
Application of drainage strategy with bi-pigtail catheters in patients undergoing lobectomy by uniportal video-assisted thoracic surgery
Highlight box
Key findings
• The drainage strategy with bi-pigtail catheters (BPCs) in patients undergoing lobectomy by uniportal video-assisted thoracic surgery (VATS) is safe and feasible.
What is known, and what is new?
• Controversy continues as to the type of thoracic drainage strategy that should be adopted after uniportal VATS. A drainage strategy with one large-bore chest tube has been confirmed to be safe and reliable in lung surgery by uniportal VATS.
• Our drainage strategy with BPCs decreased the incidence of postoperative complications in patients undergoing lobectomy by uniportal VATS. The drainage strategy with BPCs alleviated the postoperative pain in patients undergoing lobectomy by uniportal VATS.
What is the implication, and what should change now?
• A drainage strategy with BPCs in patients undergoing lobectomy by uniportal VATS is highly recommended.
Introduction
Lung cancer is a major cause of cancer-related death worldwide, and surgery is the standard treatment for most patients with early-stage non-small cell lung cancer (NSCLC) and is part of the treatment strategy for patients with locally advanced NSCLC (1,2). Due to its advantages, which include less pain, a reduced hospital stay, a more satisfactory surgical incision, and a faster recovery, the use of uniportal video-assisted thoracic surgery (VATS) in minimally invasive thoracic surgery is supported by accumulating evidence (3-6). However, controversy continues as to which type of thoracic drainage strategy to adopt after uniportal VATS. Traditional large-bore chest tubes (usually 20–32 Fr) have advantages in terms of the drainage of both fluid and air, but can cause significant pain and discomfort. Conversely, small-bore tubes may obstruct drainage, which can result in loculated pleural effusion, severe subcutaneous emphysema, or the poor re-expansion of the residual lung (7).
A pigtail catheter (PC) is a small-bore tube that has been proven to be safe in the drainage of pneumothorax, traumatic hemothorax, and wedge resection of the lung (8-13). Recently, it was reported that a drainage strategy with bi-pigtail catheters (BPCs) in uniportal VATS lung surgery safely reduced the postoperative pain of patients, but the types of surgery in that study only included wedge resection, segmentectomy, or wedge resection combined with segmentectomy (14). To date, no reports have been published on the usage of drainage strategies with BPCs in patients undergoing lobectomy only by uniportal VATS.
In this retrospective study, we introduced a drainage strategy with BPCs in uniportal lobectomy to examine whether this strategy decreased the incidence of postoperative complications and whether the patients who received the BPC strategy had lower postoperative visual analogue scale (VAS) pain scores compared to those who received a drainage strategy that used traditional chest tubes (TCTs). We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-925/rc).
Methods
Patients who underwent lung operations at the Department of Thoracic Surgery of the Cancer Hospital of Dalian University of Technology were enrolled in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Liaoning Cancer Hospital & Institute Ethics Committee (No. KY20240407). Individual consent for this retrospective analysis was waived. The patients were divided into the following two groups according to the postoperative drainage strategy adopted: (I) the TCT group; and (II) the BPC group. The choice of the specific drainage method was based on the surgeon’s preference.
Baseline data, including gender, age, body mass index (BMI), hypertension, history of smoking, Charlson Comorbidity Index (CCI), the percentage of forced expiratory in 1 second (FEV1), the percentage of forced vital capacity (FVC), the percentage of diffusion lung capacity for carbon monoxide (DLCO), the lobe involved, the operative time, the drainage duration, the postoperative drainage volume, the postoperative hospital time, the collapse rate of the residual lung after surgery, and the cumulative consumption of analgesic drugs, were collected. Operative time was defined as the time from the start of the incision to the completion of wound suturing. Postoperative drainage time was defined as the time from the day after surgery to the day of chest tube extraction. Postoperative hospital time was defined as the time spent in the hospital from the day after operation to the last day of surgery. Kircher et al. (15) described the calculation method of the collapse rate of the residual lung after surgery.
Inclusion and exclusion criteria
Patients who underwent lobectomy at the Department of Thoracic Surgery at Liaoning Cancer Hospital & Institute between August 2021 and August 2022 were included in the study. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had undergone a previous chest surgery; (II) tuberculosis, pleurisy, or trauma on the surgical side; (III) had received neoadjuvant therapy; (IV) pleural adhesion was found during the surgery; (V) had to be converted to thoracotomy; (VI) had intraoperative bleeding; (VII) had incomplete data; and/or (VIII) underwent any of the following types of surgery: segmentectomy, wedge resection, bronchoplasty angioplasty, bilobectomy, or pneumonectomy.
Perioperative management measures
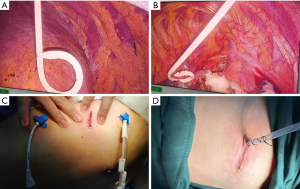
After general anesthesia with a double-lumen tube, the patients were placed in the lateral position. Intraoperative pain control strategies were implemented by the anesthesiologists. A 2.5–4-cm incision was made between the middle axillary and anterior axillary lines in the fourth or fifth intercostal space. In the BPC group, a 12-Fr × 20-cm PC was connected to a standard chest drain system, and was inserted in the third intercostal space in the anterior axillary line, and a 10-Fr × 20-cm PC which was connected to drainage bag was inserted in the seventh or eighth intercostal space at the posterior axillary line (Figure 1A-1C). The depth of the PC inserted to the thoracic cavity is 15 cm. In the TCT group, a 20-Fr chest tube was connected to a standard drainage system and was inserted in the posterior edge of the incision (Figure 1D). The depth of chest tube inserted to the thoracic cavity was 14 or 15 cm. The incisions were then closed with absorbable sutures.
All the patients received non-steroidal anti-inflammatory drugs for postoperative pain control, and the treatment was propacetamol hydrochloride by injection (2 grams, two times per day). Oral oxycodone was administered at a standard dose (with maximum dose of 20 mg per day). The VAS pain scores of the patients at 6 hours, and day 1 and day 2 following surgery were recorded. All the patients underwent chest radiography on day 1 after surgery (Figure 2), and as needed on subsequent days. The collapse rate was calculated according to the last chest X-ray obtained. The tubes were removed in accordance with our criteria that required the expansion of the residual lung, a drainage volume <300 mL per 24 hours, and no air leakage. The patients were discharged the day following the removal of the drainage tubes.

Clinical outcomes
The primary outcome was postoperative complications. The postoperative complications were recorded using the Clavien-Dindo method. The secondary outcome was the VAS pain scores collected when the most severe pain occurred during 0–6, 7–24, and 25–28 hours after surgery, respectively. The VAS pain scores ranged from 0 (no pain) to 10 (the worst pain). The verbal categories of mild, moderate, and severe pain corresponded to values of 1–3, 4–6, and 7–10, respectively, on the VAS in the same patient (16).
Statistical analysis
The collected data are expressed as the number and percentage, and the mean ± standard deviation (SD). The continuous variables are expressed as the mean ± SD. The two-sample Student’s t-test or the Mann-Whitney U test was used to compare the means between the continuous variables. The Chi-square test or Fisher’s exact test was used to compare the categorical variables. Univariable and multivariable analyses were used to assess the predictors of postoperative complications, and moderate to severe postoperative pain. All the hypothesis tests were two-sided; a P value <0.05 was considered statistically significant.
Results
From August 2021 to August 2022, 868 patients underwent lung surgery at our department. After exclusion, a total of 470 patients remained, of whom 235 patients were allocated to the TCT group and 235 patients were allocated to the BPC group (Figure 3) according to the surgeon’s preference.
The epidemiological and clinical characteristic data of the patients are shown in Table 1. No statistically significant differences were observed between the two groups in demographic characteristics (all P>0.05). In relation to the postoperative hospitalization time (4.6±1.5 vs. 5.4±4.5 days) and the collapse rate of the residual lung after operation (19.2%±9.1% vs. 20.9%±9.6%), the results of the BPC group were significantly better than those of the TCT group (all P<0.05).
Table 1
| Variables | Traditional chest tube (n=235) | Bi-pigtail catheters (n=235) | P value |
|---|---|---|---|
| Gender | 0.06 | ||
| Men | 74 (31.5) | 93 (39.6) | |
| Women | 161 (68.5) | 142 (60.4) | |
| Age (years) | 59.3±8.7 | 59.4±9.4 | 0.95 |
| BMI (kg/m2) | 23.8±2.9 | 24.2±3.2 | 0.13 |
| Hypertension | 0.90 | ||
| Yes | 47 (20.0) | 48 (20.4) | |
| No | 188 (80.0) | 187 (79.6) | |
| History of smoking | 0.17 | ||
| Yes | 58 (24.7) | 71 (30.2) | |
| No | 177 (75.3) | 164 (69.8) | |
| Charlson Comorbidity Index | 2.6±1.3 | 2.8±1.3 | 0.27 |
| FEV1% | 94.4±17.2 | 93.3±15.6 | 0.49 |
| FVC% | 92.6±18.6 | 91.2±17.5 | 0.40 |
| DLCO% | 83.5±17.9 | 82.7±18.2 | 0.64 |
| Lobe involved | 0.64 | ||
| RUL | 85 (36.2) | 77 (32.8) | |
| RML | 23 (9.8) | 19 (8.1) | |
| RLL | 47 (20.0) | 43 (18.3) | |
| LUL | 52 (22.1) | 60 (25.5) | |
| LLL | 28 (11.9) | 36 (15.3) | |
| Operating time (min) | 147.6±45.8 | 141.8±46.8 | 0.17 |
| Drainage duration (days) | 3.5±1.7 | 3.7±1.6 | 0.16 |
| Total drainage volume (mL) | 748.1±469.7 | 752.8±485.4 | 0.91 |
| Postoperative hospitalization (days) | 5.4±4.5 | 4.6±1.5 | 0.01 |
| Collapse rate of lung after operation (%) | 20.9±9.6 | 19.2±9.1 | 0.047 |
| Analgesic drug usage, per person (g) | 14.6±3.2 | 15.1±3.0 | 0.15 |
Data are presented as n (%) or mean ± standard deviation. BMI, body mass index; FEV1, forced expiratory in 1 second; FVC, forced vital capacity; DLCO, diffusion lung capacity for carbon monoxide; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe.
The results of postoperative complications and VAS pain scores are set out in Table 2. The incidence of complications was significantly higher in the TCT group than the BPC group (19.1% vs. 7.7%, P<0.001), especially in terms of arrhythmia and hypertension. In relation to the postoperative VAS pain scores, the scores at 7–24 hours (3.3±1.0 vs. 3.7±1.5) and 25–48 hours (3.1±0.8 vs. 3.6±1.5) were significantly lower in the BPC group than the TCT group (P<0.001), but there was no difference in the scores at 0–6 hours between the two groups.
Table 2
| Variables | Traditional chest tube (n=235) | Bi-pigtail catheters (n=235) | P value |
|---|---|---|---|
| Postoperative complications | 45 (19.1) | 18 (7.7) | <0.001 |
| Pneumothorax and/or subcutaneous emphysema (Grade IIIa) | 1 (2.1) | 1 (5.0) | |
| Arrhythmia (Grade II) | 7 (14.9) | 1 (5.0) | |
| Atrial fibrillation (Grade IIIa) | 21 (44.7) | 15 (75.0) | |
| Air leakage (Grade I) | 3 (6.4) | 1 (5.0) | |
| Hypertension (Grade II) | 9 (19.1) | 2 (10.0) | |
| Pleural effusion (Grade IIIa) | 3 (6.4) | 0 | |
| Chylothorax (Grade I) | 2 (4.3) | 0 | |
| Cerebral infarction (Grade II) | 1 (2.1) | 0 | |
| Pain VAS score | |||
| 0–6 hours after surgery | 3.1±0.7 | 3.1±0.5 | 0.32 |
| 7–24 hours after surgery | 3.7±1.5 | 3.3±1.0 | <0.001 |
| 25–48 hours after surgery | 3.6±1.5 | 3.1±0.8 | <0.001 |
Data are presented as n (%) or mean ± standard deviation. VAS, visual analogue scale.
The results of the univariable analysis showed that age, the CCI, the operative time, and the drainage strategy were associated with occurrence of postoperative complications (Table 3), and the results of the multivariable analysis showed that the drainage strategy was the only independent risk factor of postoperative complications (Table 4).
Table 3
| Variables | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Gender | 0.699 | 0.408–1.199 | 0.19 |
| Age | 2.193 | 1.267–3.797 | 0.005 |
| BMI | 1.532 | 0.889–2.637 | 0.12 |
| Hypertension | 1.030 | 0.534–1.987 | 0.92 |
| History of smoking | 1.166 | 0.652–2.084 | 0.60 |
| Charlson Comorbidity Index | 1.238 | 1.011–1.515 | 0.03 |
| FEV1% | 0.997 | 0.981–1.013 | 0.70 |
| FVC% | 0.991 | 0.976–1.006 | 0.22 |
| DLCO% | 1.009 | 0.995–1.022 | 0.21 |
| Lobe involved | 2.083 | 0.945–4.594 | 0.08 |
| Operating time | 1.006 | 1.001–1.012 | 0.03 |
| Drainage strategy | 0.350 | 0.196–0.626 | <0.001 |
BMI, body mass index; FEV1, forced expiratory in 1 second; FVC, forced vital capacity; DLCO, diffusion lung capacity for carbon monoxide; CI, confidence interval.
Table 4
| Variables | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Age | 1.046 | 0.994–1.101 | 0.08 |
| Charlson Comorbidity Index | 1.010 | 0.724–1.409 | 0.95 |
| Operating time | 1.005 | 0.999–1.011 | 0.08 |
| Drainage strategy | 2.885 | 1.598–5.208 | <0.001 |
CI, confidence interval.
We classified mild, moderate, and severe pain as corresponding to values of 1–3, 4–6, and 7–10, respectively, on the VAS, and compared the mild pain with the moderate or severe pain at 7–24 and 25–48 hours after surgery (Tables 5-8). The univariable and multivariable analyses showed that the lobe involved (i.e., the right lower lobe) and the drainage strategy were independent risk factors of moderate or severe pain at 7–24 hours after surgery (P<0.05), and the lobe involved (i.e., the right middle lobe) and the drainage strategy were independent risk factors of moderate or severe pain at 25–48 hours after surgery (P<0.05).
Table 5
| Variables | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Gender | 1.562 | 0.896–2.723 | 0.11 |
| Age | 0.935 | 0.539–1.624 | 0.81 |
| BMI | 0.840 | 0.473–1.491 | 0.55 |
| Hypertension | 0.623 | 0.333–1.164 | 0.13 |
| History of smoking | 0.574 | 0.323–1.019 | 0.058 |
| Charlson Comorbidity Index | 0.913 | 0.736–1.133 | 0.41 |
| FEV1% | 1.003 | 0.987–1.020 | 0.69 |
| FVC% | 1.004 | 0.989–1.019 | 0.63 |
| DLCO% | 0.992 | 0.976–1.009 | 0.37 |
| Lobe involved (RLL) | 3.121 | 1.144–8.516 | 0.02 |
| Operating time | 1.150 | 0.653–2.024 | 0.62 |
| Drainage strategy | 2.710 | 1.491–4.926 | 0.001 |
VAS, visual analogue scale; BMI, body mass index; FEV1, forced expiratory in 1 second; FVC, forced vital capacity; DLCO, diffusion lung capacity for carbon monoxide; RLL, right lower lobe; CI, confidence interval.
Table 6
| Variables | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Lobe involved (RLL) | 2.971 | 1.075–8.212 | 0.03 |
| Drainage strategy | 2.583 | 1.410–4.731 | 0.002 |
VAS, visual analogue scale; RLL, right lower lobe; CI, confidence interval.
Table 7
| Variables | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Gender | 1.031 | 0.551–1.932 | 0.92 |
| Age | 2.238 | 1.164–4.302 | 0.01 |
| BMI | 1.044 | 0.547–1.992 | 0.89 |
| Hypertension | 2.947 | 1.032–8.424 | 0.04 |
| History of smoking | 1.266 | 0.624–2.570 | 0.51 |
| Charlson Comorbidity Index | 0.670 | 0.518–0.868 | 0.002 |
| FEV1% | 0.987 | 0.969–1.006 | 0.17 |
| FVC% | 0.988 | 0.971–1.005 | 0.15 |
| DLCO% | 0.996 | 0.978–1.013 | 0.63 |
| Lobe involved (RML) | 2.868 | 1.198–6.862 | 0.01 |
| Operating time | 1.020 | 0.552–1.884 | 0.95 |
| Drainage strategy | 5.646 | 2.577–12.370 | <0.001 |
VAS, visual analogue scale; BMI, body mass index; FEV1, forced expiratory in 1 second; FVC, forced vital capacity; DLCO, diffusion lung capacity for carbon monoxide; RML, right middle lobe; CI, confidence interval.
Table 8
| Variables | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Age | 1.282 | 0.528–3.110 | 0.58 |
| Hypertension | 2.208 | 0.717–6.801 | 0.16 |
| Charlson Comorbidity Index | 0.783 | 0.543–1.131 | 0.19 |
| Lobe involved (RML) | 2.833 | 1.149–6.983 | 0.02 |
| Drainage strategy | 5.634 | 2.535–12.525 | <0.001 |
VAS, visual analogue scale; RML, right middle lobe; CI, confidence interval.
Discussion
There are no standard guidelines as to the number and sizes of the chest tubes that should be selected after thoracic surgery. In the past, thoracic surgery mainly comprised thoracotomy, and the use of two large-bore chest tubes was considered the routine and safe option (17). With the growing popularity of VATS surgery, a drainage strategy with one large-bore chest tube has been proven to be safe and is accepted by most surgeons (18), especially in uniportal VATS. Recently, the use of PCs has been reported to be effective in the drainage of pneumothorax, traumatic hemothorax, and wedge resection of the lung (8-13). It has also been reported that a drainage strategy that uses BPCs in uniportal VATS lung surgery reduces postoperative pain safely, but the type of surgery only included wedge resection, segmentectomy, or wedge resection combined with segmentectomy (14). Yang et al. (19) showed that a strategy that use one pleural catheter plus a single chest tube for drainage was safe in upper lobectomy by uniportal VATS. The present study was the first to examine the use of a drainage strategy with BPCs in patients undergoing lobectomy only by uniportal VATS, and the results showed that the drainage strategy with BPCs decreased the incidence of postoperative complications, and was accompanied by less pain at 7–24 and 25–48 hours after surgery.
Research has shown that larger-bore chest tubes cause more severe pain that negatively affects respiration, which in turn increases the risk of postoperative respiratory complications, impairs patients’ early ambulation after surgery, and consequently increases the risk of a thromboembolic event (20). Xu et al. (21) reported that placing a 12-Fr PC alone was effective and safe after uniportal VATS lobectomy and extended lymphadenectomy. In our study, the BPC group had fewer postoperative complications and lower VAS pain scores at 7–24 and 25–48 hours after surgery than the TCT group, and there were no statistically significant differences in drainage duration and postoperative drainage volumes between the two groups, which suggests that the drainage strategy with BPCs is safe and effective. We conducted univariable and multivariable analyses to examine predictors of postoperative complications and moderate to severe postoperative pain, and found that the drainage strategy with TCT was the only predictive factor.
There was no difference between the two groups in terms of the postoperative VAS pain score at 0–6 hours. This might be because the postoperative time was too short, and not sufficient for the patients to completely metabolize the analgesic drugs during anesthesia. Additionally, the surgical patients were required to stay in bed for 6 hours postoperatively after returning to the ward, and their reduced activity might have reduced the stimulation of the drainage tube to the chest wall and the residual lung.
Several studies have reported that a drainage strategy with PC improves the drainage of pleural effusion and air, but no studies have focused on the re-expansion of the residual lung (14,22,23). In our study, the collapse rate of the lung after operation in the BPC group was significantly better than that of the TCT group; thus, the re-expansion of the residual lung in the BPC group was better than that in the TCT group. The full drainage and reduced stimulation of the PCs to the lung or chest wall could reduce patients’ pain, and such patients should be able to increase their activity and respiratory function exercise faster after surgery.
It has been reported that pain and discomfort after surgery caused by chest tubes might be one of the main obstacles to enhanced recovery after surgery (ERAS) (24). Chen et al. (25) also reported that postoperative pain is a primary obstacle to ERAS, as it leads to insufficient respiratory function exercise during the initial postoperative days. In our study, the postoperative hospitalization time of the BPC group was significantly better than that of the TCT group, and patients in the BPC group also had less pain and a lower incidence of postoperative complications than those in the TCT group. Thus, a drainage strategy that uses BPCs is in accordance with the concept of ERAS.
This study had several limitations. First, it was a single-center retrospective study with a relatively small sample size and choice of the specific drainage method was based on the surgeon’s preference, which inevitably led to selection bias. Thus, further international multi-center randomized controlled trials (RCTs) are needed to verify our results. Second, there are no standard guidelines for efficient drainage, and we used the 20-Fr chest tube, 12- and 10-Fr PC based only on our experience; thus, the adequate diameters of PCs still need to be determined. Third, we did not have any data on incision healing and cosmetic outcomes, and it is possible that the incision healing results and cosmetic outcomes could have been better in the BPC group than the TCT group. Fourth, long-term outcomes, such as chronic pain and quality of life, were not examined in the current study, but we intend to analyze these outcomes in a further report.
Conclusions
Our drainage strategy with BPCs decreased the incidence of postoperative complications and alleviated the postoperative pain of patients undergoing lobectomy by uniportal VATS, and is safe and feasible. Thus, this type of drainage strategy is simply a viable option. Additionally, there’s potential for initiating a RCT to explore and establish any causal relationships between factors such as pain, complications, and incision placement.
Acknowledgments
Funding: This study was supported by funding from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-925/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-925/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-925/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-925/coif). H.C.F. serves as an unpaid editorial board member of Journal of Thoracic Disease from April 2024 to June 2026. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Chaft JE, Rimner A, Weder W, et al. Evolution of systemic therapy for stages I-III non-metastatic non-small-cell lung cancer. Nat Rev Clin Oncol 2021;18:547-57. [Crossref] [PubMed]
- Guido-Guerrero W, Bolaños-Cubillo A, González-Rivas D. Single-port video-assisted thoracic surgery (VATS)-advanced procedures & update. J Thorac Dis 2018;10:S1652-61. [Crossref] [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Gao Y, Abulimiti A, He D, et al. Comparison of single- and triple-port VATS for lung cancer: A meta-analysis. Open Med (Wars) 2021;16:1228-39. [Crossref] [PubMed]
- Mizukami Y, Takahashi Y, Adachi H. Single-Port vs Conventional Three-Port Video-Assisted Thoracoscopic Pulmonary Wedge Resection: Comparison of Postoperative Pain and Surgical Costs. Ann Thorac Cardiovasc Surg 2021;27:91-6. [Crossref] [PubMed]
- Satoh Y. Management of chest drainage tubes after lung surgery. Gen Thorac Cardiovasc Surg 2016;64:305-8. [Crossref] [PubMed]
- Chang SH, Kang YN, Chiu HY, et al. A Systematic Review and Meta-Analysis Comparing Pigtail Catheter and Chest Tube as the Initial Treatment for Pneumothorax. Chest 2018;153:1201-12. [Crossref] [PubMed]
- Kulvatunyou N, Erickson L, Vijayasekaran A, et al. Randomized clinical trial of pigtail catheter versus chest tube in injured patients with uncomplicated traumatic pneumothorax. Br J Surg 2014;101:17-22. [Crossref] [PubMed]
- Salé A, Sohier L, Campion M, et al. Exclusive ambulatory management of spontaneous pneumothorax with pigtail catheters, a prospective multicentric study. Respir Med 2020;166:105931. [Crossref] [PubMed]
- Kulvatunyou N, Bauman ZM, Zein Edine SB, et al. The small (14 Fr) percutaneous catheter (P-CAT) versus large (28-32 Fr) open chest tube for traumatic hemothorax: A multicenter randomized clinical trial. J Trauma Acute Care Surg 2021;91:809-13. [Crossref] [PubMed]
- Patel NJ, Dultz L, Ladhani HA, et al. Management of simple and retained hemothorax: A practice management guideline from the Eastern Association for the Surgery of Trauma. Am J Surg 2021;221:873-84. [Crossref] [PubMed]
- Zhang JT, Dong S, Chu XP, et al. Randomized Trial of an Improved Drainage Strategy Versus Routine Chest Tube After Lung Wedge Resection. Ann Thorac Surg 2020;109:1040-6. [Crossref] [PubMed]
- Song L, Chen X, Zhu L, et al. Perioperative outcomes of bi-pigtail catheter drainage strategy versus conventional chest tube after uniportal video-assisted thoracic lung surgery. Eur J Cardiothorac Surg 2023;64:ezad411. [Crossref] [PubMed]
- Kircher LT Jr, Swartzel RL. Spontaneous pneumothorax and its treatment. J Am Med Assoc 1954;155:24-9. [Crossref] [PubMed]
- Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth 2008;101:17-24. [Crossref] [PubMed]
- Filosso PL, Sandri A, Guerrera F, et al. Management of Chest Drains After Thoracic Resections. Thorac Surg Clin 2017;27:7-11. [Crossref] [PubMed]
- Kim SS, Khalpey Z, Daugherty SL, et al. Factors in the Selection and Management of Chest Tubes After Pulmonary Lobectomy: Results of a National Survey of Thoracic Surgeons. Ann Thorac Surg 2016;101:1082-8. [Crossref] [PubMed]
- Yang F, Wang X, Xu H, et al. A novel drainage strategy using chest tube plus pleural catheter in uniportal upper lobectomy: A randomized controlled trial. Thorac Cancer 2023;14:399-406. [Crossref] [PubMed]
- Lai Y, Wang X, Zhou H, et al. Is it safe and practical to use a Foley catheter as a chest tube for lung cancer patients after lobectomy? A prospective cohort study with 441 cases. Int J Surg 2018;56:215-20. [Crossref] [PubMed]
- Xu Y, Luo J, Ge QY, et al. Safety and feasibility of a novel chest tube placement in uniportal video-assisted thoracoscopic surgery for non-small cell lung cancer. Thorac Cancer 2023;14:2648-56. [Crossref] [PubMed]
- Li X, Chen X, He S, et al. The Application of Pigtail Catheters in Postoperative Drainage of Lung Cancer. Clin Lung Cancer 2022;23:e196-202. [Crossref] [PubMed]
- Yang Z, Hu T, Shen H, et al. Application of small-bore pigtail catheter to improve pleural drainage after single-incision thoracoscopic lobectomy for lung cancer. ANZ J Surg 2020;90:139-43. [Crossref] [PubMed]
- Batchelor TJP, Ljungqvist O. A surgical perspective of ERAS guidelines in thoracic surgery. Curr Opin Anaesthesiol 2019;32:17-22. [Crossref] [PubMed]
- Chen Z, Jiang L, Zheng H, et al. Early postoperative pain after subxiphoid uniportal thoracoscopic major lung resection: a prospective, single- blinded, randomized controlled trial. Interact Cardiovasc Thorac Surg 2022;35:ivac133. [Crossref] [PubMed]



