
The Geriatric Nutritional Risk Index as a prognostic factor in patients treated with immune checkpoint inhibitors with non-small-cell lung cancer
Highlight box
Key findings
• The Geriatric Nutritional Risk Index (GNRI) is an effective independent prognostic factors for patients with non-small cell lung cancer (NSCLC) receiving immune checkpoint inhibitors (ICIs) therapies and a higher GNRI at diagnosis in these patients is significantly associated with longer progression-free survival and overall survival.
What is known and what is new?
• Immune checkpoint inhibitors have revolutionized the field of tumor therapy and GNRI has been evaluated as a predictive and prognostic factor in different malignancies.
• There are few reports on prognostic relationship between GNRI and NSCLC patients treated with ICIs.
What is the implication, and what should change now?
• GNRI can provide a basis for nutritional support before ICI treatment and a predictive model for survival rates of NSCLC patients receiving ICI therapy can be established based on GNRI. Further prospective randomized studies are needed.
Introduction
Pulmonary cancer is the primary cause of cancer-related deaths on a global scale, with 1.8 million fatalities reported annually, making it the second most frequently diagnosed sort of cancer (1). The overall lung cancer survival rate for over five years is less than 20% (2). Non-small cell lung cancer (NSCLC), the most typical form of lung cancer diagnosis, making up approximately 85% of cases all around (3). This type of cancer is typically diagnosed in advanced stages, leading to a worse prognosis for patients.
Several immunological checkpoints have been identified after the discovery of cytotoxic T-lymphocyte antigen 4 (CTLA-4), such as programmed death-1 (PD-1), and programmed death-ligand 1 (PD-L1) (4). Targeting the immune checkpoint can result in a long-term therapeutic response in the treatment of cancer (5-7). Immune checkpoint inhibitors (ICIs) work by removing the barriers that prohibit T cells from striking tumor cells, ultimately enhancing the immunity system’s response and promoting effective anti-tumor immune reactions (8). This mechanism allows for a significant boost in the body’s ability to combat cancer, leading to promising results in cancer treatment. Immune checkpoint therapy has become the first-line therapy for a range of solid and liquid tumors including NSCLC (9-11).
For years, it has been recognized that a worse prognosis is associated with malnutrition in cancer patients (12). Early investigations have demonstrated that weight decrease, and lower body mass index (BMI) are critical predictors of worse outcomes for advanced NSCLC patients (13,14). In 2005, Bouillanne et al. initially proposed the concept of the Geriatric Nutrition Risk Index (GNRI) which is derived from the percentage of actual weight to optimal weight, and the level of serum albumin (15). GNRI has been evaluated as a predictive and prognostic variable in different malignancies, such as gastric carcinoma, colorectal cancer, renal cell cancer, esophageal carcinoma and many other malignant tumors (16-21). Peng et al. observed a substantial association between elevated GNRI scores and improved survival in advanced NSCLC individuals (22). However, researches about the prognostic relationship between GNRI and NSCLC patients treated with ICIs are lacking, particularly in Chinese population. Therefore, we conducted this investigation to figure out the influence of nutritional conditions on prognostic outcomes in NSCLC patients receiving ICIs by GNRI. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-436/rc).
Methods
Study population
We enrolled 687 primary lung cancer patients treated in Huadong Hospital between January 1, 2018 and December 31, 2021. Out of these, 148 patients who met the criteria for immunotherapy were selected for further analysis (Figure 1). We excluded patients who received neoadjuvant immunotherapy and only included those who chose immunotherapy due to lack of surgical indications or used immunotherapeutic drugs postoperatively. For enrolled patients, ICI therapy was utilized both as first-line and subsequent treatments, including second-line or higher interventions. Eligible patients had received at least one cycle of ICI therapy during treatment period. In our study, immunotherapy drugs such as camrelizumab, sintilimab, pembrolizumab, and tislelizumab were administered intravenously at a set dosage of 200 mg once every 3 weeks. Several patients accepted toripalimab (at a dose of 240 mg per 3 weeks), nivolumab (at a dose of 360 mg per 3 weeks), durvalumab (at a dose of 1,500 mg per 4 weeks) and serplulimab (at a dose of 240 mg per 3 weeks). The combination chemotherapy typically includes platinum-based drugs and taxanes. Bevacizumab was used in targeted therapy, commonly given at a dose of 400 mg per 3 weeks. At last, Patients with recurrence or metastasis typically undergo radiation therapy. Treatment was administered until the condition worsened, unacceptable side effects appeared, or the prescribed number of cycles of treatment had been completed. This study was conducted in accordance with the Declaration of Helsinki (revised in 2013) and was approved by the Ethics Committee of Huadong Hospital affiliated to Fudan University (No. 2021K010). Informed consent was obtained from all patients.
Data collection
We collected data on patient demographics when diagnosed, including age, gender, height, weight, smoking history, surgical history, extent of resection, histology, treatment therapy, lines of immunotherapy, cycles of immunotherapy, and tumor node metastasis (TNM) stage. The staging of NSCLC was determined following the criteria outlined in the 8th version of the TNM staging strategy (23). By adhering to these established guidelines, healthcare providers can accurately classify the stage of NSCLC based on tumor size, lymph node involvement, and metastasis. Additionally, laboratory parameters were collected, including albumin, peripheral lymphocyte count, peripheral neutrophil count, platelet count and tumor markers (CYFRA21, CEA). The efficacy of ICI therapy was evaluated by a superordinate doctor utilizing Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) (24). Progression-free survival (PFS) and overall survival (OS) were the main research outcomes, with the last follow-up date being November 30, 2023. PFS was defined as the period of time without illness progression or death after starting ICI therapy. The definition of OS used in the study included tracking the whole duration from diagnosis to the death from whatever cause, or noting which patients were still living at the follow-up deadline. This information is crucial in understanding the influence of GNRI on patient prognosis and can provide valuable insights for future research and treatment approaches.
Score calculation
The BMI was calculated as follows: weight (kg)/[height (m)]2. In compliance with guidelines from the World Health Organization, individuals were categorized into four groups based on their BMI (25): underweight (<18.5 kg/m2), normal weight (18.5 to <24 kg/m2), overweight (24 to <28 kg/m2), and obesity (≥28 kg/m2). The GNRI was formulated by amalgamating two nutritional variables: the proportion of real body weight to optimal body weight and albumin concentrations. The GNRI was computed as follows (15): GNRI = 1.489 × serum albumin levels (g/L) + 41.7 × actual body weight (kg)/optimal body weight (kg). The optimal body weight was calculated using the following equation (26): optimal body weight = 22 × height (m)2. In case that the patient’s actual weight surpassed the optimal weight, the ratio was standardized to 1 (15). As supplementary nutritional parameters, we also assessed neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and the prognostic nutrition index (PNI) (27-29). The following formula was used to determine the PNI (30): PNI = 5 × peripheral lymphocyte count (109/L) + serum albumin concentrations (g/L).
Statistical analysis
The receiver operating characteristic (ROC) curve was employed to establish the most suitable GNRI cutoff values for predicting OS. We assessed the predictive ability of GNRI for OS using the area under the ROC curve (AUC). Fisher’s exact test or the Chi-squared test was utilized to contrast categorical variables between the high and low GNRI groups. When comparing continuous variables that were not regularly distributed, the Mann-Whitney U test was employed. OS and PFS were analyzed using log-rank and the Kaplan-Meier approach. Our study employed both univariate and multivariate Cox regression models to analyze the factors related to PFS and OS. We then calculated hazard ratios (HRs) as well as corresponding 95% confidence intervals (CIs). Age, gender, smoking history, surgery history, BMI, histology, treatment therapy, lines of immunotherapy, TNM stage, CYFRA21, CEA, NLR, PLR, PNI as well as GNRI were involved in univariate analyses. The multivariate analysis included variables having a univariate P value <0.05. The Statistical Package for the Social Sciences (SPSS) 25.0 program (IBM Corporation, Armonk, NY, USA) was employed for all statistical analyses.
Results
Patient characteristics
The baseline characteristics, laboratory, and therapeutic information of 148 NSCLC patients are presented in Table 1. The high GNRI group included 73 (49.32%) patients and low GNRI group included 75 (50.68%) patients (Table 1). Males were predominant in the study population (85.14%, n=126), with a median age of 66 years (range, 37–87 years). Smoking history was observed in most cases (81.08%). The most prevalent histological category was adenocarcinoma, making up 53.38% of cases (n=79), squamous cell carcinoma and other types accounted for 39.86% (n=59) and 6.76% (n=10), respectively. According to the TNM classification, 79.05% of the patients (n=117) were diagnosed as stage II–III. A little over half of the patients (56.76%) underwent immunotherapy combined with chemotherapy, following by 29 (19.59%) patients underwent radiation therapy and 23 (15.54%) patients underwent targeted therapy. The remaining 12 (8.11%) patients received all treatment regimens. The median length of follow-up period was 893.5 days, ranging from 44 to 3,911 days. About half of the patients (55.41%) deceased before the follow-up period ended.
Table 1
| Variable | Total | High GNRI (n=73) | Low GNRI (n=75) | P |
|---|---|---|---|---|
| Age (years) | 64.00 [57.50, 69.00] | 68.00 [60.00, 70.00] | 0.11 | |
| Outcome | 0.003 | |||
| Live | 66 | 42 (57.5) | 24 (32.0) | |
| Dead | 82 | 31 (42.5) | 51 (68.0) | |
| Gender | 0.95 | |||
| Female | 22 | 11 (15.1) | 11 (14.7) | |
| Male | 126 | 62 (84.9) | 64 (85.3) | |
| Smoking | 0.77 | |||
| Never | 28 | 15 (20.5) | 13 (17.3) | |
| Ever or current | 120 | 58 (79.5) | 62 (82.7) | |
| Surgery | 0.25 | |||
| Yes | 69 | 38 (52.1) | 31 (41.3) | |
| No | 79 | 35 (47.9) | 44 (58.7) | |
| Extent of resection | 0.81 | |||
| Wedge resection | 18 | 8 (21.1) | 10 (32.3) | |
| Segmentectomy | 5 | 3 (7.9) | 2 (6.5) | |
| Lobectomy | 31 | 24 (63.2) | 17 (54.8) | |
| Pneumonectomy | 5 | 3 (7.9) | 2 (6.5) | |
| Histology | 0.18 | |||
| AC | 79 | 41 (56.2) | 38 (50.7) | |
| SCC | 59 | 30 (41.1) | 29 (38.7) | |
| Others | 10 | 2 (2.7) | 8 (10.7) | |
| TNM stage | 0.97 | |||
| II–III | 117 | 58 (79.5) | 59 (78.7) | |
| IV | 31 | 15 (20.5) | 16 (21.3) | |
| Lines of immunotherapy | 0.14 | |||
| First-line | 69 | 29 (39.7) | 40 (53.3) | |
| Second or later | 79 | 44 (60.3) | 35 (46.7) | |
| Cycles of immunotherapy | 5 [3.00, 10.00] | 6 [3.00, 10.00] | 0.86 | |
| Combination therapy | 0.22 | |||
| Chemotherapy | 84 | 42 (57.5) | 42 (56.0) | |
| C + T | 23 | 13 (17.8) | 10 (13.3) | |
| C + R | 29 | 10 (13.7) | 19 (25.3) | |
| C + T + R | 12 | 8 (11.0) | 4 (5.3) | |
| BMI (kg/m2) | <0.001 | |||
| Underweight (<18.5) | 14 | 3 (4.1) | 11 (14.7) | |
| Normal (18.5 to <24.0) | 95 | 36 (49.3) | 59 (78.7) | |
| Overweight (24.0 to <28.0) | 35 | 31 (42.5) | 4 (5.3) | |
| Obese (≥28.0) | 4 | 3 (4.1) | 1 (1.3) | |
| CYFRA21 (ng/mL) | 3.90 [2.39, 6.87] | 5.57 [3.17, 13.04] | 0.02 | |
| CEA (ng/mL) | 4.0 [2.60, 8.25] | 4.10 [2.20, 8.20] | 0.72 | |
| NLR | 3.30 [2.36, 5.09] | 3.91 [2.88., 5.93] | 0.02 | |
| PLR | 138.95 [109.45, 197.62] | 202.49 [131.09, 255.41] | <0.001 | |
| PNI | <0.001 | |||
| ≤51.996 | 91 | 23 (31.5) | 68 (90.7) | |
| >51.996 | 57 | 50 (68.5) | 7 (9.3) | |
| Best overall response | 0.053 | |||
| CR | 10 | 7 | 3 | |
| PR | 31 | 20 | 11 | |
| SD | 33 | 17 | 16 | |
| PD | 74 | 29 | 45 | |
| ORR (95% CI) | 37.0 (25.6–48.3) | 18.7 (9.6–27.6) | 0.01 | |
| PFS (days) | 588 [228.00, 848.50] | 225.00 [103.00, 771.00] | 0.02 | |
| OS (days) | 952.00 [671.00, 1,536.00] | 813.00 [500.00, 1,155.00] | 0.01 |
Data are presented as number (percentage) or median [IQR], unless otherwise indicated. GNRI, Geriatric Nutritional Risk Index; AC, adenocarcinoma; SCC, squamous cell carcinoma; TNM, tumor node metastasis; C, chemotherapy; R, radiotherapy; T, target therapy; BMI, body mass index; CEA, carcinoembryonic antigen; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutrition index; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; PFS, progression-free survival; ORR, overall response rate; CI, confidence interval; IQR, interquartile range; OS, overall survival.
Above all, both patient groups exhibited similar characteristics in terms of age, gender, smoking history, surgical history, histology, TNM stage and treatment therapy and CEA. Nevertheless, the high GNRI group displayed superior survival rate and decreased mortality rate (57.5% vs. 42.5%, 32.0% vs. 68.0%, P=0.003) compared to the low GNRI group. The overall response rate (ORR) was 37.0% (95% CI: 25.6–48.3%) in the high GNRI group and 18.7% (95% CI: 9.6–27.6%) in the low GNRI group (P=0.01). Additionally, the GNRI showed significant correlations with various clinicopathological variables: BMI (P<0.001), CYFRA21 (P=0.02), NLR (P=0.02), PLR (P<0.001), PNI (P<0.001) (Table 1).
Determining the optimal cutoff value
We utilized SPSS 25.0 to plot the ROC curves to ascertain the optimal cutoff value for GNRI, PLR, NLR, and PNI. The optimal cutoff value for GNRI was 108.15 (AUC =0.575, 95% CI: 0.481–0.669, P=0.048) (Figure 2). The ROC curve further demonstrated that the optimal threshold value for NLR is 4.78 (AUC =0.579, 95% CI: 0.486–0.672, P=0.047) (Figure S1) and for PLR is 174.66 (AUC =0.579, 95% CI: 0.486–0.673, P=0.048) (Figure S2). Lastly, The ROC curve also illustrates that the optimal cutoff value for PNI is 52.00 (AUC =0.553, 95% CI: 0.457–0.648, P=0.049) (Figure S3).

PFS and OS among patients with high and low GNRI
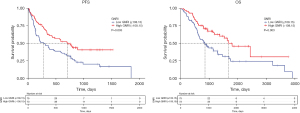
The median PFS for the high GNRI group was 588.0 days (IQR, 228.0–848.5 days, P=0.02) and the low GNRI group was 225 (IQR, 103–771 days, P=0.02). The median overall survival time for the high GNRI group was 952 days (IQR, 671–1,536, P=0.01) and the low GNRI group was 813.00 days (IQR, 500–1,155, P=0.01), demonstrating that the median PFS and median OS of high GNRI cluster were substantially exceeded that of low GNRI cluster. The Kaplan–Meier analysis also displayed those individuals with high GNRI exhibited longer PFS (P=0.005) and superior overall survival rates in contrast to those with low GNRI (P=0.003) (Figure 3). Six-month PFS rate and one-year PFS rate of the high GNRI group were 79.45% and 63.01%. Meanwhile, six-month PFS rate and one-year PFS rate of the low GNRI group were 61.33% and 45.33%. One-year OS rates of high and low GNRI groups were 93.15% and 82.67%, respectively. High and low GNRI groups’ two-year OS rates were 75.34% and 56.00%, respectively. Finally, yet importantly, the three-year OS rates within the high and low GNRI groups were 68.49% and 45.33%, respectively.
OS among patients with high and low GNRI stratified by the tumor stage
Subsequently, we assessed the prognostic influence of the GNRI based on the malignancy stage, aimed to further investigate whether GNRI has same impact on the prognosis of patients with distinct TNM stages. As the baseline table demonstrated, we grouped TNM II and TNM III stage patients because they were eligible for surgeries. Among the patients in stages II and III, the three-year OS of high and low GNRI groups were 67.7% and 51.9% respectively (P=0.02). The three-years OS of the patients with TNM stage IV were 56.3% in high GNRI cluster and 25.1% in low GNRI cluster respectively (P=0.05) (Figure 4).

OS among patients with high and low GNRI stratified by age
Cause GNRI is primarily applied in the elderly population (15), we also assessed the prognostic influence of the GNRI based on age. Elderly individuals are typically defined as those aged ≥65 years old (31). Elderly patients often have poorer nutritional status compared to younger people. Among the patients <65 years old, there was a tendency towards improved overall survival after three years for those in high GNRI cluster in contrast to low GNRI cluster, with rates of 66.67% and 48.15% respectively. While this difference showed no statistical significance (P=0.08). Conversely, among patients aged 65 and older, there was a more distinct difference in three-year overall survival based on GNRI (P=0.03). Those in high GNRI group had an outstanding greater three-year overall survival rate of 70.59%, compared to only 43.75% in the low GNRI group (Figure 5).

Univariate and multivariate analyses of PFS and OS
The results of both univariate and multivariate analyses of the factors that may be related to PFS are displayed in Table 2. Based on univariate Cox proportional hazard analyses, PFS was associated with GNRI, surgical history, TNM stage, treatment, and PLR. The multivariate analyses demonstrated that a positive prognosis PFS was independently associated with elevated GNRI (HR: 0.591, 95% CI: 0.382–0.914, P=0.02), as well as a history of surgery (HR: 0.602, 95% CI: 0.384–0.944, P=0.03) (Table 2). Table 3 shows the findings from univariate as well as multivariate analyses of variables that might be associated with OS. According to the univariate Cox proportional hazard calculations, longer OS was predicted by higher GNRI. Meanwhile, advantageous OS outcomes were also independently predicted by surgery history, TNM stage, CYFRA21, NLP, PLR and PNI.
Table 2
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (<65 vs. ≥65 years) | 0.799 (0.533–1.198) | 0.28 | |||
| Gender (male vs. female) | 0.765 (0.447–1.310) | 0.33 | |||
| Smoking (ever or current vs. never) | 0.6951 (0.428–1.130) | 0.14 | |||
| Surgery (yes vs. no) | 0.506 (0.332–0.771) | 0.002 | 0.602 (0.384–0.944) | 0.03 | |
| BMI (kg/m2) (vs. normal) | |||||
| Underweight | 1.056 (0.541–2.060) | 0.87 | |||
| Overweight | 0.880 (0.536–1.443) | 0.61 | |||
| Obesity | 1.239 (0.388–3.949) | 0.72 | |||
| Histology (vs. AC) | |||||
| SCC | 1.019 (0.677–1.536) | 0.93 | |||
| Others | 0.381 (0.119–1.221) | 0.10 | |||
| TNM stage (II–III vs. IV) | 0.583 (0.371–0.916) | 0.02 | 0.696 (0.435–1.116) | 0.13 | |
| Lines of immunotherapy (first vs. second or later) | 0.692 (0.463–1.034) | 0.07 | |||
| Treatment (vs. combined with chemotherapy) | |||||
| C + T | 1.111 (0.623–1.979) | 0.72 | 1.026 (0.575–1.830) | 0.93 | |
| C + R | 1.679 (1.008–2.797) | 0.046 | 1.659 (0.992–2.776) | 0.054 | |
| C + T + R | 2.143 (1.083–4.241) | 0.03 | 1.895 (0.938–3.827) | 0.08 | |
| CYFRA21 (>3.3 vs. ≤3.3 ng/mL) | 1.471 (0.929–2.328) | 0.10 | |||
| CEA (>5 vs. ≤5 ng/mL) | 1.358 (0.908–2.033) | 0.14 | |||
| NLR (>4.782 vs. ≤4.782) | 1.472 (0.978–2.216) | 0.06 | |||
| PLR (>174.661 vs. ≤174.661) | 1.579 (1.058–2.357) | 0.03 | 1.152 (0.746–1.777) | 0.52 | |
| PNI (>51.996 vs. ≤51.996) | 0.722 (0.473–1.103) | 0.13 | |||
| GNRI (>108.15 vs. ≤108.15) | 0.627 (0.415–0.947) | 0.03 | 0.591 (0.382–0.914) | 0.02 | |
PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; BMI, body mass index; AC, adenocarcinoma; SCC, squamous cell carcinoma; TNM, tumor node metastasis; C, chemotherapy; R, radiotherapy; T, target therapy; CEA, carcinoembryonic antigen; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutrition index; GNRI, Geriatric Nutritional Risk Index.
Table 3
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (<65 vs. ≥65 years) | 0.783 (0.503–1.220) | 0.28 | |||
| Gender (male vs. female) | 1.015 (0.549–1.878) | 0.96 | |||
| Smoking (ever or current vs. never) | 0.841 (0.492–1.438) | 0.53 | |||
| Surgery (yes vs. no) | 0.264 (0.161–0.433) | <0.001 | 0.305 (0.176-0.526) | <0.001 | |
| BMI (kg/m2) (vs. normal) | |||||
| Underweight | 1.130 (0.554–2.304) | 0.74 | 0.966 (0.455–2.054) | 0.93 | |
| Overweight | 0.866 (0.499–1.504) | 0.61 | 1.472 (0.747–2.901) | 0.26 | |
| Obesity | 2.904 (0.898–9.389) | 0.08 | 16.283 (4.510–58.792) | <0.001 | |
| Histology (vs. AC) | |||||
| SCC | 1.041 (0.661–1.637) | 0.86 | |||
| Others | 0.581 (0.180–1.870) | 0.36 | |||
| TNM stage (II–III vs. IV) | 0.506 (0.304–0.842) | 0.009 | 0.767 (0.435–1.351) | 0.37 | |
| Lines of immunotherapy (first vs. second or later) | 0.892 (0.572–1.392) | 0.62 | |||
| Treatment (vs. combined with chemotherapy) | |||||
| C + T | 1.262 (0.685–2.323) | 0.46 | |||
| C + R | 1.611 (0.921–2.818) | 0.10 | |||
| C + T + R | 1.857 (0.864–3.997) | 0.11 | |||
| CYFRA21 (>3.3 vs. ≤3.3 ng/mL) | 2.143 (1.266–3.628) | 0.005 | 1.570 (0.900–2.741) | 0.11 | |
| CEA (>5 vs. ≤5 ng/mL) | 1.445 (0.931–2.224) | 0.10 | |||
| NLR (>4.782 vs. ≤4.782) | 1.766 (1.132–2.756) | 0.01 | 0.869 (0.491–1.540) | 0.63 | |
| PLR (>174.661 vs. ≤174.661) | 2.101 (1.348–3.274) | 0.001 | 1.298 (0.758–2.223) | 0.34 | |
| PNI (>51.996 vs. ≤51.996) | 0.503 (0.312–0.813) | 0.005 | |||
| GNRI (>108.15 vs. ≤108.15) | 0.561 (0.354–0.888) | 0.004 | 0.536 (0.301–0.952) | 0.03 | |
OS, overall survival; HR, hazard ratio; CI, confidence interval; BMI, body mass index; AC, adenocarcinoma; SCC, squamous cell carcinoma; TNM, tumor node metastasis; C, chemotherapy; R, radiotherapy; T, target therapy; CEA, carcinoembryonic antigen; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutrition index; GNRI, Geriatric Nutritional Risk Index.
Consistent with the consequences of univariate analyses, multivariate Cox proportional hazard analyses revealed that elevated GNRI predicted longer OS for patients with NSCLC receiving ICIs (HR: 0.536, 95% CI: 0.301–0.952, P=0.03). Additionally, surgery history (HR: 0.305, 95% CI: 0.176–0.526, P<0.001) was a significant protective factor, while obesity (HR: 16.283, 95% CI: 4.510–58.792, P<0.001) was identified as a significant risk element for OS of NSCLC victims treated undergoing ICIs (Table 3).
Discussion
It is widely recognized that malnutrition raises the probability of progression in various cancer types and negatively impacts long-term survival (22,32-34). In the past, researchers utilized assessment tools such as skeletal muscle index (SMI), mini nutritional assessment (MNA), and bioelectric impedance analysis (BIA) for nutritional assessment (35). These indicators tended to be intricate or needed a substantial amount of time for statistical computation. Given the poor prognosis and substantial burden of NSCLC, there is an urgent requirement to identify and verify patient-specific prognostic factors for these patients. GNRI is figured out by serum albumin concentration, weight and height which are all hematologic parameters easily accessible during the treatment process. The GNRI has a broad potential for clinical applications due to its convenient data collection and straightforward calculation principles (36).
Since GNRI is calculated by albumin levels, weight, and height, it is an index that can reflect nutritional status. A tumor, especially when highly malignant, represents a chronic wasting disease and may lead to hypoalbuminemia, malnutrition and cachexia which can contribute to frailty, muscle weakness, reduced physical function and impaired immune function (37-39). Albumin is a plasma protein that is produced by the liver and is commonly used as a biomarker for various health conditions such as malnutrition, inflammation, and liver dysfunction. It is an important indicator of the overall health as well as offering important details regarding a patient’s nutritional state and liver function (40). Previous studies have indicated that hematological and biochemical parameters, including albumin levels, can predict the prognosis of NSCLC patients (41,42). The mechanisms that underlie the correlation between decreased serum albumin concentration and unfavorable outcome within carcinoma patients are probably multifactorial. One potential mechanism is the complex interplay between the tumor microenvironment (TME) and malnutrition, which causes poor infiltration of anti-tumoral immune cells in NSCLC patients (43). Additionally, serum albumin is the most abundant plasma protein and plays a crucial character as a circulating carrier. Chemotherapy drugs can be transported by binding them with albumin. Hence, patients with hypoalbuminemia are more likely to respond poorly to chemotherapy and experience more severe chemotherapy-induced toxicity symptoms (44,45). A previous study has also demonstrated that decreased albumin levels correlate with increased ICI clearance. The ascent of ICI clearance, which reflects the progression of tumor cachexia, may partially illustrate the distinctive association between decreased albumin levels and poor outcomes in ICI monotherapy (46). Therefore, serum albumin is an important indicator determining the predictive power of GNRI. Body weight and body height can be employed to calculate BMI, which has been demonstrated to relate to lower stage-specific survival rates of lung cancer patients. Studies have demonstrated a link between being morbidly obese or underweight at diagnosis and poorer outcomes for individuals with lung cancer (47-49). GNRI, formed by the integration of these three crucial factors, could be considered as one of the most valuable immunonutritional indicators.
Ever since the conception of GNRI was proposed, many previous researches have investigated the impact on patients with malignant tumors and their prognosis. GNRI ≤98 is an independent predictor of progressive renal insufficiency, 30-day readmission, septic shock, superficial incisional surgical site infection, and urinary tract infection in the setting of nephrectomy for renal cancer (17). Güç et al. have proved that GNRI has excellent prognostic ability in metastatic colorectal cancer patients with sarcopenia (16). Migita et al. studied the role of GNRI in esophageal cancer prognosis. They concluded that GNRI was a straightforward and dependable predictor of the postoperative survival in esophageal carcinoma patients and a low preoperative GNRI (<98) indicated an elevated risk of esophageal cancer death (19). In addition, Doi et al. retrospectively delved into the GNRI’s predictive significance in colorectal cancer and found that the patients with colorectal cancer who had a lower GNRI experienced a considerably inferior overall survival rate compared to others with higher GNRI (P=0.001) (50).
Our research focused on analyzing the characteristics and outcomes of NSCLC individuals undergoing ICI treatment in order to assess the potential correlation between GNRI and extended survival. The reason for selecting this target population is due to the rapid development of ICI, a revolutionary form of immunotherapy, has transformed the way numerous cancers are managed, especially in NSCLC (51). A nutritional assessment before ICI treatment is necessary to reduce the potential for poor outcomes in patients (52). We demonstrated that the GNRI exhibits discriminative ability for predicting long-term survival in NSCLC patients receiving ICI therapy. From our results, lower GNRI was substantially linked to a shorter survival duration in NSCLC patients treated with ICI therapy and GNRI was an independent prognostic predictor for PFS and OS. Our findings were consistent with the application of GNRI in other tumors.
Compared to the group with low GNRI, the high GNRI group had significantly longer median PFS (588 vs. 225 days, P=0.02) and higher ORR (37% vs. 18.7%, P=0.01) after receiving ICI treatment. Furthermore, our multivariate analysis suggested that the high GNRI group had a 40.90% and 46.4% lower probability of suffering from short PFS (HR: 0.591, 95% CI: 0.382–0.914, P=0.02) and OS (HR: 0.536, 95% CI: 0.301–0.952, P=0.03). These findings demonstrated the prognostic value of GNRI in NSCLC patients accepting immunotherapy and were agreed with earlier literature (22,26). Moreover, Surgery history was another independent prognostic factor of both PFS (HR: 0.602, 95% CI: 0.384–0.944, P=0.03) and OS (HR: 0.305, 95% CI: 0.176–0.526, P<0.001) for NSCLC sufferers receiving ICI therapy, which was consistent with clinical practice. Surgery can significantly alleviate the tumor burden in patients so that extended survival period can be attained (53,54). Obesity was another independent prognostic factor of OS in our investigation (HR: 16.283, 95% CI: 4.510–58.792, P<0.001). Obesity is an important malignancy risk factor (55). Ringel et al. performed a study revealed that tumor metabolism may considerably differ in a lean versus an obese context. Obesity might influence the function of CD8+ T cells, which leads to changed nutrition availability in the TME and immunological dysfunction (56). Another credible research conducted by Iyengar also concluded that the tumor-promoting effects of obesity occur at both the local level, through adipose inflammation and related alterations in the microenvironment, and systemically, via circulating metabolic and inflammatory mediators resulting from adipose inflammation (57). To the best of our knowledge, no study has yet explored the upper limit of GNRI. Considering the fact that GNRI is correlated to patient weight and overweight is an independent risk factor for tumor prognosis, exploring the upper cutoff value of GNRI in future studies holds significant importance.
We then stratified the study population based on age and TNM stage. The results revealed that GNRI had an enhanced predictive value when forecasting the risk of mortality and long-term outcomes in TNM II–III NSCLC patients treated with ICI therapy (P=0.02, P=0.05). This phenomenon also existed in the elderly population (P=0.03, 0.08). Interestingly, when only considering age, the three-year survival rate for the older group was 54.88%, while for the non-older group, it was 59.09%. The overall survival was worse in the elderly population, though there was no significance between them (P=0.28). Nevertheless, after grouping according to GNRI, it revealed a higher three-year survival rate among elderly patients with high GNRI (70.59% vs. 66.7%), showcasing the efficacy of GNRI in forecasting the prognosis of immunotherapy in this demographic.
Through multivariate Cox regression analysis, we also included PLR and NLR, which are index of inflammation. While both of them are factors influencing prognosis in the univariate Cox regression analysis, our research findings did not identify a correlation between NLR, PLR and patient prognosis in the multivariate regression analysis (NLR: P=0.63; PLR: P=0.34). However, earlier investigations have suggested that elevated NLR corresponds with an unfavorable prognosis among advanced NSCLC (58). The reasons for the different outcomes may be attributed to the inclusion of predominantly early-stage patients. Certainly, malnutritional status may be associated with a descent in immune response, which could be one of the explanations poor nutrition adversely affects survival (59,60).
One notable observation is that previous studies have typically set the cutoff value for GNRI at 98, consistent with the initial concept proposed by Bouillanne and colleagues. We did not adopt this classification method on account of characteristics of our study population. Only 16.2% (n=24) patients in our study scored below 98. So, we applied the ROC curve to establish the cutoff value. We have repeatedly confirmed the accuracy of the data. The potential for an elevated GNRI may be attributed to population differences. However, in a study conducted in 2022, Güç et al. utilized the ROC curve for GNRI displaying an optimum cutoff value of 107.28 (AUC =0.805, P<0.001) (16). Utilizing the similar method, Ide S’s study determined the cutoff value as 104.26 (61). Additionally, according to the median GNRI value, Tang et al. categorized all patients to the high GNRI group with GNRI greater than 107.7 and the low GNRI group with GNRI less than 107.7 (62). The numerical differences in GNRI values may be attributed to variations in the study populations. On the one hand, 44.59% patients (n=66) in our study were younger than 65 years old. From an experiential standpoint, this portion of patients had better nutritional conditions than elder (55.41%, n=82). The median GNRI for patients <65 years old was 109.16, while the median GNRI for elder was 106.98. On the other hand, the difference in cutoff value may also be due to variations in albumin measurements between different hospitals. Despite our study is consistent with most of the conclusions about GNRI and malignancy, a previous research found that while univariate analysis revealed a slight variation in OS, the results of multivariate analysis showed that OS could not be independently predicted by the GNRI score (63). The reason for the differences may be attributed to variations in the study populations, as the individuals in this study were younger (mean age, 55 years). One limitation of our research was its retrospective nature, which could have potentially skewed results because of the study’s design. The AUC and the sensitivity of the GNRI in our research were somewhat inadequate. This might be attributed to the confounders generated during the collection of retrospective data. GNRI’s reliance solely on objective parameters such as height, weight, and serum albumin could partially contribute to this issue. Secondly, our sample size was inadequate as we excluded a substantial number of patients. This included those who were treated without ICI therapy, as well as patients lost to follow-up having incomplete data. These exclusions may have impacted the generalizability of our findings and introduced potential confounding variables. We need to increase the proportion of female patients and it is essential to conduct randomized controlled trials and larger cohort studies, involving nutritional intervention, to accurately validate these findings. Additionally, considering the limited sample size of this investigation, we did not apply propensity score matching (PSM) or inverse probability of treatment weighting (IPTW) approaches, which might enhance the bias induced by potential confounding factors. Thirdly, the patients who received diverse preoperative and postoperative treatment plans might introduce interference in assessing the effectiveness of immunotherapy. Lastly, we did not record and assess adverse events related to the treatment because of the incomplete follow-up data. These limitations hinder the drawing of definitive conclusions. Comprehensive research is required to validate our results.
Conclusions
To conclude, our investigation highlights that a higher GNRI at diagnosis in NSCLC patients receiving ICI therapy is significantly associated with longer PFS and OS. GNRI is an effective independent prognostic factor for these patients, especially in elder patients with TNM II–III. According to our findings, we recommend using this index, as it is straightforward and cost-effective metrics that can be computed using the parameters routinely employed in clinical practice. Moreover, a predictive model for survival rates of NSCLC patients receiving ICI therapy can be established based on GNRI and GNRI can also provide a basis for nutritional support before treatment. However, it is essential to establish a more scientific and accurate grading scale, so further prospective studies with greater range of patients are essential to ascertain the cutoff value of GNRI.
Acknowledgments
We sincerely appreciate the patients for their active participation in this study and for their support.
Funding: This research was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-436/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-436/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-436/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-436/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (revised in 2013) and was approved by the Ethics Committee of Huadong Hospital affiliated to Fudan University (No. 2021K010). Informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019;5:1749-68. [Crossref] [PubMed]
- Chen R, Manochakian R, James L, et al. Emerging therapeutic agents for advanced non-small cell lung cancer. J Hematol Oncol 2020;13:58. [Crossref] [PubMed]
- Lahiri A, Maji A, Potdar PD, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer 2023;22:40. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med 2017;377:1824-35. [Crossref] [PubMed]
- Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol 2021;16:223-49. [Crossref] [PubMed]
- Reck M, Remon J, Hellmann MD. First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:586-97. [Crossref] [PubMed]
- Liu K, Zhu Y, Zhu H. Immunotherapy or targeted therapy as the first-line strategies for unresectable hepatocellular carcinoma: A network meta-analysis and cost-effectiveness analysis. Front Immunol 2023;13:1103055.
- Zeidan AM, Boss I, Beach CL, et al. A randomized phase 2 trial of azacitidine with or without durvalumab as first-line therapy for older patients with AML. Blood Adv 2022;6:2219-29. [Crossref] [PubMed]
- Vigano A, Donaldson N, Higginson IJ, et al. Quality of life and survival prediction in terminal cancer patients: a multicenter study. Cancer 2004;101:1090-8. [Crossref] [PubMed]
- Viganò A, Dorgan M, Buckingham J, et al. Survival prediction in terminal cancer patients: a systematic review of the medical literature. Palliat Med 2000;14:363-74. [Crossref] [PubMed]
- Chen YM, Lai CH, Lin CY, et al. Body Mass Index, Weight Loss, and Mortality Risk in Advanced-Stage Non-Small Cell Lung Cancer Patients: A Focus on EGFR Mutation. Nutrients 2021;13:3761. [Crossref] [PubMed]
- Bouillanne O, Morineau G, Dupont C, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr 2005;82:777-83. [Crossref] [PubMed]
- Güç ZG, Altay C, Özgül HA, et al. GNRI And Conut Scores: Simple Predictors of Sarcopenia in Metastatic Colorectal Cancer Patients. Support Care Cancer 2022;30:7845-52. [Crossref] [PubMed]
- Riveros C, Chalfant V, Bazargani S, et al. The geriatric nutritional risk index predicts complications after nephrectomy for renal cancer. Int Braz J Urol 2023;49:97-109. [Crossref] [PubMed]
- Hu SP, Chen L, Lin CY, et al. The Prognostic Value of Preoperative Geriatric Nutritional Risk Index in Patients with Pancreatic Ductal Adenocarcinoma. Cancer Manag Res 2020;12:385-95. [Crossref] [PubMed]
- Migita K, Matsumoto S, Wakatsuki K, et al. The Prognostic Significance of the Geriatric Nutritional Risk Index in Patients with Esophageal Squamous Cell Carcinoma. Nutr Cancer 2018;70:1237-45. [Crossref] [PubMed]
- Yiu CY, Liu CC, Wu JY, et al. Efficacy of the Geriatric Nutritional Risk Index for Predicting Overall Survival in Patients with Head and Neck Cancer: A Meta-Analysis. Nutrients 2023;15:4348. [Crossref] [PubMed]
- Hirahara N, Matsubara T, Fujii Y, et al. Preoperative geriatric nutritional risk index is a useful prognostic indicator in elderly patients with gastric cancer. Oncotarget 2020;11:2345-56. [Crossref] [PubMed]
- Peng SM, Yu N, Ren JJ, et al. The Geriatric Nutritional Risk Index as a Prognostic Factor in Patients with Advanced Non-Small-Cell Lung Cancer. Nutr Cancer 2021;73:2832-41. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Chen K, Shen Z, Gu W, et al. Prevalence of obesity and associated complications in China: A cross-sectional, real-world study in 15.8 million adults. Diabetes Obes Metab 2023;25:3390-9. [Crossref] [PubMed]
- Sonehara K, Tateishi K, Araki T, et al. Prognostic value of the geriatric nutritional risk index among patients with previously treated advanced non-small cell lung cancer who subsequently underwent immunotherapy. Thorac Cancer 2021;12:1366-72. [Crossref] [PubMed]
- Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176-81. [Crossref] [PubMed]
- Mandaliya H, Jones M, Oldmeadow C, et al. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res 2019;8:886-94. [Crossref] [PubMed]
- Peng L, Wang Y, Liu F, et al. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother 2020;69:1813-22. [Crossref] [PubMed]
- Zhang L, Ma W, Qiu Z, et al. Prognostic nutritional index as a prognostic biomarker for gastrointestinal cancer patients treated with immune checkpoint inhibitors. Front Immunol 2023;14:1219929. [Crossref] [PubMed]
- Sieber CC. The elderly patient--who is that? Internist (Berl) 2007;48:1190-4. [Crossref] [PubMed]
- Kang HW, Seo SP, Kim WT, et al. A Low Geriatric Nutritional Risk Index is Associated with Aggressive Pathologic Characteristics and Poor Survival after Nephrectomy in Clear Renal Cell Carcinoma: A Multicenter Retrospective Study. Nutr Cancer 2020;72:88-97. [Crossref] [PubMed]
- Fujiya K, Kawamura T, Omae K, et al. Impact of Malnutrition After Gastrectomy for Gastric Cancer on Long-Term Survival. Ann Surg Oncol 2018;25:974-83. [Crossref] [PubMed]
- Imaoka Y, Ohira M, Kobayashi T, et al. Impact of Geriatric Nutritional Risk Index After Initial Hepatectomy for Hepatocellular Carcinoma: a Retrospective Cohort Study with the Hiroshima Surgical Study Group of Clinical Oncology (HiSCO). J Gastrointest Surg 2023;27:1152-8. [Crossref] [PubMed]
- Aprile G, Basile D, Giaretta R, et al. The Clinical Value of Nutritional Care before and during Active Cancer Treatment. Nutrients 2021;13:1196. [Crossref] [PubMed]
- Hirahara N, Tajima Y, Fujii Y, et al. Prediction of postoperative complications and survival after laparoscopic gastrectomy using preoperative Geriatric Nutritional Risk Index in elderly gastric cancer patients. Surg Endosc 2021;35:1202-9. [Crossref] [PubMed]
- Nishikawa H, Goto M, Fukunishi S, et al. Cancer Cachexia: Its Mechanism and Clinical Significance. Int J Mol Sci 2021;22:8491. [Crossref] [PubMed]
- Byrnes A, Banks M, Mudge A, et al. Enhanced Recovery After Surgery as an auditing framework for identifying improvements to perioperative nutrition care of older surgical patients. Eur J Clin Nutr 2018;72:913-6. [Crossref] [PubMed]
- Potter J, Klipstein K, Reilly JJ, Roberts M. The nutritional status and clinical course of acute admissions to a geriatric unit. Age Ageing 1995;24:131-6. [Crossref] [PubMed]
- Zhang X, Xing P, Hao X, et al. Clinical value of serum albumin level in patients with non-small cell lung cancer and anaplastic lymphoma kinase (ALK) rearrangement. Ann Palliat Med 2021;10:12403-11. [Crossref] [PubMed]
- Zhao B, Guo H, Wu W, et al. Hemoglobin, albumin, lymphocyte and platelet (HALP) score can predict the prognosis of patients with non-small cell lung cancer (NSCLC). Asian J Surg 2023;46:4891-2. [Crossref] [PubMed]
- Matsubara T, Okamoto T. ASO Author Reflections: The C-Reactive Protein (CRP)-Albumin Ratio May Be Useful as the Most Prognostic Index Among the Immuno-nutritional Parameters Using CRP and Albumin for Resected NSCLC. Ann Surg Oncol 2021;28:3055-6. [Crossref] [PubMed]
- Alifano M, Mansuet-Lupo A, Lococo F, et al. Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PLoS One 2014;9:e106914. [Crossref] [PubMed]
- Espinosa E, Feliu J, Zamora P, et al. Serum albumin and other prognostic factors related to response and survival in patients with advanced non-small cell lung cancer. Lung Cancer 1995;12:67-76. [Crossref] [PubMed]
- Wang X, Han H, Duan Q, et al. Changes of serum albumin level and systemic inflammatory response in inoperable non-small cell lung cancer patients after chemotherapy. J Cancer Res Ther 2014;10:1019-23. [Crossref] [PubMed]
- Guo Y, Wei L, Patel SH, et al. Serum Albumin: Early Prognostic Marker of Benefit for Immune Checkpoint Inhibitor Monotherapy But Not Chemoimmunotherapy. Clin Lung Cancer 2022;23:345-55. [Crossref] [PubMed]
- Shepshelovich D, Xu W, Lu L, et al. Body Mass Index (BMI), BMI Change, and Overall Survival in Patients With SCLC and NSCLC: A Pooled Analysis of the International Lung Cancer Consortium. J Thorac Oncol 2019;14:1594-607. [Crossref] [PubMed]
- Yuan Q, Du M, Loehrer E, et al. Postdiagnosis BMI Change Is Associated with Non-Small Cell Lung Cancer Survival. Cancer Epidemiol Biomarkers Prev 2022;31:262-8. [Crossref] [PubMed]
- Cortellini A, Ricciuti B, Tiseo M, et al. Baseline BMI and BMI variation during first line pembrolizumab in NSCLC patients with a PD-L1 expression ≥ 50%: a multicenter study with external validation. J Immunother Cancer 2020;8:e001403. [Crossref] [PubMed]
- Doi S, Migita K, Ueno M, et al. The Prognostic Significance of the Geriatric Nutritional Risk Index in Colorectal Cancer Patients. Nutr Cancer 2022;74:2838-45. [Crossref] [PubMed]
- Garon EB, Hellmann MD, Rizvi NA, et al. Five-Year Overall Survival for Patients With Advanced Non-Small-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J Clin Oncol 2019;37:2518-27. [Crossref] [PubMed]
- Güç ZG, Altay C, Özgül HA, Ellidokuz H, et al. GNRI And Conut Scores: Simple Predictors of Sarcopenia in Metastatic Colorectal Cancer Patients. Support Care Cancer 2022;30:7845-52. [Crossref] [PubMed]
- Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 2016;5:288-300. [Crossref] [PubMed]
- Guo Q, Hu S, Ye J, et al. Surgery offers survival advantage over radiotherapy in patients who are 80 years and older with Stage I and II NSCLC: A retrospective cohort study of 7,045 patients. Front Surg 2022;9:1018320. [Crossref] [PubMed]
- Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med 2016;375:794-8. [Crossref] [PubMed]
- Ringel AE, Drijvers JM, Baker GJ, et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell 2020;183:1848-1866.e26. [Crossref] [PubMed]
- Iyengar NM, Gucalp A, Dannenberg AJ, et al. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J Clin Oncol 2016;34:4270-6. [Crossref] [PubMed]
- Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017;106:1-7. [Crossref] [PubMed]
- Hara K, Shiozawa T, Kohno S, et al. Kansenshogaku Zasshi 1998;72:569-74. [Crossref] [PubMed]
- Rivadeneira DE, Grobmyer SR, Naama HA, et al. Malnutrition-induced macrophage apoptosis. Surgery 2001;129:617-25. [Crossref] [PubMed]
- Ide S, Okugawa Y, Omura Y, et al. Geriatric nutritional risk index predicts cancer prognosis in patients with local advanced rectal cancer undergoing chemoradiotherapy followed by curative surgery. World J Surg Oncol 2021;19:34. [Crossref] [PubMed]
- Tang QN, Qiu HZ, Sun XQ, et al. Geriatric nutritional risk index as an independent prognostic factor in locally advanced nasopharyngeal carcinoma treated using radical concurrent chemoradiotherapy: a retrospective cohort study. Ann Transl Med 2021;9:532. [Crossref] [PubMed]
- Li Z, Guo Q, Wei J, et al. Geriatric nutritional risk index is not an independent predictor in patients with diffuse large B-cell lymphoma. Cancer Biomark 2018;21:813-20. [Crossref] [PubMed]



