhere to the follow-up management (61). Patients may not fully understand the importance of regular follow-ups, particularly if they are asymptomatic and have nodules with low malignancy risk. Establishing support programs such as patient groups or commercial management programs may enhance the follow-up adherence and, consequently, the overall effectiveness of pulmonary nodule management.
Future direction
The large variation in the prevalence across varying studies underscores the necessity for a more standardized and harmonized approach in the future pulmonary nodules research. Future studies could consider the following directions. Firstly, the establishment of a more generalized definition for pulmonary nodules could reduce the variation observed across the studies. Adhering to widely accept guidelines such as the BTS guidelines may provide a more consistent understanding of pulmonary nodules. Secondly, utilizing a prospective longitudinal study design across multiple regions with diverse populations may provide a better understanding of the pulmonary nodules in diverse communities. A longitudinal design could also allow for a better assessment of the long-term impact of lifestyle factors on the risk of pulmonary nodules. Thirdly, few studies discuss the long-term effect of the proper follow-up management for pulmonary nodules patients. A future research direction would be to compare the malignant risk of the pulmonary nodules between patients with management and those with no proper management.
Limitation
This study has several limitations. Firstly, the significant heterogeneity of definition, population characteristics, and methodologies makes it challenging to provide a reliable conclusion for the results. Secondly, several included studies have relatively small sample sizes which could cause less accurate estimates for the results. Thirdly, most included studies are cross-section, the lack of long-term follow-up in some studies limits the understanding of the progression pulmonary nodules over time.
Conclusions
In this study, we systematically reviewed 32 studies across 11 regions. Overall, about 27% of the participants were positive for pulmonary nodules with significant heterogeneity across studies. These findings underscore the challenge in generating a unified conclusion for pulmonary nodules. Although several studies reported different results, advancing age was shown to be highly correlated with pulmonary nodules incidence in most of studies. Meanwhile, gender differences had more varied results across studies, although the overall pooled analysis shows a slightly higher risk in males. Smoking plays a significant role in the risk of pulmonary nodules which emphasize the importance of smoking cessation. Management strategies are different across studies, but recent guidance emphasized personalized approaches based on nodule size, characteristics, and individual risk factors.
Acknowledgments
We thank Shanghai Universal Medical Imaging Diagnostic Center for helping us with data clean up.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-874/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-874/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-874/coif). P.E.Y.V.S. receives personal and/or institutional honoraria for lectures and advisory boards from BMS, MSD, AstraZeneca, Roche, Janssen; and serves as the IASLC president (2023-2025). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;307:2418-29. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Bai C, Choi CM, Chu CM, et al. Evaluation of Pulmonary Nodules: Clinical Practice Consensus Guidelines for Asia. Chest 2016;150:877-93. [Crossref] [PubMed]
- Gould MK, Tang T, Liu IL, et al. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am J Respir Crit Care Med 2015;192:1208-14. [Crossref] [PubMed]
- Zhao J, Cui L, He J, et al. Lung cancer screening by low-dose CT in asymptomatic population undergoing physical examination: preliminary analysis of 22 351 cases in Shanghai. Journal of Diagnostics Concepts & Practice 2019;18:183-8.
- Hammerschlag G, Cao J, Gumm K, et al. Prevalence of incidental pulmonary nodules on computed tomography of the thorax in trauma patients. Intern Med J 2015;45:630-3. [Crossref] [PubMed]
- Vindum HH, Kristensen K, Christensen NL, et al. Outcome of Incidental Pulmonary Nodules in a Real-World Setting. Clin Lung Cancer 2023;24:673-81. [Crossref] [PubMed]
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer; 2020.
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Di Girolamo C, Walters S, Benitez Majano S, et al. Characteristics of patients with missing information on stage: a population-based study of patients diagnosed with colon, lung or breast cancer in England in 2013. BMC Cancer 2018;18:492. [Crossref] [PubMed]
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- Becker N, Motsch E, Trotter A, et al. Lung cancer mortality reduction by LDCT screening-Results from the randomized German LUSI trial. Int J Cancer 2020;146:1503-13. [Crossref] [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [Crossref] [PubMed]
- Rotenstein LS, Torre M, Ramos MA, et al. Prevalence of Burnout Among Physicians: A Systematic Review. JAMA 2018;320:1131-50. [Crossref] [PubMed]
- Tufanaru C, Munn Z, Stephenson M, et al. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc 2015;13:196-207. [Crossref] [PubMed]
- Schmid-Bindert G, Vogel-Claussen J, Gütz S, et al. Incidental Pulmonary Nodules - What Do We Know in 2022. Respiration 2022;101:1024-34. [Crossref] [PubMed]
- Ouyang BH, Guo J, Zhou W, et al. Lung cancer screening with low-dose spiral CT in a unit staff: Results of the baseline screening. Journal of Central South University 2019;44:1252-7. (Medical Science). [Crossref] [PubMed]
- Xu SF, Yue SL, Xu YS. Analysis of The pulmonary Nodule Detection Rate Among Health Check-up Population in Caoxian. Chinese Manipulation and Rehabilitation Medicine 2020;11:45-7.
- Pan Y, Jiang QC. Analysis of the Detection of Pulmonary Nodules and Its Influencing Factors in Physical Examination Population. Journal of Preventive Medicine Information 2020;36:356-64.
- Wei ZY, Zhao HM, Fu S, et al. Screening status of pulmonary nodule and its correlation with living habits among 2,311 staff members from public institutions in a city. Practical Preventive Medicine 2022;29:778-81.
- Ji M, Wang YH, Zhou J, et al. Analysis on the result and influencing factors of pulmonary nodules in teachers' physical examination. Modern Oncology 2022;30:2933-7.
- Wu JB, Jiang L, Xu GH, et al. Discussion on the Screening Status and Risk Factors of Pulmonary Nodules in 9776 Health Examination Personnel. Medical Theory and Practice 2022;35:2496-9.
- Wu HH, Di YH, Li X, et al. Analysis of Pulmonary Nodule Detection Results in University Faculty and Staff. Chinese Journal of Preventive Medicine 2023;24:747-9.
- Zhang XF, Hua WJ, Wang T. Construction of nomogram predictive model for pulmonary nodule risk in healthy people. Clinical Pulmonary Medicine Journal 2023;28:1151-5.
- Vehmas T. Factors influencing the prevalence of pulmonary nodules in lung cancer screening trials: re-evaluation of a CT study. Ups J Med Sci 2008;113:279-86. [Crossref] [PubMed]
- Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009;169:1961-5. [Crossref] [PubMed]
- Sigel KM, Xu D, Weber J, et al. Prevalence of Pulmonary Nodules Detected by Computed Tomography in World Trade Center Rescue and Recovery Workers. Ann Am Thorac Soc 2020;17:125-8. [Crossref] [PubMed]
- Rinaldi MF, Bartalena T, Giannelli G, et al. Incidental lung nodules on CT examinations of the abdomen: prevalence and reporting rates in the PACS era. Eur J Radiol 2010;74:e84-8. [Crossref] [PubMed]
- Lin DD, Wu XQ, Liu T, et al. Analysis of the Results of Routine Low-Dose High-Resolution Spiral CT Lung Examinations in 2082 Health Examination Participants. China Journal of Lung Diseases 2020;13:687-9. (Electronic Edition).
- Xu GH, Huang HX, Chen B, et al. A study on the first chest low-dose CT screening and susceptible factors of pulmonary nodules in 23 695 physical examinees in a medical examination center. Fudan University Journal of Medical Science 2020;47:654-9.
- Li DX, Wei WT, Han GL, et al. Detection and Influencing Factors of Pulmonary Nodules in Physical Examination Population of a Tertiary A Hospital in Beijing. Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease 2022;30:23-8.
- Zhang QQ. Analysis of Lung Examination Results Using Low-Dose Spiral CT in 19,923 Health Examination Participants. Journal of Imaging Research and Medical Applications 2022;6:176-8.
- He YT, Zhang YC, Shi GF, et al. Risk factors for pulmonary nodules in north China: A prospective cohort study. Lung Cancer 2018;120:122-9. [Crossref] [PubMed]
- Yorgun H, Kaya EB, Hazirolan T, et al. Prevalence of incidental pulmonary findings and early follow-up results in patients undergoing dual-source 64-slice computed tomography coronary angiography. J Comput Assist Tomogr 2010;34:296-301. [Crossref] [PubMed]
- Infante M, Lutman FR, Cavuto S, et al. Lung cancer screening with spiral CT: baseline results of the randomized DANTE trial. Lung Cancer 2008;59:355-63. [Crossref] [PubMed]
- Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. [Crossref] [PubMed]
- Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Wilson DO, Weissfeld JL, Fuhrman CR, et al. The Pittsburgh Lung Screening Study (PLuSS): outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med 2008;178:956-61. [Crossref] [PubMed]
- Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99-105. [Crossref] [PubMed]
- Pedersen JH, Ashraf H, Dirksen A, et al. The Danish randomized lung cancer CT screening trial--overall design and results of the prevalence round. J Thorac Oncol 2009;4:608-14. [Crossref] [PubMed]
- Field JK, Duffy SW, Baldwin DR, et al. UK Lung Cancer RCT Pilot Screening Trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161-70. [Crossref] [PubMed]
- Hendrix W, Rutten M, Hendrix N, et al. Trends in the incidence of pulmonary nodules in chest computed tomography: 10-year results from two Dutch hospitals. Eur Radiol 2023;33:8279-88. [Crossref] [PubMed]
- Becker N, Motsch E, Gross ML, et al. Randomized study on early detection of lung cancer with MSCT in Germany: study design and results of the first screening round. J Cancer Res Clin Oncol 2012;138:1475-86. [Crossref] [PubMed]
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. [Crossref] [PubMed]
- Lopes Pegna A, Picozzi G, Mascalchi M, et al. Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer 2009;64:34-40. [Crossref] [PubMed]
- Henschke CI, Yip R, Shaham D, et al. A 20-year Follow-up of the International Early Lung Cancer Action Program (I-ELCAP). Radiology 2023;309:e231988. [Crossref] [PubMed]
- Bueno J, Landeras L, Chung JH. Updated Fleischner Society Guidelines for Managing Incidental Pulmonary Nodules: Common Questions and Challenging Scenarios. Radiographics 2018;38:1337-50. [Crossref] [PubMed]
- Callister ME, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70:ii1-ii54. [Crossref] [PubMed]
- Divisi D, Di Tommaso S, Di Leonardo G, et al. 18-fluorine fluorodeoxyglucose positron emission tomography with computerized tomography versus computerized tomography alone for the management of solitary lung nodules with diameters inferior to 1.5 cm. Thorac Cardiovasc Surg 2010;58:422-6. [Crossref] [PubMed]
- Groheux D, Quere G, Blanc E, et al. FDG PET-CT for solitary pulmonary nodule and lung cancer: Literature review. Diagn Interv Imaging 2016;97:1003-17. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-e120S.
- Chen X, Zhou J, Yuan Q, et al. Challenge of ending TB in China: tuberculosis control in primary healthcare sectors under integrated TB control model-a systematic review and meta-analysis. BMC Public Health 2024;24:163. [Crossref] [PubMed]
- Ren J, Chen F, Liu Q, et al. Management of pulmonary nodules in non-high-risk population: initial evidence from a real-world prospective cohort study in China. Chin Med J (Engl) 2022;135:994-6. [Crossref] [PubMed]
- Jamal A, King BA, Neff LJ, et al. Current Cigarette Smoking Among Adults - United States, 2005-2015. MMWR Morb Mortal Wkly Rep 2016;65:1205-11. [Crossref] [PubMed]
- White AM. Gender Differences in the Epidemiology of Alcohol Use and Related Harms in the United States. Alcohol Res 2020;40:01.
- Tammemägi MC, Berg CD, Riley TL, et al. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst 2014;106:dju084. [Crossref] [PubMed]
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206-52. [Crossref] [PubMed]
- Prosper AE, Kammer MN, Maldonado F, et al. Expanding Role of Advanced Image Analysis in CT-detected Indeterminate Pulmonary Nodules and Early Lung Cancer Characterization. Radiology 2023;309:e222904. [Crossref] [PubMed]
- Ding Y, Zhang J, Zhuang W, et al. Improving the efficiency of identifying malignant pulmonary nodules before surgery via a combination of artificial intelligence CT image recognition and serum autoantibodies. Eur Radiol 2023;33:3092-102. [Crossref] [PubMed]
- Mankidy BJ, Mohammad G, Trinh K, et al. High risk lung nodule: A multidisciplinary approach to diagnosis and management. Respir Med 2023;214:107277. [Crossref] [PubMed]
- Iaccarino JM, Steiling K, Slatore CG, et al. Patient characteristics associated with adherence to pulmonary nodule guidelines. Respir Med 2020;171:106075. [Crossref] [PubMed]



