
The prognostic value of controlling nutritional status score on esophageal squamous cell carcinoma patients with neoadjuvant therapy followed by esophagectomy—a retrospective research
Highlight box
Key findings
• This study represents the first attempt to assess the prognostic significance of controlling nutritional status (CONUT) scores at distinct time intervals and the delta CONUT (ΔCONUT) score in patients diagnosed with esophageal squamous cell carcinoma (ESCC).
What is known and what is new?
• The CONUT score has been linked to clinical and survival outcomes across various cancer types. Yet, it remains uncertain whether calculating the CONUT score could yield improved predictive capabilities concerning clinical and survival outcomes in ESCC patients who have undergone multidisciplinary treatments.
• Calculating the CONUT score at the preoperative stage could potentially serve as a superior index. Furthermore, calculating the ΔCONUT value by subtracting the preoperative CONUT score from the pretreatment CONUT score may prove beneficial in predicting both postoperative survival outcomes and overall survival in patients.
What is the implication, and what should change now?
• The CONUT score can serve not only as a predictor of tumor progression but also as a tool for identifying ESCC patients with diminished immune-nutritional status and those in need of nutritional support following neoadjuvant therapy.
Introduction
Esophageal cancer (EC) is the ninth most common aggressive malignant tumors around the world (1,2). Esophageal squamous cell carcinoma (ESCC) represents the main pathological type of EC (3,4). Due to the aggressiveness of EC, it has a poor prognosis, and the overall five-year survival rate of EC is still lower than 30% (5,6). While esophagectomy represents a promising therapeutic avenue for patients with EC, it is notably invasive and associated with a heightened risk of postoperative complications, including anastomotic leak and pneumonia. For patients with locally advanced EC, neoadjuvant chemoradiation has emerged as a standard treatment modality. This approach holds the potential to downstage the primary tumor and extend the survival of patients diagnosed with EC (7), and neoadjuvant therapy combined with surgery emerges as the primary treatment option for patients with advanced EC (8-10).
However, many patients with EC experience weight loss and dysphagia, which are closely associated with malnutrition due to the malignant and invasive nature of EC. Additionally, neoadjuvant therapy can exacerbate nutritional or functional impairments. Various risk factors such as dyscrasia and obstruction induced by neoadjuvant therapy contribute to poor nutritional status in patients with EC (11,12). Hence, nutritional assessment and support are crucial components in optimizing multidisciplinary treatment strategies (13,14).
Recently, controlling nutritional status (CONUT) score was reported to be associated with clinical and survival outcomes in several types of cancer (15-17). The CONUT score could be easily calculated based on the albumin, lymphocyte, and cholesterol levels in peripheral blood. Previous reports had shown that CONUT score had the prognostic importance in EC patients who had only undergone esophagectomy.
Previous research demonstrated that in EC patients, CONUT was a predictor of cancer-specific survival (CSS) after esophagectomy (18). In that study, only 148 EC patients were included, and the sample size was limited. In 2016, scholars conducted a retrospective study to examine the predictive significance of pretreatment CONUT scores in patients with ESCC. However, within that research cohort, the majority of patients exclusively received surgical intervention, resulting in an insufficient sample size to adequately assess the prognostic utility of CONUT scores in EC patients who underwent neoadjuvant therapy prior to surgery (19). Therefore, this retrospective study aims to assess the clinical utility and prognostic value of the CONUT score in ESCC patients who underwent neoadjuvant therapy prior to esophagectomy. Our objective is to elucidate whether the CONUT score could serve as a valuable metric, aiding in the formulation of more effective treatment strategies and timely adjustments to therapy. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-187/rc).
Methods
Patient eligibility
The study cohort consisted of 314 individuals diagnosed with ESCC confirmed through pathological examination. Between August 2016 and August 2021, all patients underwent preoperative neoadjuvant treatment. Following completion of neoadjuvant therapy, radical esophagectomy was performed on all patients at West China Hospital, Sichuan University. Inclusion criteria comprised: (I) histologically confirmed ESCC diagnosis; (II) receipt of esophagectomy; (III) administration of neoadjuvant chemoradiotherapy or combined immunotherapy before esophagectomy; and (IV) adequate follow-up. Exclusion criteria included distant tumor metastases, receipt of adjuvant treatment, and inadequate medical records. Comprehensive medical, pathological, and clinical data of each ESCC patient were retrospectively retrieved from our database, with all patients providing informed consent. Pathological diagnosis and disease classification employed the 8th edition TNM Classification of Malignant Tumors for ESCC (20). Patients’ survival outcomes were computed from the date of esophagectomy, and follow-up data were updated until September 2022. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of West China Hospital, Sichuan University (No. 2019632) and individual consent for this retrospective analysis was waived.
Neoadjuvant chemoradiotherapy and surgery
Patients received neoadjuvant treatment according to national guidelines. Typically, the entire neoadjuvant chemotherapy regimen comprised two cycles. After completing each cycle of chemotherapy, patients were required to undergo a three-week rest period before commencing the subsequent cycle. All patients received paclitaxel (175 mg/m2 body-surface area, D1) and cisplatin (75 mg/m2 body-surface area, D1) intravenously during a two-cycle period. Patients also received radiotherapy with the radiation dosage of 1.8–2.0 Gy/fraction. Among all the included ESCC patients, during the therapeutic period of neoadjuvant chemotherapy, 25 patients had been synchronously treated with two cycles of immunotherapy administered by camrelizumab (200 mg, D1), tislelizumab (200 mg, D1), pembrolizumab (200 mg, D1), or sintilimab (200 mg, D1).
Surgical resection was performed approximately 6–8 weeks after the completion of neoadjuvant therapy. All patients had undergone radical esophagectomy with two or three-field lymphadenectomy, utilizing either minimally invasive or open techniques. Subsequently, conduit reconstruction via the stomach was carried out. In cases where preoperative computed tomography (CT) scans indicated potential cervical lymph node metastases, cervical lymph node dissection was performed.
CONUT score assessment
The assessment of the CONUT score in all cases was conducted via laboratory peripheral blood tests. CONUT scores were calculated at two distinct time points: before treatment initiation and before surgery. The CONUT score was derived from the cholesterol level, serum albumin level, and total lymphocyte level of each ESCC patient. The total CONUT score was categorized as either moderate malnutrition or severe malnutrition based on a predefined cutoff value set at 4. Furthermore, the delta CONUT (ΔCONUT) score was calculated as the preoperative CONUT score minus the pretreatment CONUT score. The pretreatment blood samples were obtained from patients upon their initial presentation to our institution, preceding the commencement of any treatment regimen. Subsequently, the preoperative blood samples were collected from patients following neoadjuvant therapy, one week prior to esophagectomy. Additionally, we calculated the classic nutritional index, and body mass index (BMI), at the same time points. The cutoff value for BMI was set at 18.5 kg/m2, consistent with previous studies (21). Furthermore, we also calculated the delta BMI (ΔBMI) by preoperative BMI value subtracting pretreatment BMI value. The details of scoring system and nutritional status assessment of CONUT are shown in Table 1.
Table 1
| Parameters | Nutritional level | ||||
|---|---|---|---|---|---|
| Moderate malnutrition | Severe malnutrition | ||||
| High grade | Low grade | High grade | Low grade | ||
| Serum albumin (g/dL) | ≥3.50 | 3.00–3.49 | 2.50–2.99 | <2.50 | |
| Score | 0 | 2 | 4 | 6 | |
| Total lymphocyte (/μL) | ≥1,600 | 1,200–1,599 | 800–1,199 | <800 | |
| Score | 0 | 1 | 2 | 3 | |
| Total cholesterol (mg/dL) | ≥180 | 140–179 | 100–139 | <100 | |
| Score | 0 | 1 | 2 | 3 | |
| Total score | ≤4 | >4 | |||
CONUT, controlling nutrition status.
Statistical analysis
SPSS 26.0 software was utilized for statistical analyses. The association of CONUT score on clinical or pathologic features was calculated through Chi-square. The survival curves were achieved by Kaplan-Meier method and group comparison was conducted through long-rank test. Cox proportional hazards model was used to confirm independent prognostic factors of ESCC patients. The hazard ratio (HR) and their 95% confidence interval (CI) were also calculated and P value lower than 0.05 indicated statistical significance.
Results
Clinical characteristics based on different CONUT score
The entire cohort consisted of 314 patients, with a mean age of 62.2 years. Among them, there were 252 male patients (80.3%) with a mean age of 61.8 years (range, 44–79 years), and 62 female patients (19.7%) with a mean age of 63.1 years (range, 44–80 years). According to the CONUT score, with a cutoff value set at 4, nutritional levels were categorized as moderate or severe malnutrition. Specifically, 279 patients were assigned to the pretreatment moderate malnutrition group, while 35 patients were assigned to the severe malnutrition group. Tumors located in the upper, middle, and lower thoracic esophagus were observed in 48 (15.3%), 187 (59.6%), and 79 (25.2%) of the cases, respectively. The pathological tumor T stage was ypT0, ypT1 or ypT2 for 213 patients and ypT3 or ypT4 for 101 patients. Regarding the pathological N stage, 197 patients were N negative, while 117 patients were N positive. Furthermore, there were 232 patients with ΔCONUT ≥0 and 82 patients with ΔCONUT <0. The details of patient characteristics for the three CONUT groups are presented in Table 2 and the details of the entire cohort were demonstrated in Table S1.
Table 2
| Factors | Pretreatment CONUT | Preoperative CONUT | ΔCONUT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (n=279) | High (n=35) | P | Low (n=247) | High (n=67) | P | Δ≥0 (n=232) | Δ<0 (n=82) | P | |||
| Sex | 0.39 | 0.19 | 0.70 | ||||||||
| Female | 57 (20.4) | 5 (14.3) | 45 (18.2) | 17 (25.4) | 47 (20.3) | 15 (18.3) | |||||
| Male | 222 (79.6) | 30 (85.7) | 202 (81.8) | 50 (74.6) | 185 (79.7) | 67 (81.7) | |||||
| Age (years) | 0.20 | 0.01* | 0.49 | ||||||||
| >60 | 111 (39.8) | 10 (28.6) | 104 (42.1) | 17 (25.4) | 92 (39.7) | 29 (35.4) | |||||
| ≤60 | 168 (60.2) | 25 (71.4) | 143 (57.9) | 50 (74.6) | 140 (60.3) | 53 (64.6) | |||||
| Pretreatment-BMI (kg/m2) | 0.58 | – | 0.29 | ||||||||
| >18.5 | 247 (88.5) | 30 (85.7) | – | – | 202 (87.1) | 75 (91.5) | |||||
| ≤18.5 | 32 (11.5) | 5 (14.3) | – | – | 30 (12.9) | 7 (8.5) | |||||
| Preoperative-BMI (kg/m2) | – | 0.01* | 0.12 | ||||||||
| >18.5 | – | – | 223 (90.3) | 53 (79.1) | 200 (86.2) | 76 (92.7) | |||||
| ≤18.5 | – | – | 24 (9.7) | 14 (20.9) | 32 (13.8) | 6 (7.3) | |||||
| ΔBMI | – | 0.31 | 0.48 | ||||||||
| >0 | – | – | 195 (78.9) | 49 (73.1) | 178 (76.7) | 66 (80.5) | |||||
| ≤0 | – | – | 52 (21.1) | 18 (26.9) | 54 (23.3) | 16 (19.5) | |||||
| Smoke | 0.98 | 0.67 | 0.40 | ||||||||
| No | 135 (48.4) | 17 (48.6) | 118 (47.8) | 34 (50.7) | 109 (47.0) | 43 (52.4) | |||||
| Yes | 144 (51.6) | 18 (51.4) | 129 (52.2) | 33 (49.3) | 123 (53.0) | 39 (47.6) | |||||
| Coronary artery disease | 0.39 | 0.53 | >0.99 | ||||||||
| No | 264 (94.6) | 35 (100.0) | 236 (95.5) | 63 (94.0) | 221 (95.3) | 78 (95.1) | |||||
| Yes | 15 (5.4) | 0 (0.0) | 11 (4.5) | 4 (6.0) | 11 (4.7) | 4 (4.9) | |||||
| Hypertension | 0.89 | 0.53 | 0.28 | ||||||||
| No | 226 (81.0) | 28 (80.0) | 198 (80.2) | 56 (83.6) | 191 (82.3) | 63 (76.8) | |||||
| Yes | 53 (19.0) | 7 (20.0) | 49 (19.8) | 11 (16.4) | 41 (17.7) | 19 (23.2) | |||||
| COPD | >0.99 | 0.57 | 0.79 | ||||||||
| No | 262 (93.9) | 33 (94.3) | 233 (94.3) | 62 (92.5) | 217 (93.5) | 78 (95.1) | |||||
| Yes | 17 (6.1) | 2 (5.7) | 14 (5.7) | 5 (7.5) | 15 (6.5) | 4 (4.9) | |||||
| Tumor location | 0.008* | 0.89 | 0.42 | ||||||||
| Upper | 46 (16.5) | 2 (5.7) | 39 (15.8) | 9 (13.4) | 37 (15.9) | 11 (13.4) | |||||
| Middle | 170 (60.9) | 17 (48.6) | 146 (59.1) | 41 (61.2) | 141 (60.8) | 46 (56.1) | |||||
| Lower | 63 (22.6) | 16 (45.7) | 62 (25.1) | 17 (25.4) | 54 (23.3) | 25 (30.5) | |||||
| Tumor diameter (cm) | 0.09 | 0.03* | 0.65 | ||||||||
| ≤3 | 184 (65.9) | 28 (80.0) | 174 (70.4) | 38 (56.7) | 155 (66.8) | 57 (69.5) | |||||
| >3 | 95 (34.1) | 7 (20.0) | 73 (29.6) | 29 (43.3) | 77 (33.2) | 25 (30.5) | |||||
| Pathological T stage | 0.63 | 0.67 | 0.35 | ||||||||
| ypT 0, 1, 2 | 188 (67.4) | 25 (71.4) | 169 (68.4) | 44 (65.7) | 154 (66.4) | 59 (72.0) | |||||
| ypT 3, 4 | 91 (32.6) | 10 (28.6) | 78 (31.6) | 23 (34.3) | 78 (33.6) | 23 (28.0) | |||||
| Pathological N stage | 0.99 | 0.09 | 0.23 | ||||||||
| ypN negative | 175 (62.7) | 22 (62.9) | 161 (65.2) | 36 (53.7) | 141 (60.8) | 56 (68.3) | |||||
| ypN positive | 104 (37.3) | 13 (37.1) | 86 (34.8) | 31 (46.3) | 91 (39.2) | 26 (31.7) | |||||
| Differentiation | 0.78 | 0.62 | 0.24 | ||||||||
| Well differentiated | 127 (45.5) | 18 (51.4) | 113 (45.7) | 32 (47.8) | 104 (44.8) | 41 (50.0) | |||||
| Moderate differentiated | 68 (24.4) | 7 (20.0) | 57 (23.1) | 18 (26.9) | 61 (26.3) | 14 (17.1) | |||||
| Poor differentiated | 84 (30.1) | 10 (28.6) | 77 (31.2) | 17 (25.4) | 67 (28.9) | 27 (32.9) | |||||
| Preoperative treatment | >0.99 | 0.74 | 0.80 | ||||||||
| nCRT | 256 (91.8) | 33 (94.3) | 228 (92.3) | 61 (91.0) | 213 (91.8) | 76 (92.7) | |||||
| IMT | 23 (8.2) | 2 (5.7) | 19 (7.7) | 6 (9.0) | 19 (8.2) | 6 (7.3) | |||||
| TRG | 0.37 | 0.87 | 0.23 | ||||||||
| 0, 1 | 150 (53.8) | 16 (45.7) | 130 (52.6) | 36 (53.7) | 118 (50.9) | 48 (58.5) | |||||
| 2, 3 | 129 (46.2) | 19 (54.3) | 117 (47.4) | 31 (46.3) | 114 (49.1) | 34 (41.5) | |||||
Data are presented as n (%). *, P<0.05. CONUT, controlling nutritional status; BMI, body mass index; COPD, chronic obstructive pulmonary disease; nCRT, neoadjuvant chemoradiotherapy; IMT, immunotherapy; TRG, tumor regression grade.
CONUT score and postoperative complications
Table 3 delineated various factors, including preoperative chronic diseases and different CONUT scores of ESCC patients calculated at different time points, and examined their impact on postoperative complications. The results indicated that the complication rates following esophagectomy were significantly higher in patients with chronic obstructive pulmonary disease (COPD) [odds ratio (OR)=2.656, 95% CI: 1.043–6.764, P=0.04], high preoperative CONUT scores (OR =2.009, 95% CI: 1.150–3.510, P=0.01), and ΔCONUT ≥0 (OR =3.373, 95% CI: 1.729–6.579, P<0.001). For patients with high pretreatment CONUT scores, no significant association was found with postoperative complications. Additionally, the results of multivariate logistic regression further demonstrated that ΔCONUT ≥0 was a strong independent predictor of postoperative complications (OR =3.008, 95% CI: 1.509–5.999, P=0.002). Conversely, BMI was not identified as an independent risk factor for postoperative complications (Table 3).
Table 3
| Factors | Univariable analyses | Multivariable analyses | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Sex (male/female) | 1.231 (0.664–2.284) | 0.51 | |||
| Age (≥60/<60 years) | 1.240 (0.754–2.040) | 0.40 | |||
| Smoke (yes/no) | 1.430 (0.883–2.318) | 0.15 | |||
| Coronary artery disease (present/absent) | 0.805 (0.250–2.596) | 0.72 | |||
| Hypertension (present/absent) | 0.777 (0.414–1.458) | 0.43 | |||
| COPD (present/absent) | 2.656 (1.043–6.764) | 0.04* | 2.604 (0.991–6.845) | 0.052 | |
| Preoperative treatment (IMT/nCRT) | 2.215 (0.971–5.052) | 0.06 | |||
| Pretreatment BMI (low/high) | 1.844 (0.915–3.713) | 0.09 | |||
| Preoperative BMI (low/high) | 1.751 (0.875–3.505) | 0.11 | |||
| ΔBMI (>0/≤0) | 1.561 (0.896–2.720) | 0.12 | |||
| Pretreatment CONUT (high/low) | 0.883 (0.406–1.918) | 0.75 | |||
| Preoperative CONUT (high/low) | 2.009 (1.150–3.510) | 0.01* | 1.526 (0.853–2.730) | 0.16 | |
| ΔCONUT (≥0/<0) | 3.373 (1.729–6.579) | <0.001* | 3.008 (1.509–5.999) | 0.002* | |
| TRG (2, 3/0, 1) | 1.080 (0.669–1.743) | 0.75 | |||
*, P<0.05. OR, odds ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease; IMT, immunotherapy; nCRT, neoadjuvant chemoradiotherapy; BMI, body mass index; CONUT, controlling nutritional status; TRG, tumor regression grade.
CONUT score and survival outcomes of esophagectomy after EC neoadjuvant therapy
Figure 1 depicts the Kaplan-Meier curves for overall survival (OS) and disease-free survival (DFS) based on the cutoff value of the CONUT score. Patients with a preoperative CONUT score higher than 4 and ΔCONUT score ≥0 exhibited significantly decreased OS and DFS. However, no significant differences in OS and DFS were observed among ESCC patients with different pretreatment CONUT groups (Figure 1). According to the univariate analysis, several factors including sex (P=0.03), smoking status (P=0.04), tumor diameter (P=0.001), pathological T stage (P<0.001), pathological N stage (P<0.001), tumor differentiation (P<0.001), tumor regression grade (TRG) (P<0.001), pretreatment BMI (P=0.009), preoperative BMI (P=0.002), ΔBMI (P=0.02), preoperative CONUT score (P=0.01), and ΔCONUT score ≥0 (P=0.01) significantly impacted the OS of ESCC patients. Furthermore, multivariable analysis revealed that pathological N stage (HR =2.550, 95% CI: 1.404–4.631, P=0.002) and ΔCONUT score (HR =2.388, 95% CI: 1.052–5.422, P=0.04) were independent prognostic factors for OS (Table 4). Furthermore, the results of univariate analysis revealed that smoking status (P=0.01), tumor diameter (P<0.001), pathological T stage (P<0.001), pathological N stage (P<0.001), tumor differentiation (P<0.001), TRG (P<0.001), preoperative BMI (P=0.01), ΔBMI (P=0.04), preoperative CONUT score (P=0.001), and ΔCONUT score ≥0 (P=0.002) were significantly associated with poorer DFS in ESCC patients. Additionally, smoking status (HR =1.698, 95% CI: 1.073–2.688, P=0.02), tumor diameter (HR =1.588, 95% CI: 1.007–2.506, P=0.047), pathological N stage (HR =2.335, 95% CI: 1.435–3.800, P=0.001), and ΔCONUT score (HR =2.459, 95% CI: 1.237–4.889, P=0.01) were identified as independent prognostic factors for DFS (Table 5).
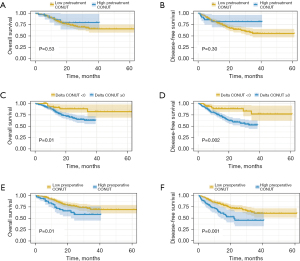
Table 4
| Factors | Univariable analyses | Multivariable analyses | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex (male/female) | 2.608 (1.123–6.055) | 0.03* | 1.555 (0.585–4.131) | 0.38 | |
| Age (≥60/<60 years) | 1.078 (0.646–1.799) | 0.77 | |||
| Smoke (yes/no) | 1.719 (1.016–2.909) | 0.04* | 1.387 (0.746–2.581) | 0.30 | |
| Coronary artery disease (present/absent) | 2.733 (0.853–6.538) | 0.10 | |||
| Hypertension (present/absent) | 0.745 (0.368–1.512) | 0.42 | |||
| COPD (present/absent) | 0.545 (0.075–3.961) | 0.55 | |||
| Tumor location | 1.034 (0.680–1.574) | 0.88 | |||
| Tumor diameter (≥3/<3 cm) | 2.317 (1.403–3.825) | 0.001* | 1.683 (0.979–2.894) | 0.06 | |
| ypT (3, 4/0, 1, 2) | 2.934 (1.776–4.848) | <0.001* | 1.173 (0.531–2.592) | 0.69 | |
| ypN (positive/negative) | 4.066 (2.398–6.897) | <0.001* | 2.550 (1.404–4.631) | 0.002* | |
| Differentiation (poor/moderate or well) | 1.709 (1.277–2.287) | <0.001* | 1.202 (0.744–1.942) | 0.45 | |
| TRG (2, 3/0, 1) | 2.866 (1.670–4.921) | <0.001* | 1.102 (0.432–2.815) | 0.84 | |
| Preoperative treatment (IMT/nCRT) | 0.970 (0.233–4.044) | 0.97 | |||
| Pretreatment BMI (low/high) | 2.275 (1.233–4.197) | 0.009* | 1.072 (0.258–4.450) | 0.92 | |
| Preoperative BMI (low/high) | 2.563 (1.412–4.653) | 0.002* | 1.440 (0.376–5.515) | 0.60 | |
| ΔBMI (>0/≤0) | 2.143 (1.153–3.986) | 0.02* | 1.876 (0.933–3.772) | 0.08 | |
| Pretreatment CONUT (high/low) | 0.744 (0.298–1.857) | 0.53 | |||
| Preoperative CONUT (high/low) | 1.967 (1.154–3.353) | 0.01* | 1.338 (0.756–2.368) | 0.32 | |
| ΔCONUT (≥0/<0) | 2.796 (1.273–6.143) | 0.01* | 2.388 (1.052–5.422) | 0.04* | |
*, P<0.05. ESCC, esophageal squamous cell carcinoma; HR, hazard ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease; TRG, tumor regression grade; IMT, immunotherapy; nCRT, neoadjuvant chemoradiotherapy; BMI, body mass index; CONUT, controlling nutritional status.
Table 5
| Factors | Univariable analyses | Multivariable analyses | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex (male/female) | 1.865 (0.989–3.516) | 0.05 | |||
| Age (≥60/<60 years) | 1.060 (0.685–1.641) | 0.79 | |||
| Smoke (yes/no) | 1.775 (1.133–2.780) | 0.01* | 1.698 (1.073–2.688) | 0.02* | |
| Coronary artery disease (present/absent) | 1.820 (0.734–4.513) | 0.20 | |||
| Hypertension (present/absent) | 0.798 (0.442–1.441) | 0.46 | |||
| COPD (present/absent) | 1.297 (0.471–3.568) | 0.62 | |||
| Tumor location | 1.064 (0.748–1.515) | 0.73 | |||
| Tumor diameter (≥3/<3 cm) | 2.138 (1.396–3.273) | <0.001* | 1.588 (1.007–2.506) | 0.047* | |
| ypT (3, 4/0, 1, 2) | 2.876 (1.877–4.408) | <0.001* | 1.581 (0.812–3.081) | 0.18 | |
| ypN (positive/negative) | 3.372 (2.175–5.228) | <0.001* | 2.335 (1.435–3.800) | 0.001* | |
| Differentiation (poor/moderate or well) | 1.627 (1.270–2.083) | <0.001* | 1.125 (0.755–1.678) | 0.56 | |
| TRG (2, 3/0, 1) | 2.565 (1.637–4.019) | <0.001* | 0.974 (0.445–2.132) | 0.95 | |
| Preoperative treatment (IMT/nCRT) | 0.531 (0.129–2.179) | 0.38 | |||
| Pretreatment BMI (low/high) | 1.701 (0.958–3.020) | 0.07 | |||
| Preoperative BMI (low/high) | 2.055 (1.192–3.544) | 0.01* | 1.306 (0.726–2.347) | 0.37 | |
| ΔBMI (>0/≤0) | 1.768 (1.041–3.004) | 0.04* | 1.469 (0.832–2.593) | 0.19 | |
| Pretreatment CONUT (high/low) | 0.642 (0.280–1.473) | 0.30 | |||
| Preoperative CONUT (high/low) | 2.104 (1.338–3.311) | 0.001* | 1.463 (0.903–2.372) | 0.12 | |
| ΔCONUT (≥0/<0) | 2.813 (1.453–5.446) | 0.002* | 2.459 (1.237–4.889) | 0.01* | |
*, P<0.05. ESCC, esophageal squamous cell carcinoma; HR, hazard ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease; TRG, tumor regression grade; IMT, immunotherapy; nCRT, neoadjuvant chemoradiotherapy; BMI, body mass index; CONUT, controlling nutritional status.
Subgroup analysis of CONUT
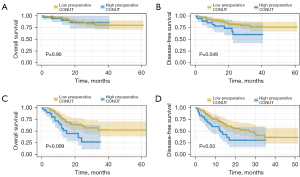
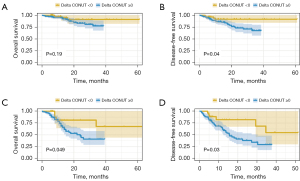
Moreover, subgroup analysis was conducted to investigate the prognostic value of preoperative CONUT score (Figure 2) and ΔCONUT score (Figure 3) in ESCC patients with or without lymph node metastasis. Interestingly, the results revealed no statistical significance in patients without lymph node metastasis for OS (P=0.90). However, in ESCC patients with lymph node metastasis, a higher preoperative CONUT score was associated with poorer survival for both OS (P=0.009) and DFS (P=0.03). The ΔCONUT score was demonstrated to be associated with worse DFS regardless of lymph node metastasis.
Discussion
To our knowledge, our research encompassed a large sample size, providing valuable insights into the prognostic significance of the nutritional parameter CONUT score at different time points, including ΔCONUT score, in ESCC patients. Traditional nutritional indices such as BMI have been validated as indicators of body composition and have also been shown to correlate with EC progression and prognosis in previous studies (22,23). In our study, owing to its acceptable individual variability, the CONUT score was chosen as a nutritional index. We evaluated the correlation between the CONUT score, clinicopathological factors, and survival outcomes in ESCC patients who underwent neoadjuvant therapy followed by esophagectomy. The findings of our study suggested that ESCC patients with high preoperative CONUT scores were more likely to experience worse postoperative outcomes. These results align with those of leading studies in EC patients (24). Furthermore, our study demonstrated that the ΔCONUT score remained an independent prognostic factor for both OS and DFS. Additionally, we found that the ΔCONUT score was an independent risk factor for postoperative complications, with patients having a ΔCONUT score ≥0 being more prone to higher postoperative complication rates. Interestingly, pretreatment CONUT scores showed no association with the survival outcomes of ESCC patients or postoperative complication rates. These findings suggest that the CONUT score calculated at the preoperative time point may be a superior index and calculating the ΔCONUT value by preoperative CONUT value subtracting pretreatment CONUT value may be helpful in predicting both postoperative survival outcomes and patients’ survival.
On the contrary, according to the results of subgroup analysis, preoperative CONUT and ΔCONUT scores were not associated with OS in ESCC patients without lymph node metastasis. However, for ESCC patients with a positive N stage, the preoperative CONUT score was shown to correlate with worse prognosis. Such a discrepancy might be attributed to differences in nutritional status among diverse stages of ESCC. For ESCC patients in advanced stages, factors such as invasiveness, obstruction, dysphagia, inflammatory responses, gastrointestinal adverse events, and cachexia of ESCC may lead to the development of severe malnutrition. Consequently, the preoperative CONUT score may emerge as a more sensitive indicator in advanced ESCC patients.
Generally, patients with EC typically present with the characteristic symptom of progressive dysphagia, initially for solids and later for liquids. These symptoms often lead to frequent weight loss and are associated with poor survival outcomes (25). Esophagectomy together with neoadjuvant chemoradiation now becomes the first choice to treat patients with progressive EC (26,27). However, EC patients who finished neoadjuvant therapy were still found to have lower nutritional level (28). Due to the side effects of chemoradiation such as esophageal edema, obstruction, and inflammation, patients with EC are more prone to experiencing insufficient nutrition intake (29). Thus, patients with EC face a severe challenge in nutritional supplement. In recent years, the importance of nutritional level and dietary support has become a common recognition and covers the whole progression of EC treatment: from diagnostic phase to long-term nutrition support. Therefore, it is vital to confirm EC patients who need intensive nutritional support. On the other hand, previous studies have suggested that the progression of EC may be associated with a decline in the immune system. Malnutrition is commonly believed to be associated with defective immune function (30-33). Considering both immune and nutritional indexes consist of CONUT score, and the strong ability of CONUT score to predict the survival outcomes in EC patients, CONUT score could not only utilized as a detector of predicting tumor progression but also for identifying ESCC patients with decreased immune-nutritional level and for patients requiring nutritional support after neoadjuvant therapy.
Currently, two types of nutritional status evaluation systems are applied in clinical practice. One relies mostly on subjective parameters, while the other is entirely based on objective parameters. Above the evaluating systems mainly based on subjective parameters, patients are required to recall their food intake condition, or the loss of appetite during the last few months (34). However, such evaluation systems may be prone to bias due to the subjective nature of various patients’ parameters, potentially leading to inaccurate results. Furthermore, while nutritional evaluation indexes based on objective parameters like BMI and serum albumin can reflect the nutritional status of patients to some extent, they may not fully capture the immune function of ESCC patients. CONUT is a simple objective parameter which is calculated through cholesterol, serum albumin and total lymphocyte level in peripheral blood that is easily obtained from a routine blood examination (35). Cholesterol has been reported to be associated with various metabolism diseases and cancers (36). Serum albumin was commonly believed as an indicator of both immune and nutritional status, and hypoalbuminemia was also reported to correlate with worse postoperative outcomes and cachexia in malignancies (37). Total lymphocyte, which was proved as an immune function detector to predict survival outcomes in various cancers (38). Therefore, based on the evidence provided, the CONUT score has been demonstrated to be a superior index in evaluating immune-nutritional function in patients with EC.
Improving the nutritional status of patients with ESCC is crucial. This is because maintaining good nutrition not only enhances patients’ tolerance to treatment but also improves treatment efficacy and reduces adverse reactions during therapy. Given the strong prognostic role of the CONUT score in EC, some scholars have attempted to counter malnutrition by providing nutritional support to EC patients during the whole disease management. Oral nutritional supplements, such as liquid meal replacements, can serve as convenient and effective means of increasing calorie and protein intake in patients with compromised nutritional status. These supplements are often fortified with essential nutrients and micronutrients to address specific nutritional deficiencies (39,40). In cases where oral intake is insufficient or compromised, enteral nutrition via tube feeding may be indicated to provide adequate nutrition. Enteral feeding can be administered either nasogastrically or via gastrostomy tube, delivering a balanced formula directly into the gastrointestinal tract (41,42). Even after surgery, nutritional support remains critically important for patients with ESCC. EC patients treated with enteral nutrition support after esophagectomy experienced greater benefits in terms of immune function recovery and nutritional improvement. Furthermore, for EC patients receiving nutritional support, it was associated with a shortened duration of hospitalization (30).
Although our research demonstrated the prognostic value of CONUT score in ESCC patients, caution should be taken for the following reasons. First, the study was a single-center retrospective cohort study with a huge samples amount, all patients included were from single institution. Secondly, it is important to recognize that CONUT serves as a single assessment tool among many in evaluating the nutritional status and prognosis of patients with ESCC. It is crucial to acknowledge that the nutritional status of individuals is multifaceted and encompasses various parameters beyond those assessed by CONUT alone. The utility of CONUT may be enhanced when considered in conjunction with other relevant nutritional parameters. Finally, it is important to acknowledge that there is no consensus on the exact cutoff value of the CONUT score, and the optimal evaluation standard for CONUT scores remains unclear. Therefore, the significance of the CONUT score requires further validation through prospective studies in the future.
Conclusions
This study suggests that CONUT score can be utilized in nutritional status evaluation and CONUT score is also a robust prognostic factor of postoperative complications and long-term survival of ESCC patients.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-187/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-187/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-187/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-187/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baiu I, Backhus L. Esophageal Cancer Surgery. JAMA 2020;324:1580. [Crossref] [PubMed]
- Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013;19:5598-606. [Crossref] [PubMed]
- Ono T. Review of clinical results of charged-particle therapy for esophageal cancer. Esophagus 2021;18:33-40. [Crossref] [PubMed]
- Thrift AP. The epidemic of oesophageal carcinoma: Where are we now? Cancer Epidemiol 2016;41:88-95. [Crossref] [PubMed]
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Zhu H, Ma X, Ye T, et al. Esophageal cancer in China: Practice and research in the new era. Int J Cancer 2023;152:1741-51. [Crossref] [PubMed]
- Watanabe M, Otake R, Kozuki R, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today 2020;50:12-20. [Crossref] [PubMed]
- Kato H, Nakajima M. Treatments for esophageal cancer: a review. Gen Thorac Cardiovasc Surg 2013;61:330-5. [Crossref] [PubMed]
- Wang Y, Yang W, Wang Q, et al. Mechanisms of esophageal cancer metastasis and treatment progress. Front Immunol 2023;14:1206504. [Crossref] [PubMed]
- Luan S, Xie R, Yang Y, et al. Acid-Responsive Aggregated Gold Nanoparticles for Radiosensitization and Synergistic Chemoradiotherapy in the Treatment of Esophageal Cancer. Small 2022;18:e2200115. [Crossref] [PubMed]
- Wujanto C, Tey J, Vellayappan B, et al. Outcomes of oesophageal cancer treated with neoadjuvant compared with definitive chemoradiotherapy. Ann Acad Med Singap 2021;50:536-47. [Crossref] [PubMed]
- Pellen MG, Sabri S, Razack A, et al. Safety and efficacy of self-expanding removable metal esophageal stents during neoadjuvant chemotherapy for resectable esophageal cancer. Dis Esophagus 2012;25:48-53. [Crossref] [PubMed]
- Cao J, Xu H, Li W, et al. Nutritional assessment and risk factors associated to malnutrition in patients with esophageal cancer. Curr Probl Cancer 2021;45:100638. [Crossref] [PubMed]
- Okadome K, Baba Y, Yagi T, et al. Prognostic Nutritional Index, Tumor-infiltrating Lymphocytes, and Prognosis in Patients with Esophageal Cancer. Ann Surg 2020;271:693-700. [Crossref] [PubMed]
- Zhou H, Chao W, Cui L, et al. Controlling Nutritional Status (CONUT) score as immune-nutritional predictor of outcomes in patients undergoing peritoneal dialysis. Clin Nutr 2020;39:2564-70. [Crossref] [PubMed]
- Akagunduz B, Demir M, Atcı MM. Controlling Nutritional Status (CONUT) Score Is a Prognostic Factor for Patients with Gastric Cancer Treated by Perioperative FLOT. J Gastrointest Cancer 2022;53:571-80. [Crossref] [PubMed]
- Zhang Z, Wang D, Zhang J, et al. Comparison of the effectiveness of chemotherapy combined with immunotherapy and chemotherapy alone in advanced biliary tract cancer and construction of the nomogram for survival prediction based on the inflammatory index and controlling nutritional status score. Cancer Immunol Immunother 2023;72:3635-49. [Crossref] [PubMed]
- Hirahara N, Matsubara T, Hayashi H, et al. Prognostic Importance of Controlling Nutritional Status in Patients Undergoing Curative Thoracoscopic Esophagectomy for Esophageal Cancer. Am J Ther 2018;25:e524-32. [Crossref] [PubMed]
- Toyokawa T, Kubo N, Tamura T, et al. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer 2016;16:722. [Crossref] [PubMed]
- Obermannová R, Alsina M, Cervantes A, et al. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33:992-1004. [Crossref] [PubMed]
- Fang P, Yang Q, Zhou J, et al. The impact of geriatric nutritional risk index on esophageal squamous cell carcinoma patients with neoadjuvant therapy followed by esophagectomy. Front Nutr 2022;9:983038. [Crossref] [PubMed]
- Gu WS, Fang WZ, Liu CY, et al. Prognostic significance of combined pretreatment body mass index (BMI) and BMI loss in patients with esophageal cancer. Cancer Manag Res 2019;11:3029-41. [Crossref] [PubMed]
- Li P, Jing J, Liu W, et al. Spatiotemporal Patterns of Esophageal Cancer Burden Attributable to Behavioral, Metabolic, and Dietary Risk Factors From 1990 to 2019: Longitudinal Observational Study. JMIR Public Health Surveill 2023;9:e46051. [Crossref] [PubMed]
- Wang PY, Chen XK, Liu Q, et al. Application of four nutritional risk indexes in perioperative management for esophageal cancer patients. J Cancer Res Clin Oncol 2021;147:3099-111. [Crossref] [PubMed]
- Mariette C, De Botton ML, Piessen G. Surgery in esophageal and gastric cancer patients: what is the role for nutrition support in your daily practice? Ann Surg Oncol 2012;19:2128-34. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Tang H, Wang H, Fang Y, et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy followed by minimally invasive esophagectomy for locally advanced esophageal squamous cell carcinoma: a prospective multicenter randomized clinical trial. Ann Oncol 2023;34:163-72. [Crossref] [PubMed]
- Smith ZL, Gonzaga JE, Haasler GB, et al. Self-Expanding Metal Stents Improve Swallowing and Maintain Nutrition During Neoadjuvant Therapy for Esophageal Cancer. Dig Dis Sci 2017;62:1647-56. [Crossref] [PubMed]
- Lund M, Tsai JA, Nilsson M, et al. Effects of neoadjuvant chemo or chemoradiotherapy for oesophageal cancer on perioperative haemodynamics: A prospective cohort study within a randomised clinical trial. Eur J Anaesthesiol 2016;33:653-61. [Crossref] [PubMed]
- Ding H, Xu J, You J, et al. Effects of enteral nutrition support combined with enhanced recovery after surgery on the nutritional status, immune function, and prognosis of patients with esophageal cancer after Ivor-Lewis operation. J Thorac Dis 2020;12:7337-45. [Crossref] [PubMed]
- Zheng Y, Chen Z, Han Y, et al. Immune suppressive landscape in the human esophageal squamous cell carcinoma microenvironment. Nat Commun 2020;11:6268. [Crossref] [PubMed]
- Huang TX, Fu L. The immune landscape of esophageal cancer. Cancer Commun (Lond) 2019;39:79. [Crossref] [PubMed]
- Fang P, Zhou J, Xiao X, et al. The prognostic value of sarcopenia in oesophageal cancer: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2023;14:3-16. [Crossref] [PubMed]
- Kondrup J, Rasmussen HH, Hamberg O, et al. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr 2003;22:321-36. [Crossref] [PubMed]
- Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005;20:38-45.
- Buchwald H. Cholesterol inhibition, cancer, and chemotherapy. Lancet 1992;339:1154-6. [Crossref] [PubMed]
- Haskins IN, Baginsky M, Amdur RL, et al. Preoperative hypoalbuminemia is associated with worse outcomes in colon cancer patients. Clin Nutr 2017;36:1333-8. [Crossref] [PubMed]
- Fogar P, Sperti C, Basso D, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas 2006;32:22-8. [Crossref] [PubMed]
- Yang L, Gao J, Zhou Y, et al. Effect of Oral Nutritional Supplements on Patients with Esophageal Cancer During Radiotherapy. Cancer Biother Radiopharm 2023;38:89-94. [Crossref] [PubMed]
- De Moura A, Turpin A, Neuzillet C. Nutritional supportive care in the course of patients with esophagogastric cancers. Bull Cancer 2023;110:540-51. [Crossref] [PubMed]
- Song HX, Wei SH, An GH, et al. Effect of sequential vs. non-sequential early enteral nutrition therapy on nutritional status, recovery, and quality of life of patients with esophageal cancer. Eur Rev Med Pharmacol Sci 2023;27:7590-6. [Crossref] [PubMed]
- Wang SA, Dai WS, Zhu JY, et al. Nasogastric tube feeding improves nutritional status and physical state in esophageal cancer patients during chemoradiotherapy: a retrospective study. Support Care Cancer 2023;31:341. [Crossref] [PubMed]


