
Contribution of fluorescence imaging to thoracoscopic anatomical segmentectomy: a multicenter propensity matching analysis
Highlight box
Key findings
• Single-port thoracoscopic anatomical segmentectomy with fluorescence visualization of intersegmental planes (ISPs) demonstrated safety and effectiveness comparable to the modified inflation-deflation method. In complex segmentectomies, specifically, fluorescence imaging conferred improved perioperative outcomes.
What is known and what is new?
• Fluorescence imaging is generally considered capable of shortening operative time and improving efficiency, but empirical data attesting to these benefits have been lacking.
• Compared to the traditional modified inflation-deflation method, fluorescence imaging shortened segmental resection time, reduced intraoperative blood loss and improved postoperative pain, albeit only for complex cases.
What is the implication, and what should change now?
• Fluorescence imaging is a viable option for identifying ISPs in single-port thoracoscopic anatomical segmentectomy and could be considered the method of choice for complex segmentectomies.
Introduction
Lung cancer is currently the most common cancer worldwide, Traditionally, lobectomy with systemic lymph node dissection or sampling has been the standard surgical treatment. Segmentectomy was considered only a “compromised” option for patients with poor cardiopulmonary reserve who could not tolerate a lobectomy. However, many groups have demonstrated that an intentional segmentectomy appears to be an equally effective alternative to lobectomy for a select subset of patients, while offering improved preservation of lung function (1-5). Three randomized controlled trials (CALGB140503/Alliance, JCOG0802, and DRKS00004897) (6-8) have confirmed the non-inferiority of segmentectomy compared to lobectomy for patients with peripheral early-stage (cIA <2.0 cm) non-small cell lung cancer (NSCLC).
For thoracic surgeons performing anatomical segmentectomies, accurately identifying the intersegmental plane (ISP) may pose significant challenges. Various intraoperative methods (9) are also available to delineate ISP including jet ventilation techniques, endobronchial methylene blue injection, modified inflation-deflation, and systemic injection of indocyanine green (ICG), three-dimensional (3D) simulation using multidetector computed tomography (CT) and virtual-assisted lung mapping. Multiple studies have investigated the application of systemic ICG injection for identifying ISP (10-12). However, data comparing the safety and effectiveness of this method with other techniques have been limited, In the following discussion, we will compare the results with the few similar studies (13,14) published to date.
To address this knowledge gap, we compared the safety and effectiveness of ICG fluorescence imaging with the modified inflation-deflation when performing single-port thoracoscopic anatomical segmentectomy. Surgical procedures were categorized operations into simple and complex segmentectomies based on the complexity of the lung segment resection site (15,16) and propensity score matching (PSM) was used to balance baseline characteristics between patients in the latter group. We subsequently analyzed and compared the perioperative and long-term outcomes between the groups. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-986/rc).
Methods
Patient population
This multicenter retrospective study included 402 patients [group a: 345 from Anhui Chest Hospital; group b: 29 from the First Affiliated Hospital of Wannan Medical College (Yijishan Hospital of Wannan Medical College); group c: 28 from the First Affiliated Hospital of Bengbu Medical University], who underwent single-port thoracoscopic anatomical segmentectomy between June 2020 and December 2022. Of these, 191 underwent the procedure using systemic ICG injection (fluorescence subgroup), while 211 patients underwent the modified inflation-deflation method (modified inflation-deflation subgroup). All three participating hospitals are regional medical centers with highly skilled thoracic surgeons, each performing more than 1,200 thoracic surgeries per year. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the leading institution Anhui Chest Hospital, Anhui, China (No. KJ2024-039) and informed consent was taken from all the patients. All participating hospitals/institutes were informed and agreed with the study.
Inclusion criteria
All consecutive patients who underwent thoracoscopic segmentectomy were included in the study. For malignant pulmonary nodules, segmentectomy was indicated based on the following criteria, as outlined by the National Comprehensive Cancer Network (NCCN) guidelines (17) and Asia expert consensus (16): (I) for stage 1A NSCLC with a favorable combination of tumor diameter, consolidation-to-tumor (C:T) ratio, and maximum standardized uptake value (SUVmax); (II) poor lung function or inability to tolerate lobectomy due to significant comorbidities; (III) CT findings suggestive of peripheral, noninvasive lung lesions with a maximum diameter of 2.0 cm or less, with intraoperative frozen section confirming atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), or invasive adenocarcinoma (IA) with clear resection margins; (IV) negative findings from intraoperative lymph node dissection/sampling; (V) CT follow-up over 1 year showing high suspicion of malignancy with a ground glass-like component ≥50%; and (VI) imaging-confirmed nodule volume doubling time ≥400 days. Additionally, patients with benign but central and segmentally confined lung nodules, which were not amenable to wedge resection, were also considered candidates for segmentectomy.
Exclusion criteria
Patients were excluded if they met any of the following criteria: (I) history of malignancy or thoracic surgery, or postoperative pathology confirming metastatic carcinoma; (II) known allergy to iodine or other contrast agents; (III) preoperative suspicion of lymph node metastasis; (IV) frozen section unable to determine whether the nodule was stage IA NSCLC with a tumor diameter of 2.0 cm or less; (V) centrally located malignant nodules for which adequate resection margins could not be ensured; (VI) frozen section suggesting malignancy within 2.0 cm of the surgical margin or less than the maximum lesion diameter, requiring additional margin sampling; and (VII) incomplete clinical data records unsuitable for statistical analysis.
Classification criteria
The 402 patients were categorized into two groups based on the complexity of the segmentectomy (16): simple segmentectomy group (n=130; na=123, nb=4, nc=3) and the complex segmentectomy group (n=272; na=222, nb=25, nc=25). Simple segmentectomy involved resection of segments forming only one ISP: LS1–3, LS4–5, LS6, and RS6 segments (65 patients in the fluorescence subgroup and 65 patients in the modified inflation-deflation subgroup). Complex segmentectomy included all other lung segments, requiring resection across two or more ISPs, including combined segmentectomies and sub-segmentectomies (126 patients in the fluorescence subgroup and 146 patients in the modified inflation-deflation subgroup).
Variables and outcomes
Demographic and clinicopathologic characteristics of the patients [sex, age, body mass index (BMI), forced expiratory volume in 1 second % predicted (FEV1%), smoking history, comorbidities], as well as perioperative data (hook-wire localization, pleural adhesions, development of interlobar fissure, mediastinal lymph node dissection/sampling, segmentectomy site, and postoperative pathology) were collected and analyzed. It should be pointed out that we defined three degrees of pleural adhesions based on the relevant literature (18): A, no adhesions, existence of an interval space between the visceral and parietal pleura due to lung collapse; B, mild adhesions, adhesion area occupies 1–50%, blunt and sharp dissection required; C, severe adhesions, adhesion area occupies 51–100%, extrapleural dissection required because dissection between the visceral and parietal pleura is difficult. Similarly, development of interlobar fissure based on the Fissure Development Score (FDS) (19) and degree of completeness was defined as follows: grade 0, complete fissure; grade 1, fusion between 0 and 30%; grade 2, fusion between 30% and 70%; and grade 3, fusion more than 70%, and we divided the patients into three groups: A, well developed (grade 0); B, moderately developed (grade 1–2); C, undeveloped (grade 3). Outcomes included segmental resection time (i.e., time taken to perform dissection of the target segmental bronchovascular structures until the excision of the target segment), intraoperative estimated blood loss, chest tube drainage (volume and duration), visual analogue scale (VAS) pain scores assessed at 8:00 am for the first three postoperative days, complications, length of hospital stay, and total hospital costs.
Perioperative management
Preoperatively, patients were advised to cease smoking at least 4 weeks and received education regarding pre- and post-operative precautions, psychological counseling, and instructions on proper coughing and sputum clearance techniques to reduce the occurrence of postoperative pulmonary complications. Patients with underlying lung diseases (e.g., infection, chronic obstructive pulmonary disease) underwent appropriate treatment, including antibiotics and bronchodilators, before proceeding with surgery after symptom resolution.
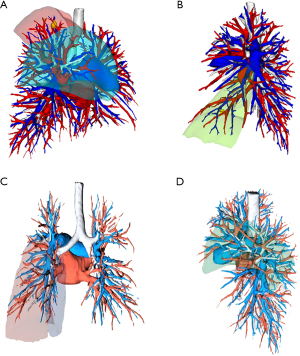
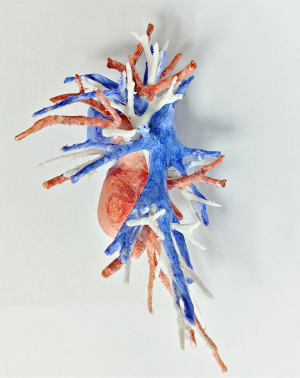
All patients underwent preoperative thin-section thoracic CT. For complex segmentectomies, chest imaging data were reconstructed using KINGSTAR 3D (Anhui King Star Digital S&T Co., Ltd., Hefei, China) or PVmed (Guangzhou Perception Vision Medical Technology Co., Ltd., Guangzhou, China) software to identify the target segmental vessels and bronchi (Figure 1). Moreover, 3D printed models of the tracheobronchial tree and pulmonary arteriovenous systems were created for select complex cases to aid in surgical planning and identification of segmental bronchovascular structures during surgery (Figure 2). For smaller, (size <1 cm), central (depth >1 cm), or predominantly ground-glass (C:T ratio <0.5) nodules preoperative CT-guided hook-wire localization was performed to precisely locate the pulmonary nodules for complete surgical resection.
Surgical procedures
All procedures were performed by a senior surgeon [a total of eight senior surgeons participated in this multicenter retrospective study, four senior surgeons from Anhui Chest Hospital, two senior surgeons from the First Affiliated Hospital of Wannan Medical College (Yijishan Hospital of Wannan Medical College), and two senior surgeons from the First Affiliated Hospital of Bengbu Medical University] with an annual thoracoscopic anatomical segmentectomy exceeding 200 cases. Patients underwent general anesthesia with one-lung ventilation. A single 3–4 cm incision was made in the fifth intercostal space anterior to the midaxillary line, with the surgeon standing ventrally. A 30° high-definition thoracoscope/fluoroscope (Karl Storz Endoskope, Tuttlingen, Germany) was inserted through this incision to visualize the thoracic cavity. Following dissection and division of the target segmental pulmonary vessels, and bronchi, either fluorescence imaging or the modified inflation-deflation technique was employed to define ISP (which technique to take depended on the surgeon’s habits or equipment conditions).
For the fluorescence method, 2 mL of diluted (5 mg/mL) Ridu ICG (25 mg/unit; Dandong Yichuang Pharmaceutical Co., Ltd., Dandong, China) was injected intravenously after the target segment arteriovenous and segmental bronchus were cut off. After 10–15 seconds, the lung tissue for preservation appeared green on the fluorescence mode of the thoracoscope, while the target segment for resection did not show green color (Figure 3A,3B).
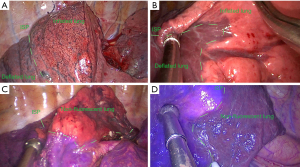
For the modified inflation-deflation technique, consistent with the operation steps of fluorescence method before injected ICG, then double-lung ventilation with 20 cmH2O airway pressure was initially used to achieve complete expansion of the operated lung. This was followed by transition to single-lung ventilation of the non-operated lung. After 15–20 min (it was generally necessary to wait for such a long time, but it was not ruled out that some patients have a slightly shorter or longer time to achieve resectable criterion), the target segment for resection appeared inflated and light pink in contrast to the deflated lung tissue for preservation (Figure 3C,3D).
In this study, we defined the criterion for inter-segmental visualizations as the display of ISP curve for more than 90% of the target lung segment using both techniques. Additionally, the target lung segment with a marginal bulge height >1 mm was required to meet through the modified inflation-deflation technique.
After ISP marking (surgeons decided whether to mark ISP if they choose the modified inflation-deflation method), energy devices or mechanical staplers were used to divide ISP. The specimen was removed in a bag and sent for rapid frozen section to assess the surgical resection margin. Subsequent lymph node dissection/sampling was performed if pathological examination revealed IA. If the lesion was reported as AIS or MIA, lymph node dissection/sampling was not undertaken. The operative field was inspected for bleeding, and the lung was assessed for air leaks following re-expansion. Air leaks from the lung surface were repaired with a polypropylene suture (PROLENE polypropylene monofilament non-absorbable suture with EVERPOINT cardiovascular needles, Ethicon, Johnson & Johnson, San Lorenzo, Puerto Rico, USA). A 24-French chest tube was then inserted through the incision and connected to a water-seal drainage system. Additionally, a microcatheter (8-French) was placed in the mid-axillary line of the seventh intercostal space and connected to a drainage bag to facilitate further postoperative drainage.
Postoperative management and follow-up
Routine postoperative care included administration antibiotics for a maximum duration of 72 hours. Pain control was achieved with a patient-controlled pump (including opioid analgesic, e.g., sufentanil; nonsteroidal analgesic, e.g., flurbiprofen axetil; antiemetic, e.g., tropisetron), which was supplemented by short-acting oral or intravenous analgesics for breakthrough pain as needed. Postoperative chest radiographs were monitored along with drainage output to guide chest tube removal. Patients without complications were discharged the day after chest tube removal.
Follow-up was conducted via telephone or outpatient clinic visits at 1 and 3 months postoperatively, then every 3 months up to 2 years, every 6 months from 2 to 5 years, and annually thereafter. Post-operative evaluation included blood laboratory investigations, tumor markers, chest radiography, thoracic CT, fiberoptic bronchoscopy, whole-body nuclear bone scan, brain magnetic resonance imaging, and positron emission tomography-CT, as indicated.
Statistical analysis
Normally distributed continuous variables were presented as mean ± standard deviation (SD), and groups were compared using Student’s t-test or Welch’s t-test based on the assumption of equal or unequal variances, respectively. Categorical variables were reported as frequencies and percentages, with between-group comparisons conducted using the Chi-squared test or Fisher’s exact test for unordered variables, and the Wilcoxon rank-sum test for ordered variables. Statistical significance was set at P<0.05.
In real-world studies, the results were susceptible to bias due to broad inclusion and exclusion criteria as well as numerous confounding factors. To address this issue, the PSM was employed in this study. PSM was primarily utilized for subgroup analysis of observational clinical research or clinical trial data. The fundamental principle involved replacing multiple covariates with a single score to balance their distribution between the treatment and control groups, thereby equalizing confounding factors in nonrandomized studies in a randomization-like manner. Its advantage lies in transforming confounding factor control into propensity values, achieving ‘dimensionality reduction’, and controlling for confounding bias. Simultaneously, it could resolve the ‘multi-dimensional’ problem posed by various non-research factors and potential independent variable collinearity. Furthermore, we also understood that PSM was only useful for recreating randomization and cannot simulate true randomized complete block designs, the latter of which being stronger at removing imbalance and bias (20).
In the simple segmentectomy group, baseline characteristics were comparable between the fluorescence and modified inflation-deflation methods (all P>0.05), and thus no further matching was required (Table 1). In the complex segmentectomy group, however, significant differences were observed in BMI (P=0.042) and smoking status (P=0.043). PSM (1:1) was therefore performed, successfully matching 110 pairs of patients with comparable profiles in the complex fluorescence and modified inflation-deflation subgroups (Table 2).
Table 1
| Characteristics | Fluorescence method (n=65) | Modified inflation-deflation method (n=65) | t/χ2 value | P value |
|---|---|---|---|---|
| Sex | 0.290‡ | 0.59 | ||
| Male | 24 (36.92) | 27 (41.54) | ||
| Female | 41 (63.08) | 38 (58.46) | ||
| Age (years) | 52.06±13.89 | 53.80±11.36 | 0.788† | 0.43 |
| BMI (kg/m2) | 23.12±3.00 | 23.11±2.72 | −0.044† | 0.97 |
| FEV1% | 79.27±6.87 | 80.08±7.52 | 0.638† | 0.53 |
| Smoking history | 5 (7.69) | 11 (16.92) | 2.566‡ | 0.11 |
| Underlying lung diseases | 4 (6.15) | 5 (7.69) | 0.000‡ | >0.99 |
| Hypertension | 19 (29.23) | 15 (23.08) | 0.637‡ | 0.43 |
| Diabetes | 8 (12.31) | 6 (9.23) | 0.320‡ | 0.57 |
| Cardiac disease | 2 (3.08) | 4 (6.15) | 0.175‡ | 0.68 |
| Hook-wire localization | 5 (7.69) | 11 (16.92) | 2.566‡ | 0.11 |
| Pleural adhesions | 3.545‡ | 0.23 | ||
| No adhesions | 50 (76.92) | 58 (89.23) | ||
| Mild adhesions | 10 (15.38) | 5 (7.69) | ||
| Severe adhesions | 5 (7.69) | 2 (3.08) | ||
| Development of interlobar fissure | 3.170‡ | 0.21 | ||
| Undeveloped | 6 (9.23) | 8 (12.31) | ||
| Moderately developed | 32 (49.23) | 22 (33.85) | ||
| Well developed | 27 (41.54) | 35 (53.85) | ||
| Mediastinal lymph node dissection/sampling | 33 (50.77) | 27 (41.54) | 1.114‡ | 0.29 |
| Site of segmentectomy | 5.822‡ | 0.13 | ||
| LS1+2+3 | 28 (43.08) | 19 (29.23) | ||
| LS4+5 | 8 (12.31) | 11 (16.92) | ||
| LS6 | 18 (27.69) | 14 (21.54) | ||
| RS6 | 11 (16.92) | 21 (32.31) |
Data are presented as n (%) or mean ± SD. †, Student’s t-test or Welch’s t-test; ‡, χ2 test value. ICG, indocyanine green; BMI, body mass index; FEV1%, forced expiratory volume in 1 second % predicted; SD, standard deviation.
Table 2
| Characteristics | Before PSM matching | After PSM matching | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fluorescence method (n=126) | Modified inflation-deflation method (n=146) | t/χ2 value | P value | Fluorescence method (n=110) | Modified inflation-deflation method (n=110) | t/χ2 value | P value | ||
| Sex | 2.607‡ | 0.11 | 0.077‡ | 0.78 | |||||
| Male | 57 (45.24) | 52 (35.62) | 41 (37.27) | 43 (39.09) | |||||
| Female | 69 (54.76) | 94 (64.38) | 69 (62.73) | 67 (60.91) | |||||
| Age (years) | 52.42±11.98 | 52.68±12.62 | 0.176† | 0.86 | 52.15±12.44 | 51.63±12.49 | 0.908† | 0.76 | |
| BMI (kg/m2) | 24.22±3.21 | 23.44±3.09 | −2.044† | 0.042 | 23.98±3.21 | 23.85±3.01 | −0.313† | 0.76 | |
| FEV1% | 79.29±8.56 | 80.95±7.57 | 1.694† | 0.09 | 79.96±8.53 | 79.61±6.87 | −0.328† | 0.74 | |
| Smoking history | 26 (20.63) | 17 (11.64) | 4.108‡ | 0.043 | 16 (14.55) | 16 (14.55) | 0.000‡ | >0.99 | |
| Underlying lung diseases | 11 (8.73) | 6 (4.11) | 2.464‡ | 0.12 | 5 (4.55) | 5 (4.55) | 0.000‡ | >0.99 | |
| Hypertension | 31 (24.60) | 31 (21.23) | 0.397‡ | 0.53 | 26 (23.64) | 24 (21.82) | 0.107‡ | 0.74 | |
| Diabetes | 12 (9.52) | 13 (8.90) | 0.031‡ | 0.86 | 9 (8.18) | 10 (9.09) | 0.058‡ | 0.81 | |
| Cardiac disease | 2 (1.59) | 4 (2.74) | 0.054‡ | 0.82 | 2 (1.82) | 2 (1.82) | 0.000‡ | >0.99 | |
| Hook-wire localization | 21 (16.67) | 31 (21.23) | 0.912‡ | 0.34 | 20 (18.18) | 23 (20.91) | 0.260‡ | 0.61 | |
| Pleural adhesions | 3.151‡ | 0.21 | 0.103§ | >0.99 | |||||
| No adhesions | 98 (77.78) | 103 (70.55) | 85 (77.27) | 84 (76.36) | |||||
| Mild adhesions | 23 (18.25) | 30 (20.55) | 21 (19.09) | 22 (20.00) | |||||
| Severe adhesions | 5 (3.97) | 13 (8.90) | 4 (3.64) | 4 (3.64) | |||||
| Development of interlobar fissure | 0.522‡ | 0.77 | 1.046‡ | 0.59 | |||||
| Undeveloped | 17 (13.49) | 21 (14.38) | 15 (13.64) | 11 (10.00) | |||||
| Moderately developed | 57 (45.24) | 71 (48.63) | 52 (47.27) | 50 (45.45) | |||||
| Well developed | 52 (41.27) | 54 (36.99) | 43 (39.09) | 49 (44.55) | |||||
| Mediastinal lymph node dissection/sampling | 59 (46.83) | 69 (47.26) | 0.005‡ | 0.94 | 54 (49.09) | 40 (36.36) | 3.641‡ | 0.056 | |
| Site of segmentectomy | 9.033§ | 0.32 | 7.740§ | 0.46 | |||||
| LS1+2 | 26 (20.63) | 36 (24.66) | 22 (20.00) | 29 (26.36) | |||||
| LS3 | 6 (4.76) | 14 (9.59) | 6 (5.45) | 10 (9.09) | |||||
| LS8 | 1 (0.79) | 2 (1.37) | 1 (0.91) | 1 (0.91) | |||||
| LS10 | 2 (1.59) | 2 (1.37) | 2 (1.82) | 2 (1.82) | |||||
| RS1 | 31 (24.60) | 30 (20.55) | 26 (23.64) | 25 (22.73) | |||||
| RS2 | 14 (11.11) | 13 (8.90) | 10 (9.09) | 13 (11.82) | |||||
| RS3 | 8 (6.35) | 18 (12.33) | 8 (7.27) | 10 (9.09) | |||||
| RS8 | 9 (7.14) | 5 (3.42) | 9 (8.18) | 3 (2.73) | |||||
| Subsegments/combined segments | 29 (23.02) | 26 (17.81) | 26 (23.64) | 17 (15.45) | |||||
Data are presented as n (%) or mean ± SD. †, Student’s t-test or Welch’s t-test; ‡, χ2 test value; §, Fisher’s exact test value. PSM, propensity score matching; ICG, indocyanine green; BMI, body mass index; FEV1%, forced expiratory volume in 1 second % predicted; SD, standard deviation.
All statistical analyses were performed using SPSS software version 22.0 (IBM Corp., Armonk, NY, USA).
Results
The clinical and surgical characteristics of the patients in the simple segmentectomy group are summarized in Table 1. Postoperative pathology was predominantly MIA (53.85% and 43.08% in the fluorescence and modified inflation-deflation subgroups, respectively), with the remaining cases included precancerous lesions, IA, and benign nodules [organizing pneumonia (n=8), benign tumors (n=4), granulomatous inflammation (n=2), bronchiectasis (n=2), novel cryptococcosis (n=1), Castleman disease (n=1), tuberculosis (n=1)]. In the simple segmentectomy group, there were no statistically significant differences in perioperative outcomes, including segmental resection time, between the fluorescence and modified inflation-deflation methods (all P>0.05) (Table 3).
Table 3
| Outcomes | Fluorescence method (n=65) | Modified inflation-deflation method (n=65) | t/χ2 value | P value |
|---|---|---|---|---|
| Segmental resection time (min) | 66.51±24.77 | 69.11±29.84 | 0.541† | 0.59 |
| Intraoperative blood loss (mL) | 17.46±9.53 | 19.46±26.71 | 0.569† | 0.57 |
| Maximum nodule diameter (cm) | 1.14±0.39 | 1.20±0.58 | 0.691† | 0.49 |
| Postoperative drainage (mL) | ||||
| Day 1 | 162.08±102.99 | 175.69±115.86 | 0.708† | 0.48 |
| Day 2 | 153.92±97.57 | 158.15±93.66 | 0.252† | 0.80 |
| Day 3 | 126.49±83.86 | 126.31±104.86 | −0.011† | 0.99 |
| Mean drainage at 3 days after surgery (mL) | 147.50±76.82 | 153.90±80.22 | 0.465† | 0.64 |
| Postoperative VAS pain score | ||||
| Day 1 | 2.97±0.35 | 3.03±0.39 | 0.939† | 0.35 |
| Day 2 | 2.68±0.53 | 2.66±0.59 | −0.155† | 0.98 |
| Day 3 | 2.37±0.55 | 2.18±0.56 | −1.909† | 0.059 |
| Postoperative chest tube duration (days) | 5.38±2.60 | 4.78±1.80 | −1.532† | 0.13 |
| Postoperative hospital stay (days) | 6.42±2.62 | 5.78±2.18 | −1.493† | 0.14 |
| Postoperative complications | 4.819§ | 0.31 | ||
| Pneumonia | 6 (9.23) | 2 (3.08) | 1.199‡ | 0.27 |
| Prolonged air leaks (>3 days) | 7 (10.77) | 8 (12.31) | 0.078‡ | 0.78 |
| Massive postoperative drainage (≥200 mL/day for 5 days) | 2 (3.08) | 7 (10.77) | 1.910‡ | 0.17 |
| Atelectasis | 2 (3.08) | 2 (3.08) | 0.00‡ | 1.00 |
| Lung lesion pathology | 1.938§ | 0.78 | ||
| AAH | 1 (1.54) | 2 (3.08) | ||
| AIS | 4 (6.15) | 5 (7.69) | ||
| MIA | 35 (53.85) | 28 (43.08) | ||
| IA | 17 (26.15) | 19 (29.23) | ||
| Benign | 8 (12.31) | 11 (16.92) | ||
| Total hospital cost (CNY) | 42,965.74±5,043.28 | 43,850.36±6,922.78 | 0.833† | 0.41 |
Data are presented as mean ± SD or n (%). †, Student’s t-test or Welch’s t-test; ‡, χ2 test value; §, Fisher’s exact test value. ICG, indocyanine green; VAS, visual analogue scale; AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; IA, invasive adenocarcinoma; CNY, Chinese Yuan; SD, standard deviation.
Table 2 details the characteristics of the patients in the complex segmentectomy group before and after PSM by surgical method (fluorescence vs. modified inflation-deflation). MIA comprised over half of the postoperative pathologies (58.18% and 61.82% in the fluorescence and modified inflation-deflation subgroups, respectively). The remaining cases included precancerous lesions (n=13 vs. 13), IA (n=27 vs. 22), and benign nodules [organizing pneumonia (n=8), benign tumors (n=3), tuberculosis (n=2), granulomatous inflammation (n=1), and Aspergillus infection (n=1)]. In the complex segmentectomy group, the fluorescence method demonstrated significant reductions in segmental resection time (69.37±28.22 vs. 78.80±34.66 min, P=0.03), intraoperative estimated blood loss (17.00±8.84 vs. 20.64±16.83 mL, P=0.046), and VAS pain scores on the first postoperative day (2.92±0.36 vs. 3.05±0.31, P=0.006) compared to the modified inflation-deflation technique. All other outcomes were comparable between the subgroups (P>0.05), and in particular no differences were identified between the two groups regarding overall complication rate and prolonged air leak (Table 4).
Table 4
| Outcomes | Fluorescence method (n=110) | Modified inflation-deflation method (n=110) | t/χ2 value | P value |
|---|---|---|---|---|
| Segmental resection time (min) | 69.37±28.22 | 78.80±34.66 | 2.212† | 0.03 |
| Intraoperative blood loss (mL) | 17.00±8.84 | 20.64±16.83 | 2.007† | 0.046 |
| Maximum nodule diameter (cm) | 1.06±0.39 | 1.08±0.43 | 0.247† | 0.81 |
| Postoperative drainage (mL) | ||||
| Day 1 | 144.59±86.00 | 128.77±82.38 | −1.393† | 0.17 |
| Day 2 | 144.55±124.12 | 139.91±88.99 | −0.318† | 0.75 |
| Day 3 | 100.27±91.80 | 104.50±82.37 | 0.359† | 0.72 |
| Mean drainage at 3 days after surgery (mL) | 131.55±79.19 | 124.40±61.01 | −0.749† | 0.45 |
| Postoperative VAS pain score | ||||
| Day 1 | 2.92±0.36 | 3.05±0.31 | 2.785† | 0.006 |
| Day 2 | 2.64±0.54 | 2.70±0.58 | 0.842† | 0.40 |
| Day 3 | 2.25±0.64 | 2.28±0.56 | 0.449† | 0.65 |
| Postoperative chest tube duration (days) | 4.59±2.23 | 4.48±1.28 | −0.445† | 0.66 |
| Postoperative hospital stay (days) | 5.55±2.36 | 5.55±1.45 | −0.413† | 0.68 |
| Postoperative complications | 3.457§ | 0.66 | ||
| Pneumonia | 5 (4.55) | 3 (2.73) | 0.130‡ | 0.72 |
| Prolonged air leak (>3 days) | 7 (6.36) | 5 (4.55) | 0.353‡ | 0.55 |
| Massive postoperative drainage (≥200 mL/day for 5 day) | 3 (2.73) | 7 (6.36) | 1.676‡ | 0.20 |
| Atelectasis | 1 (0.91) | 0 (0.00) | –§ | >0.99 |
| Other | 2 (1.82) | 2 (1.82) | 0.000‡ | >0.99 |
| Lung lesion pathology | 1.764‡ | 0.62 | ||
| AIS | 13 (11.82) | 13 (11.82) | ||
| MIA | 64 (58.18) | 68 (61.82) | ||
| IA | 27 (24.55) | 20 (18.18) | ||
| Benign | 6 (5.45) | 9 (8.18) | ||
| Total hospital cost (CNY) | 43,997.20±8,192.24 | 44,192.39±6,054.58 | 0.201† | 0.84 |
Data are presented as mean ± SD or n (%). †, Student’s t-test or Welch’s t-test; ‡, χ2 test value; §, Fisher’s exact test value. ICG, indocyanine green; VAS, visual analogue scale; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; IA, invasive adenocarcinoma; CNY, Chinese Yuan; SD, standard deviation.
A total of 350 patients were included in both the simple segmentectomy group and the matched complex segmentectomy group, with a follow-up deadline set for January 20, 2024, (range, 52 to 172 weeks). Of these, 33 patients were lost to follow-up, resulting in an overall follow-up rate of 90.57%. All patients who were followed up remained disease free. Long-term complications were observed in 22 cases, predominantly chest discomfort/dyspnea (n=10) and chronic cough (n=9) (Table 5).
Table 5
| Outcomes | Simple segmentectomy group | Complex segmentectomy group after PSM | Total (n=350) | |||
|---|---|---|---|---|---|---|
| Fluorescence method (n=65) | Modified inflation-deflation method (n=65) | Fluorescence method (n=110) | Modified inflation-deflation method (n=110) | |||
| Follow-up completion | 61 (93.85) | 60 (92.31) | 94 (85.45) | 102 (92.73) | 317 (90.57) | |
| Chest discomfort/dyspnea | 1/61 (1.64) | 4/60 (6.67) | 1/94 (1.06) | 4/102 (3.92) | 10/317 (3.15) | |
| Chronic cough (>3 months) | 4/61 (6.56) | 1/60 (1.67) | 2/94 (2.13) | 2/102 (1.96) | 9/317 (2.84) | |
| Chronic pain (>3 months) | 1/61 (1.64) | 0/60 (0.00) | 1/94 (1.06) | 1/102 (0.98) | 2/317 (0.63) | |
| Excessive sputum | 1/61 (1.64) | 0/60 (0.00) | 0/94 (0.00) | 0/102 (0.00) | 1/317 (0.32) | |
Data are presented as n (%) or n/follow-up completion (%). ICG, indocyanine green; PSM, propensity score matching.
Discussion
The use of ICG fluorescence imaging for surgical guidance dates back to 1948 when it was initially employed for the identification and localization of brain tumors (21). Since then, this technology has found applications across various surgical disciplines, including ophthalmic surgery, hepatobiliary surgery, gynecology, gastrointestinal surgery, and urology (22-26). The potential of fluorescence imaging to delineate lung ISPs was first demonstrated by Misaki et al. (27) through animal experiments in 2009. The following year, his team successfully identified ISPs using ICG in eight patients undergoing open segmentectomy (28), gradually expanding its application to thoracic surgical procedures. ICG is currently the sole contrast agent approved by the US Food and Drug Administration (FDA) for this purpose, owing to its established safety and effectiveness (29).
In the design of this multicenter study, we excluded the outcomes of operative time and intersegmental marking time which have been commonly used in similar studies (13,14). Operative time can be significantly affected by variations in intraoperative steps, including degrees of pleural adhesion, frozen section analysis, lymph node dissection/sampling, lung trauma repair, and hemostasis. Similarly, determining the endpoint for intersegmental marking time can be challenging, particularly with the modified inflation-deflation method. Instead, we selected segmental resection time as our comparison index since it is less influenced by the aforementioned and other external factors and can provide more relevant results.
Based on the study results, there was no statistically significant difference in any of the outcomes between the fluorescence and modified inflation-deflation methods in the simple segmentectomy group. This included segmental resection time, where the fluorescence method did not demonstrate an advantage. We hypothesize that this is due to the low anatomical complexity of the lung segment and the lower likelihood of arteriovenous and bronchial variants encountered during simple segmentectomy. The modified inflation-deflation technique likely resulted in faster lung deflation and greater operator familiarity with the plane of simple lung segments. This might have caused the surgeons to proceed with resecting the target segments before the surrounding segments were fully deflated, leading to shorter but statistically non-significant differences in resection times compared to the fluorescence method.
In the complex segmentectomy group, the fluorescence method (69.37±28.22 min) was associated with significantly shorter segmental resection time compared to the modified inflation-deflation technique (78.80±34.66 min, P=0.03). Considering the increased variance and irregularity of ISPs in complex lung segments, complete inflation and deflation is required to clearly delineate ISP when using this method. Among the 110 matched patients who underwent the modified inflation-deflation method, 17 patients with an inflation-deflation time exceeding 20 min still did not present a fully visible ISP. This may be attributed to emphysema or collateral ventilation between adjacent lung segments. In contrast, among the 110 matched patients who underwent fluorescence imaging, ICG revealed significantly enlarged ISP in five cases of RS1 resection, one case of RS2 resection, and one case of RS3 resection compared to experience-based observations. However, ISP revealed by ICG was found to be significantly reduced in three cases of LS1+2 resection. This discrepancy could be attributed to variations in right and left upper pulmonary arteries that were not detectable by preoperative 3D imaging techniques and incomplete intraoperative dissection.
The findings of our study also revealed that the fluorescence method resulted in significantly less intraoperative blood loss (17.00±8.84 mL) compared to the modified inflation-deflation method (20.64±16.83 mL, P=0.046). This suggests that during the complex procedure of modified inflation-deflation, there may be increased blood and interstitial fluid leakage due to arteriovenous stump and lung wound, as well as the release of inflammatory factors such as prostaglandins and cytokines during the prolonged operation time and lung inflation-deflation process, leading to systemic inflammatory response, pain, or hyperalgesia. This also could explain why the modified method yielded a higher VAS on the first postoperative day (3.05±0.31) compared to the fluorescence method (2.92±0.36, P=0.006). It is worth noting that, while these differences were statistically significant, they may have not necessarily translated into clinically meaningful improvements for all patients. The statistically significant but small reduction in blood loss and pain scores, although beneficial, may not substantially impact the overall clinical outcomes in every case.
For operations involving resection of lung subsegments or combined segments/subsegments, the fluorescence method seemed to be more accurate and efficient than the modified inflation-deflation technique. Due to the small number of lung subsegments or combined segments/subsegments cases, we did not conduct further comparative analysis, and only drew the above conclusion from the intuitive experience of these senior surgeons participating in this study. Indeed, the fluorescence method provides enhanced visualization that allows clear and immediate delineation of these complex borders, minimizing the risk of damage to adjacent healthy lung tissue. Additionally, this method reduces the likelihood of incomplete deflation, a common issue in the inflation-deflation technique, especially in subsegmental resections.
Other postoperative outcomes, including chest tube drainage and duration, complication rate, and length of hospitalization, showed no statistically significant differences between the fluorescence and the modified inflation-deflation methods in both the simple and complex segmentectomy groups. The three most common postoperative complications for the fluorescence and modified inflation-deflation methods were air leaks (n=14 and n=13, respectively), large fluid drainage (n=5 and n=14, respectively), and pneumonia (n=11 and n=5, respectively). Not only in the overall complications, but also in the individual complications, there was no significant difference between patients by the two methods in respective subgroups. In particular, the overall incidence of postoperative pneumonia appeared to be higher in the fluorescence subgroup (6.29%, 6+5/65+110) than in the modified inflation-deflation subgroup (2.86%, 2+3/65+110), but there was no statistically significant difference (χ2 test value =2.358, P=0.13). Regarding massive postoperative drainage, the total postoperative incidence of 2.86% (2+3/65+110) in the fluorescence subgroup seemed to be lower than that of 8.00% (7+7/65+110) by the modified inflation-deflation subgroup, with statistically significant differences observed (χ2 test value =4.508, P=0.03<0.05). Although such statistical analysis might be confused by surgical complexity assessment, resulting in unconvincing results, however, they did suggest potential advantages of using fluorescent techniques for reducing postoperative complications. Luckily, none of these complications were severe, and all patients with complications were discharged after extended hospitalization for symptomatic treatment. These findings indicate that the fluorescence method is comparable to the modified inflation-deflation technique in terms of safety. This finding is consistent with the results of another study (13).
An important consideration when adopting surgical technologies is cost-effectiveness, especially in healthcare settings with limited resources. Although a formal economic evaluation of the compared methods was outside the scope of this study, it is worth noting that there was no significant increase in the total cost of hospitalization with the fluorescence method in either groups [Chinese Yuan (CNY) ¥42,965.74±¥5,043.28 vs. ¥43,850.36±¥6,922.78, for the fluorescence and modified inflation-deflation methods, respectively, in the simple segmentectomy groups, P=0.41; CNY ¥43,997.20±¥8,192.24 vs. ¥44,192.39±¥6,054.58, for the fluorescence and modified inflation-deflation methods, respectively, in the complex segmentectomy groups, P=0.84]. Therefore, the fluorescence method does not impose a greater financial burden on patients and healthcare systems.
During long-term follow-up of approximately 90% of patients, no disease recurrence or cancer-related mortality was recorded. This demonstrates the sound oncological outcomes conferred by both the fluorescence and modified inflation-deflation methods, which ensured adequate resection margins. However, a small number of patients experienced long-term complications, such as chest discomfort/dyspnea and recurrent cough. These complications did not exhibit distinct associations with either method; rather, they were closely related to the extent of surgical resection and postoperative respiratory function recovery.
In a study by Liu et al. (14) comparing the near-infrared fluorescence method with the inflation-deflation method in segmentectomy for patients with chronic lung diseases, several advantages were observed including reduced operation time, improved clarity of ISPs, shorter chest tube drainage duration and length of hospital stay after surgery, as well as decreased incidence of prolonged air leak. However, it should be noted that because the patient population studied consisted of elderly individuals with chronic lung diseases, the benefit of near-infrared fluorescence over the inflation-deflation method have been accentuated in their study (e.g., in terms of chest tube drainage duration and length of hospital stay). One limitation of Liu’s study was its small sample size and short follow-up period. In contrast, our multicenter study encompassed a larger sample size and broader range of indications within the study population. We also considered additional confounding factors to minimize statistical bias and conducted a longer follow-up observation period. Despite these differences, our results were overall fairly similar to Liu’s findings, further highlighting the advantages of utilizing the fluorescence method.
Some limitations of this study should be acknowledged. First, the retrospective design of the study may introduce selection bias and limit the ability to establish causality. Furthermore, although we employed a multicenter design and used PSM to balance the baseline characteristics of complex segmentectomy patients and reduce confounding factors, certain data were not collected or compared. These include postoperative lung function tests, perioperative inflammatory markers, management measures for postoperative complications, and whether the techniques of ISP dissection were equivalent in the subgroups. Additionally, some patients were lost to follow-up, mainly due to changes in contact information, potentially resulting in missing adverse events related to either method. There has been an analysis of 5-year disease-free survival rate and risk factors of worse oncological in the study of simple and complex thoracoscopic segmentectomies for NSCLC (30), but our study was lacking in this regard. Future studies should aim to prospectively compare methods for identifying ISPs during segmentectomy, with long-term patient follow-up to better evaluate oncologic outcomes and late complications.
Conclusions
This multicenter retrospective study revealed no significant differences in short- and long-term outcomes between patients undergoing single-port thoracoscopic simple segmentectomy using the ICG fluorescence method vs. the modified inflation-deflation method. However, for complex segmentectomies, the fluorescence method demonstrated advantages in lung segmental resection time, intraoperative blood loss, and postoperative pain without compromising other perioperative or oncologic outcomes. These findings confirm the safety and effectiveness of systemic ICG injection for performing single-port thoracoscopic anatomical segmentectomy and support its application as the method of choice in complex cases.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-986/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-986/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-986/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-986/coif). B.H.O. reports honoraria from AstraZeneca, Bristol-Myers Squibb, Medtronic, MSD and Roche, and meeting support from MSD, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the leading institution Anhui Chest Hospital, Anhui, China (No. KJ2024-039) and informed consent was taken from all the patients. All participating hospitals/institutes were informed and agreed with the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tsutani Y, Miyata Y, Nakayama H, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest 2014;145:66-71. [Crossref] [PubMed]
- Mimae T, Okada M. Are segmentectomy and lobectomy comparable in terms of curative intent for early stage non-small cell lung cancer? Gen Thorac Cardiovasc Surg 2020;68:703-6. [Crossref] [PubMed]
- Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449-55. [Crossref] [PubMed]
- Tane S, Nishio W, Nishioka Y, et al. Evaluation of the Residual Lung Function After Thoracoscopic Segmentectomy Compared With Lobectomy. Ann Thorac Surg 2019;108:1543-50. [Crossref] [PubMed]
- Onaitis MW, Furnary AP, Kosinski AS, et al. Equivalent Survival Between Lobectomy and Segmentectomy for Clinical Stage IA Lung Cancer. Ann Thorac Surg 2020;110:1882-91. [Crossref] [PubMed]
- Altorki N, Wang X, Damman B, et al. Lobectomy, segmentectomy, or wedge resection for peripheral clinical T1aN0 non-small cell lung cancer: A post hoc analysis of CALGB 140503 (Alliance). J Thorac Cardiovasc Surg 2024;167:338-347.e1. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Stamatis G, Leschber G, Schwarz B, et al. Survival outcomes in a prospective randomized multicenter Phase III trial comparing patients undergoing anatomical segmentectomy versus standard lobectomy for non-small cell lung cancer up to 2 cm. Lung Cancer 2022;172:108-16. [Crossref] [PubMed]
- Andolfi M, Potenza R, Seguin-Givelet A, et al. Identification of the intersegmental plane during thoracoscopic segmentectomy: state of the art. Interact Cardiovasc Thorac Surg 2020;30:329-36. [Crossref] [PubMed]
- Iizuka S, Kuroda H, Yoshimura K, et al. Predictors of indocyanine green visualization during fluorescence imaging for segmental plane formation in thoracoscopic anatomical segmentectomy. J Thorac Dis 2016;8:985-91. [Crossref] [PubMed]
- Bédat B, Triponez F, Sadowski SM, et al. Impact of near-infrared angiography on the quality of anatomical resection during video-assisted thoracic surgery segmentectomy. J Thorac Dis 2018;10:S1229-34. [Crossref] [PubMed]
- Pischik VG, Kovalenko A. The role of indocyanine green fluorescence for intersegmental plane identification during video-assisted thoracoscopic surgery segmentectomies. J Thorac Dis 2018;10:S3704-11. [Crossref] [PubMed]
- Mun M, Okumura S, Nakao M, et al. Indocyanine green fluorescence-navigated thoracoscopic anatomical segmentectomy. J Vis Surg 2017;3:80. [Crossref] [PubMed]
- Liu Z, Yang R, Cao H. Near-infrared intraoperative imaging with indocyanine green is beneficial in video-assisted thoracoscopic segmentectomy for patients with chronic lung diseases: a retrospective single-center propensity-score matched analysis. J Cardiothorac Surg 2020;15:303. [Crossref] [PubMed]
- Handa Y, Tsutani Y, Mimae T, et al. Surgical Outcomes of Complex Versus Simple Segmentectomy for Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2019;107:1032-9. [Crossref] [PubMed]
- Liu L, Aokage K, Chen C, et al. Asia expert consensus on segmentectomy in non-small cell lung cancer: A modified Delphi study. JTCVS Open 2023;14:483-501. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:497-530. [Crossref] [PubMed]
- Kobayashi N, Kawamura T, Yanagihara T, et al. Impacts of pleural adhesions on lobectomies for malignant lung tumors. Gen Thorac Cardiovasc Surg 2022;70:1042-7. [Crossref] [PubMed]
- Lee S, Lee JG, Lee CY, et al. Pulmonary fissure development is a prognostic factor for patients with resected stage I lung adenocarcinoma. J Surg Oncol 2016;114:848-52. [Crossref] [PubMed]
- Kane LT, Fang T, Galetta MS, et al. Propensity Score Matching: A Statistical Method. Clin Spine Surg 2020;33:120-2. [Crossref] [PubMed]
- Moore GE, Peyton WT. The clinical use of fluorescein in neurosurgery; the localization of brain tumors. J Neurosurg 1948;5:392-8. [Crossref] [PubMed]
- Tran AQ, Park RB, Lee DK, et al. Qualitative use of ICG angiography and lymphography in periorbital surgery. Orbit 2024;43:196-202. [Crossref] [PubMed]
- Piccolo G, Barabino M, Lecchi F, et al. Laparoscopic Indocyanine Green Fluorescence Imaging for Intrahepatic Cholangiocarcinoma. Am Surg 2023;89:2577-82. [Crossref] [PubMed]
- Ditto A, Ferla S, Martinelli F, et al. Real-time Fluorescent ICG and 99m-Tc Nanocolloid Tracer Navigation in Bilateral Sentinel Lymph Node Mapping of Vulvar Cancer. J Minim Invasive Gynecol 2023;30:780-1. [Crossref] [PubMed]
- Chen QY, Zhong Q, Liu ZY, et al. Indocyanine green fluorescence imaging-guided versus conventional laparoscopic lymphadenectomy for gastric cancer: long-term outcomes of a phase 3 randomised clinical trial. Nat Commun 2023;14:7413. [Crossref] [PubMed]
- Wit EMK. A hybrid radioactive and fluorescence approach is more than the sum of its parts; outcome of a phase II randomized sentinel node trial in prostate cancer patients. Eur J Nucl Med Mol Imaging 2023;50:2861-71. [Crossref] [PubMed]
- Misaki N, Chang SS, Gotoh M, et al. A novel method for determining adjacent lung segments with infrared thoracoscopy. J Thorac Cardiovasc Surg 2009;138:613-8. [Crossref] [PubMed]
- Misaki N, Chang SS, Igai H, et al. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg 2010;140:752-6. [Crossref] [PubMed]
- Muraleedharan S, Tripathy K. Indocyanine Green (ICG) Angiography. In: StatPearls. Treasure Island: StatPearls Publishing. 2023.
- Bongiolatti S, Salvicchi A, Indino R, et al. Post-operative and early oncological results of simple and complex full thoracoscopic segmentectomies for non-small-cell lung cancer. Asian Cardiovasc Thorac Ann 2023;31:123-32. [Crossref] [PubMed]
(English Language Editor: J. Gray)


