
Does the guide sheath outperform the non-guide sheath method in endobronchial ultrasound-guided biopsy of peripheral pulmonary lesions?—a meta-analysis
Highlight box
Key findings
• Endobronchial ultrasound-guided transbronchial biopsy with and without a guide sheath (EBUS-GS and EBUS-nGS) have similar overall diagnostic rates for peripheral pulmonary lesions (PPLs), but EBUS-nGS demonstrated a higher diagnostic rate for lesions ≤30 mm, lower lobe lesions, and using an ultrathin bronchoscope, while EBUS-GS is safer.
What is known and what is new?
• The existing literature has yet to definitively show differences in the diagnostic capabilities of EBUS-GS and EBUS-nGS for PPLs.
• This study aims to investigate the diagnostic efficacy of EBUS-GS and EBUS-nGS for PPLs, in order to provide a rational, informed basis for decision-making.
What is the implication, and what should change now?
• In clinical practice, both EBUS-GS and EBUS-nGS methods should be considered complementary and used flexibly in different situations.
Introduction
Peripheral pulmonary lesions (PPLs) are common lung lesions, defined as abnormalities located in the distal branches of segmental bronchi, surrounded by lung parenchyma, and not visible during routine bronchoscopy examination. They exclude intraluminal lesions, submucosal tumor infiltration, intraluminal inflammation, or bleeding and so on (1). Early detection of PPLs and obtaining histopathological diagnosis are critical factors in improving the survival rates of lung cancer patients; endobronchial ultrasound-guided transbronchial biopsy with or without a guide sheath (EBUS-GS or EBUS-nGS) is commonly used for diagnosing PPLs.
EBUS-GS is designed by introducing a microprobe covered by a guide sheath (GS) into the target bronchus; after lesion detection, the microprobe is retracted, leaving the GS in place as a working channel for obtaining biopsy samples; it can be considered as an extension of the bronchoscope (2,3). Subsequently, biopsy samples can be obtained using brushes, forceps, or other devices through the GS. In theory, this approach can improve the diagnostic yield of PPLs. Hence, it has been extensively employed in studies investigating ultrasound-guided bronchopulmonary biopsy (4,5). EBUS-nGS methods include endobronchial ultrasound-guided transbronchial lung biopsy distance measurement (EBUS-D-TBLB), ultrathin bronchoscopy (UTB), etc. EBUS-D-TBLB is a non-real-time guided technique. When the lesion is visualized on ultrasound scanning, the distance between the lesion and the target bronchus is measured to determine the advancement length of the biopsy forceps for better specimen collection. Numerous studies have confirmed its safety and efficacy (6-9). UTB has good accessibility through the small bronchus and can make effective contact with the surrounding lung lesions; when combined with EBUS, this utility is especially enhanced, with studies showing that it has a high diagnostic rate and a certain level of safety (10,11).
However, the existing literature is yet to definitively show differences in the diagnostic capabilities of EBUS-GS and EBUS-nGS for PPLs. Whether using GS during EBUS improves the diagnosis rate of PPLs remains controversial (12). Therefore, this meta-analysis aims to consolidate studies comparing the diagnostic efficacy of EBUS-GS and EBUS-nGS for PPLs, elucidating the value of EBUS with or without a GS, to provide a foundation for future research endeavors. We present this article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-845/rc).
Methods
This systematic review was conducted following the guiding principles of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (13). It has been registered in the PROSPERO database (https://www.crd.york.ac.uk/PROSPERO/) with the registration number CRD42024512784.
Search strategy
Searches were performed in four databases—PubMed, Embase, Web of Science, and the Cochrane Library—using the following terms as titles, abstracts, and keywords: “Bronchoscopy”, “endobronchial ultrasound-guided biopsy”, “EBUS”, “Bronchoscopic transbronchial biopsy”, “TBB”, “peripheral pulmonary lesions”, “PPLs”, “Guide sheath”, “GS”, “non-guide sheath”, and “nGS”. The search period was extended from the inception of each database to January 2024. Additionally, manual retrieval of the reference lists of relevant studies was conducted to identify any potentially relevant studies that might not have been captured through the database searches. Details of the search strategy are provided in Appendix 1.
Study selection
Two authors independently assessed all articles identified through the search strategy for eligibility. The inclusion criteria were as follows: (I) patients with PPLs confirmed by radiological evidence; (II) studies comparing the diagnostic efficacy of EBUS-GS or EBUS-nGS for PPLs; (III) diagnosis confirmed by histology or close clinical follow-up; (IV) studies including a minimum of twenty patients. The exclusion criteria were: (I) reviews, systematic reviews, and animal experiments; (II) studies with irrelevant content, inability to obtain full-text articles, or small sample sizes; (III) studies with inconsistent outcome measures; (IV) studies not published in English.
When the same author had published two or more studies, the articles were reviewed to ensure no overlap in study dates. If there was an overlap in dates or the author explicitly stated that the data came from a previously published study, the study with the largest number of patients relevant to this meta-analysis was included. Two reviewers independently conducted literature screening based on predefined inclusion and exclusion criteria, and a third reviewer was consulted if eligibility was uncertain.
Data extraction
Information and data extracted from eligible studies meeting inclusion criteria by two authors included the first author, research methodology [randomized controlled trial (RCT), prospective, retrospective, or unknown], year of study, study group, sample size, participant age, bronchoscope channel size, biopsy forceps size, lesion size, presence or absence of bronchial signs, lobar location of lesions, diagnostic rate, complications, surgical duration, etc.
Study quality assessment
The quality of the RCTs included in this meta-analysis was assessed using the Cochrane Risk of Bias Assessment Tool (14) and the modified Jadad Composite Scale (15). RCTs with a Jadad score of ≥4 were considered high-quality studies (16). Non-RCT studies were evaluated using the Newcastle-Ottawa Scale (NOS), which assesses three key domains: selection of study groups, comparability of groups, and ascertainment of either exposure for case-control studies or outcome for cohort studies. Studies with a NOS score of ≥6 were considered high-quality research (17).
Statistical analysis
Data analysis was performed using Review Manager 5.4 and STATA 14.0. The odds ratio (OR) with a 95% confidence interval (CI) was used to compare binary variables. The mean difference (MD) and 95% CI were calculated for continuous outcomes. The heterogeneity among included studies was assessed using the Chi-squared test, with a significance level generally set at P value (P) <0.1; the degree of heterogeneity was evaluated based on I2, where P≤0.1 and I2 >50% indicated significant heterogeneity, in such cases, a random-effects model was employed for analysis; otherwise, a fixed-effects model was used (18-20). Subgroup analyses and sensitivity analyses were conducted to identify the sources of heterogeneity. A funnel plot was used to assess the risk of publication bias (21). A P<0.05 was considered statistically significant for differences.
Results
Research characteristics
The overview of our study selection process is illustrated in Figure 1. Baseline characteristics of the studies and corresponding data are summarized in Table 1. Overall, out of the 581 studies initially retrieved from electronic databases, we selected nine relevant studies (7,9,11,22-27) to be included in this meta-analysis. All studies were conducted in either China or Japan. The anesthesia methods across nine articles included conscious sedation or conscious sedation combined with local anesthesia. These studies encompassed 2,898 patients, comprising five RCTs and four non-RCTs. The Cochrane Risk of Bias assessment for the five included RCT studies is depicted in Figure 2, where green represents low risk, yellow indicates medium risk, and red signifies high risk. Lower risk in each aspect of the assessment corresponds to higher quality and more excellent reliability of the research. Based on the modified Jadad Composite Scale, three RCTs were rated with 3 points, indicating low-quality research, while two RCTs scored 4 points, indicating high-quality research. According to the NOS, all four non-RCTs included in the analysis received a score indicative of high-quality research (7 points). Due to the specific nature of bronchoscopy examination and cost variations, blinding was not feasible, thus, this aspect was not scored.
Table 1
| Study/design | Country | Interventions | Number of patients |
Age (years) | Working channel/forceps diameter (mm) | Lesion size (mm) | Operative time (min) | Diagnostic yields (%) | Complications | Quality assessments | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Malignant | Benign | Jade score | NOS | |||||||||
| Guan 2022/RCT (9) | China | EBUS-GS | 287 | – | – | 42.9±13.3 | – | 74.91 | 78.9 | 65.06 | 3 H | 4 | – |
| EBUS-nGS | 282 | – | – | 43.3±13.6 | – | 76.95 | 82.09 | 64.20 | 14 H/1 CP | ||||
| Huang 2021/Retro (22) | China | EBUS-GS | 360 | 65±13 | 2/1.5 | – | 29±11 | 78 | 78 | – | 2 PTX/21 H | – | 7 |
| EBUS-nGS | 360 | 68±11 | 2/1.8 | – | 24±11 | 79 | 79 | – | 7 PTX/26 H | ||||
| Ito 2021/Retro (23) | Japan | EBUS-GS | 39 | 70±7.9 | 2/– | 16.0±3.0 | 28±8.3 | 71.7 | 74.07 | 66.67 | – | – | 7 |
| EBUS-nGS | 41 | 68±9.7 | 2/1.9 | 14.0±3.5 | 20±7.5 | 82.9 | 80.06 | 90.00 | – | ||||
| Oki 2012/RCT (24) | Japan | EBUS-GS | 102 | 67.4±10.2 | 2/1.5 | 27.4±11.3 | 27.4±11.3 | 61.76 | 70.73 | 30.00 | 2 P | 3 | – |
| EBUS-nGS | 101 | 65.6±11.0 | 1.7/1.5 | 33±13.8 | 33±13.8 | 65.34 | 75.00 | 40.90 | 3 PTX/1 H/1 P | ||||
| Oki 2015/RCT (25) | Japan | EBUS-GS | 155 | 70.04±10.76 | 2/1.5 | 30.13±12.45 | 30.13±12.45 | 59 | 69.72 | 36.36 | 5 PTX/2 H | 3 | – |
| EBUS-nGS | 150 | 69.49±10.99 | 1.7/1.5 | 28.95±12.32 | 28.95±12.32 | 74 | 81.30 | 42.31 | 3 PTX | ||||
| Oki 2022/RCT (12) | Japan | EBUS-GS | 300 | 71.19±11.34 | 2/1.5 | 19.91±4.07 | 31.10±13.60 | 55.3 | 49.7 | 18 | 3 PTX/1 H/4 P | 3 | – |
| EBUS-nGS | 296 | 71.73±8.04 | 2/1.8 or 1.9 | 18.97±4.04 | 30.34±15.97 | 46.6 | 55.4 | 16.7 | 5 PTX/3 H/3 P | ||||
| Sumi 2020/Retro (26) | Japan | EBUS-GS | 66 | 72.25±9.80 | 2/1.5 | 20.74±6.39 | 20.74±6.39 | 59.1 | – | – | 1 H/1 P | – | 7 |
| EBUS-nGS | 102 | 72.83±8.98 | 1.7/1.5 | 24.50±6.59 | 24.50±6.59 | 74.5 | – | – | 1 PTX/1 H/1 P | ||||
| Yatani 2023/Retro (27) | Japan | EBUS-GS | 93 | – | 2.0/1.5 | 29.94±14.36 | – | 78.5 | – | – | – | – | 7 |
| EBUS-nGS | 56 | – | 1.7/1.5 | 26.15±10.28 | – | 78.6 | – | – | – | ||||
| Zhang 2016/RCT (7) | China | EBUS-GS | 54 | 60.85±9.12 | 2.0/1.5 | 30.2±13.5 | 5.17±2.34 | 72.2 | 78.95 | 56.25 | – | 4 | – |
| EBUS-nGS | 54 | 60.85±9.12 | 2.0/1.8 | 30.2±13.5 | 7.36±3.18 | 75.9 | 78.95 | 68.75 | – | ||||
Numerical variables are presented as mean ± standard deviation. RCT, randomized controlled trial; Retro, retrospective study; EBUS-GS, endobronchial ultrasound-guided transbronchial biopsy with a guide sheath; EBUS-nGS, endobronchial ultrasound-guided transbronchial biopsy without a guide sheath; NOS, Newcastle-Ottawa Scale; PTX, pneumothorax; CP, chest pain; H, hemorrhage; P, pneumonia.
Diagnostic rate
The heterogeneity test revealed that the I2 statistic was 53%, with a significant Q test result (P=0.03<0.1) for the nine studies included in this analysis. This suggested notable heterogeneity among the selected studies, which was statistically significant. Therefore, a random-effects model was utilized to conduct a meta-analysis of the diagnostic rate. The meta-analysis of the nine studies indicated that the overall diagnostic rate of EBUS-GS for PPLs was slightly lower compared to EBUS-nGS; still, the difference was not statistically meaningfulness [OR: 0.83, 95% CI: 0.64–1.08, Z-score (Z) =1.37, P=0.17]. Based on current research, these findings show no substantial difference between EBUS-GS and EBUS-nGS in terms of the overall diagnostic rate for PPLs (Figure 3).

Subgroup analysis was conducted to explore heterogeneity within the study. Among the nine studies, there were variations in the types of bronchoscopes used. Except for one study that did not report the manufacturer, all others employed bronchoscopes produced by Olympus. Regarding bronchoscopic external diameter, five studies used bronchoscopes with the same diameter (4.0 or 4.2 mm) for both the EBUS-GS and EBUS-nGS groups. Three articles used bronchoscopes with an outer diameter of 3.0 mm, while Oki et al. 2012 (24) used an EBUS-nGS with an outer diameter of 3.4 mm and a channel diameter of 1.7 mm. Subgroup analyses based on bronchoscopic outer diameter revealed that when the EBUS-GS and EBUS-nGS groups used bronchoscopes with the same external diameter, there was no significant difference in diagnostic rates between the two groups (OR: 1.00, 95% CI: 0.77–1.31, Z=0.02, P=0.98). However, when the EBUS-nGS group used bronchoscopes with an external diameter of 3.0 mm, that is, ultrathin bronchoscopes (UTB), the diagnostic rate was significantly higher compared to the EBUS-GS group, with statistical significance (OR: 0.58, 95% CI: 0.40–0.84, Z=2.86, P=0.004) (Figure 4). Among the eight reports on bronchoscopic working channel diameter, four mentioned the use of bronchoscopes with a working channel of 2.0 mm for both EBUS-GS and EBUS-nGS, with biopsy forceps sizes of 1.5 and 1.8 or 1.9 mm, respectively. In another four reports, EBUS-GS employed bronchoscopes with a working channel of 2.0 mm. At the same time, EBUS-nGS utilized bronchoscopes with a working channel of 1.7 mm, with consistent sizes for biopsy forceps. Subgroup analysis based on the working channel diameter revealed that when both groups employed bronchoscopes with a 2.0 mm working channel diameter (2.0/2.0 mm), EBUS-GS exhibited a higher diagnostic rate for PPLs compared to EBUS-nGS; however, this difference lacked statistical significance (OR: 1.10, 95% CI: 0.88–1.38, Z=0.83, P=0.41). In studies where EBUS-GS and EBUS-nGS respectively utilized bronchoscopes with working channel diameters of 2.0 and 1.7 mm (2.0/1.7 mm), EBUS-GS demonstrated a lower diagnostic rate for PPLs than EBUS-nGS, with the difference being statistically significant (OR: 0.70, 95% CI: 0.51–0.96, Z=2.21, P=0.03) (Figure 5).
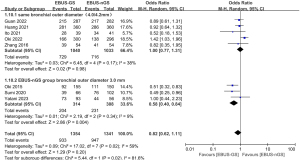

In the subgroup analysis stratified by lesion size, the meta-analysis showed that when lesions exceeded 30 mm in size, there was no noteworthy difference in diagnostic rates between the two groups (OR: 0.85, 95% CI: 0.58–1.24, Z=0.84, P=0.40). However, for lesions measuring 30 mm or less, EBUS-GS demonstrated a lower diagnostic rate for PPLs compared to EBUS-nGS, with this disparity was statistically meaningful (Figure 6). When subgroup analysis was performed based on the different locations of pulmonary nodules within lung lobes, it was found that when nodules were located in the upper lobe, EBUS-GS exhibited a higher diagnostic rate for PPLs compared to EBUS-nGS, although this difference did not reach statistical significance (OR: 1.29, 95% CI: 0.98–1.69, Z=1.79, P=0.07). Conversely, for nodules located in the lower lobe, the diagnostic rate of PPLs by EBUS-GS was significantly lower than that by EBUS-nGS (OR: 0.59, 95% CI: 0.38–0.91, Z=2.36, P=0.02) (Figure 7).


As shown in Table 2, there was no difference in diagnostic rates between EBUS-GS and EBUS-nGS in the subgroup analysis based on study type (RCT vs. non-RCT), histologic diagnosis (benign vs. malignant), location of lesions (different lung bands), bronchial signs (present vs. absent), lesion texture on computed tomography (CT) (solid vs. part-solid), EBUS probe position (adjacent vs. or outside) and navigational tools or ancillary imaging [using virtual bronchoscopic navigation (VBN) or fluoroscopy vs. no VBN and fluoroscopy]. The subgroup analysis suggests that bronchoscope type, lesion size, and lesion location (across different lung lobes) were potential sources of heterogeneity among the nine articles.
Table 2
| Subgroup | Subgroups | EBUS-GS | EBUS-nGS | Weight (%) | OR | 95% CI | P | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||||||
| Study type | RCT | 575 | 898 | 573 | 883 | 64.8 | 0.96 | 0.79–1.17 | 0.69 | |
| No RCT | 421 | 558 | 440 | 559 | 35.2 | 0.80 | 0.60–1.06 | 0.12 | ||
| Histologic diagnosis | Malignant | 781 | 1,057 | 791 | 1,060 | 80.2 | 0.97 | 0.79–1.18 | 0.74 | |
| Benign | 104 | 236 | 102 | 261 | 19.8 | 0.87 | 0.58–1.30 | 0.49 | ||
| Lesion location (lung bands) | Inner and middle | 167 | 236 | 166 | 219 | 24.5 | 0.75 | 0.49–1.15 | 0.18 | |
| Outer | 419 | 665 | 427 | 668 | 75.5 | 0.98 | 0.78–1.23 | 0.27 | ||
| Bronchus sign | Present | 280 | 436 | 230 | 284 | 61.8 | 0.53 | 0.24–1.16 | 0.11 | |
| Absent | 52 | 112 | 42 | 99 | 38.2 | 0.96 | 0.54–1.72 | 0.90 | ||
| Lesion texture on CT | Solid | 245 | 382 | 180 | 332 | 67.8 | 1.12 | 0.61–2.07 | 0.71 | |
| Part-solid | 22 | 50 | 36 | 58 | 32.2 | 0.51 | 0.23–1.13 | 0.46 | ||
| EBUS probe position | Within | 46 | 54 | 81 | 87 | 35.9 | 0.38 | 0.12–1.19 | 0.10 | |
| Adjacent to or outside | 21 | 51 | 30 | 56 | 64.1 | 0.59 | 0.27–1.28 | 0.18 | ||
| Navigational tools or ancillary imaging | Using VBN or fluoroscopy | 461 | 755 | 469 | 746 | 61.9 | 0.77 | 0.50–1.20 | 0.25 | |
| No VBN and fluoroscopy | 535 | 701 | 544 | 696 | 38.1 | 0.90 | 0.70–1.16 | 0.41 | ||
EBUS-GS, endobronchial ultrasound-guided transbronchial biopsy with a guide sheath; EBUS-nGS, endobronchial ultrasound-guided transbronchial biopsy without a guide sheath; OR, odds ratio; CI, confidence interval; RCT, randomized controlled trial; CT, computed tomography; VBN, virtual bronchoscopic navigation.
Surgical duration
Of the nine studies included, seven provided data on surgical duration. Heterogeneity testing was performed, with I2=94% and P<0.00001 for the Q test, indicating substantial heterogeneity among the selected literature; consequently, a random-effects model was employed for meta-analysis. The pooled analysis of seven studies suggested no statistical difference in operation time between EBUS-GS and EBUS-nGS procedures, with a MD of 0.45 and 95% CI of −2.96, 3.59 (Figure 8).

Complications
Out of the nine studies incorporated, complications were documented in six studies. Analysis using a fixed-effects model revealed that the probability of complications occurring in EBUS-GS was significantly lower than in EBUS-nGS, with statistical significance (OR: 0.64, 95% CI: 0.44–0.93, Z=2.33, P=0.02) (Figure 9). In the EBUS-GS group, among a total of 1,270 patients included in the meta-analysis, there were ten cases of pneumothorax, 28 cases of bleeding, seven cases of pneumonia, one case of transient hypoxemia, one case of catheter displacement, and one case of arrhythmia. Among the 1,293 patients in the EBUS-nGS group, pneumothorax occurred in 19 cases, hemorrhage in 45 cases, pneumonia in seven cases, transient hypoxemia in one case, and postoperative chest pain in two cases. Based on subgroup analysis according to the main types of complications, it was found that the incidence of pneumothorax in the EBUS-GS group was lower than that in the EBUS-nGS, but the difference was not statistically significant (OR: 0.55, 95% CI: 0.26–1.16, Z=1.56, P=0.12). Conversely, the incidence of pneumonia in the EBUS-GS group was higher than that in the EBUS-nGS group; still, the difference did not attain statistical significance (OR: 1.08, 95% CI: 0.39–3.02, Z=0.15, P=0.88). Notably, the bleeding risk of EBUS-GS was markedly decreased in the EBUS-GS group compared to the EBUS-nGS group (OR: 0.62, 95% CI: 0.39–1.00, Z=1.97, P=0.05) (Figure 10). Safety subgroup analysis of different bronchoscopic types used in EBUS-GS and EBUS-nGS revealed that, when the same kind of conventional bronchoscope was used, the EBUS-GS group had fewer complications compared to the EBUS-nGS group (OR: 0.59, 95% CI: 0.39–0.91, Z=2.42, P=0.02). However, when EBUS-nGS used a thinner bronchoscope, the complications between the two groups were not statistically significant (OR: 1.14, 95% CI: 0.44–2.95, Z=0.27, P=0.79) (Figure 11).



Publication bias and sensitivity analysis
We further analyzed publication bias regarding overall diagnostic rate, surgical duration, and safety through funnel plots. The results showed relatively symmetric distributions in studies concerning overall diagnostic rate and safety, indicating no significant publication bias. This finding bolsters our confidence in the results on diagnostic rate and safety. However, considerable publication bias was observed in operation time. Further sensitivity analysis indicated a certain degree of robustness in the studies (Figures 12,13).
Discussion
In diagnosing PPLs, both EBUS-GS and EBUS-nGS have their respective advantages. Due to the feasibility of repeated biopsy with EBUS-GS, many experts have adopted this approach (3,28,29). However, the existing literature has not definitively demonstrated differences between the diagnostic capabilities of EBUS-GS and EBUS-nGS for diagnosing PPLs. In this study, we performed a meta-analysis on the diagnostic application of EBUS-GS and EBUS-nGS in PPLs, covering multiple aspects such as diagnostic rate, surgical duration, and complications. Subgroup analysis was employed to explore the sources of heterogeneity in diagnostic rates and complications.
The results of the analysis regarding diagnostic rates indicated that no substantial disparity was observed in the overall diagnostic rates between EBUS-GS and EBUS-nGS. The funnel plot’s symmetry further strengthened the research findings’ reliability and mitigated evident publication bias. However, it is worth noting that the overall diagnostic rate results merely provide a macroscopic view. Subgroup analyses focusing on factors such as bronchus type, lesion size, and location offered us more specific insights. In terms of bronchus type, our meta-analysis indicated that when both EBUS-GS and EBUS-nGS used bronchoscopes with the same outer diameter (4.0/4.2 mm) and working channel diameter (2.0 mm), which refers to conventional bronchoscopy, there was no statistically significant difference in the diagnostic rate for PPLs between the two techniques, despite the use of larger biopsy forceps in EBUS-nGS. Interestingly, in the EBUS-nGS group, bronchoscopes with an outer diameter of 3.0 mm all had a working channel diameter of 1.7 mm. In this context, EBUS-GS exhibited a lower diagnostic rate for PPLs than EBUS-nGS, and this variance reached statistical significance. This may be attributed to a 3.0-mm UTB with a 1.7-mm working channel, which can traverse smaller airways with higher resistance, enhancing accessibility to PPLs and improving operator performance and convenience. UTB appears to be a potential tool for better biopsy and diagnosis of PPLs by reaching more distal bronchi (10,11). Therefore, to achieve a higher diagnostic rate for PPLs, under the condition of both employing EBUS, choosing a 3.0-mm outer diameter and 1.7-mm working channel bronchoscope may lead to a higher diagnostic rate, even without a GS (26,27,30). Regarding subgroup analysis based on lesion size, our inclusion of six studies revealed that there was no difference in diagnostic rates between the two groups when the lesion size was >30 mm. However, for lesions ≤30 mm, the diagnostic rate of EBUS-GS was consistently lower than that of EBUS-nGS; this finding aligns with the results reported by Ito et al. 2021 (23), underscoring the greater clinical relevance of EBUS-nGS for diagnosing small PPLs. This might be attributed to the size constraints imposed by GS on sampling instruments, which could potentially lower the diagnostic rate (12). Notably, in the subgroup analysis, according to the location of lesions in different lung lobes, when the lesions were located in the lower lobe, the diagnostic rate of EBUS-GS was significantly lower than that of EBUS-nGS. However, there was no difference between the two groups for lesions in the upper and middle lobes. This could potentially offer insights for future clinical practice. In subsequent studies, it may be discovered that employing the EBUS-nGS method for lesions in the upper lobe could enhance the diagnostic rate of PPLs. Based on the above considerations, when focusing solely on diagnostic purposes, non-selective application of GS guidance during EBUS-transbronchial biopsy (TBB) should not be encouraged; they should only be employed in very select cases. Furthermore, reducing the use of GSs can help save costs and resources.
Regarding surgical duration, Huang et al. 2021 (22) and Ito et al. 2021 (23) have suggested that EBUS-GS procedures may exhibit prolonged operating times. Conversely, Oki et al. 2012 (24), Sumi et al. 2020 (26), and Zhang et al. 2016 (7) have reported shorter durations for EBUS-GS surgeries. However, our meta-analysis findings indicated that there is no statistically notable disparity between the two groups in terms of surgical time. High heterogeneity may introduce a certain degree of interference with the analysis results. Considering the varying levels of technical proficiency among operators and differences in sample sizes across studies, we refrained from conducting further subgroup analysis on factors influencing surgical duration. In future comparisons of surgical duration between EBUS-GS and EBUS-nGS, it is essential to focus more on assessing the operators’ proficiency levels and considering constraints associated with sample acquisition. This strategy will facilitate an in-depth investigation into the disparities in surgical duration between the two techniques. In the realm of safety, when using the same conventional bronchoscopes, the meta-analysis highlighted a clear superiority of EBUS-GS over EBUS-nGS. This implies that GS technology may provide greater protection and reduce surgical complications. The superiority of EBUS-GS in safety might stem from its capability to securely fixate within the appropriate bronchus after removing the EBUS probe, facilitating repetitive biopsies and minimizing complications. Its safety, stability, repeatability, and suitability for novice users contribute to its advantageous position (3,31). Furthermore, as noted in the text, bleeding was the most frequently reported complication in both groups. Therefore, in future bronchoscopic lung biopsy procedures guided by ultrasound, operators should be familiar with hemostatic techniques such as wedging the bronchoscope or locally injecting adrenaline (22).
Admittedly, there were some limitations to this study. Firstly, the limited number of studies and the variations in bronchoscope types present certain constraints on the results and subgrouping of this meta-analysis. More studies in this area may be published in the future, at which time an updated meta-analysis will be conducted. Secondly, in assessing the quality of studies, the non-feasibility of blinding due to the unique nature and cost differences of bronchoscopy examinations resulted in a lack of scoring in this regard, leading to three RCTs scoring lower on the Jadad scale. The inconsistency in study quality introduced a certain degree of heterogeneity in diagnostic rates. Consequently, subgroup analysis was conducted to address this issue and explore factors contributing to the observed heterogeneity. Thirdly, our study excluded systematic reviews and conference abstracts, which potentially excluded some relevant studies and thus affected the analysis of results. Lastly, the differences in diagnostic rates and procedural times may be related to operator experience, techniques, hospital equipment, and the number of specimens collected; however, these factors were not thoroughly analyzed in the nine articles we included, which somewhat limits our ability to interpret the study results.
Conclusions
In summary, there is no significant difference in the overall diagnostic rate of PPLs between EBUS-GS and EBUS-nGS. However, when utilizing a bronchoscope with an outer diameter of 3.0 mm, a 1.7-mm bronchoscope channel, or for lesions ≤30 mm in size and lesions located in the lower lobe of the lung, EBUS-nGS demonstrated a higher diagnostic rate. When using the same conventional bronchoscopes, EBUS-GS provides greater safety advantages but is associated with a higher cost. Therefore, in clinical practice, both methods should be considered complementary and used flexibly in different situations. We anticipate the development of bronchoscopic methods that enhance diagnostic rates while also prioritizing high safety standards and cost-effectiveness.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-845/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-845/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-845/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xie F, Yang H, Huang R, et al. Chinese expert consensus on technical specifications of electromagnetic navigation bronchoscopy in diagnosing peripheral pulmonary lesions. J Thorac Dis 2021;13:2087-98. [Crossref] [PubMed]
- Zhang L, Wu H, Wang G. Endobronchial ultrasonography using a guide sheath technique for diagnosis of peripheral pulmonary lesions. Endosc Ultrasound 2017;6:292-9. [Crossref] [PubMed]
- Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959-65. [Crossref] [PubMed]
- Ali MS, Trick W, Mba BI, et al. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: A systematic review and meta-analysis. Respirology 2017;22:443-53. [Crossref] [PubMed]
- Steinfort DP, Khor YH, Manser RL, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011;37:902-10. [Crossref] [PubMed]
- Chung YH, Lie CH, Chao TY, et al. Endobronchial ultrasonography with distance for peripheral pulmonary lesions. Respir Med 2007;101:738-45. [Crossref] [PubMed]
- Zhang SJ, Zhang M, Zhou J, et al. Comparison of radial endobronchial ultrasound with a guide sheath and with distance by thin bronchoscopy for the diagnosis of peripheral pulmonary lesions: a prospective randomized crossover trial. J Thorac Dis 2016;8:3112-8. [Crossref] [PubMed]
- Zhang SJ, Zhang M, Zhou J, et al. Radial endobronchial ultrasonography with distance measurement through a thin bronchoscope for the diagnosis of malignant peripheral pulmonary lesions. Transl Lung Cancer Res 2018;7:80-7. [Crossref] [PubMed]
- Guan S, Zhou J, Zhang Q, et al. Comparison of radial endobronchial ultrasound-guided transbronchial lung biopsy with distance measurement versus with guide sheath in diagnosing peripheral pulmonary lesions with a diameter ≥3 cm by thin bronchoscope. Ann Thorac Med 2022;17:151-8. [Crossref] [PubMed]
- Oki M, Saka H. Diagnostic value of ultrathin bronchoscopy in peripheral pulmonary lesions: a narrative review. J Thorac Dis 2020;12:7675-82. [Crossref] [PubMed]
- Kim SH, Kim J, Pak K, et al. Ultrathin Bronchoscopy for the Diagnosis of Peripheral Pulmonary Lesions: A Meta-Analysis. Respiration 2023;102:34-45. [Crossref] [PubMed]
- Oki M, Saka H, Imabayashi T, et al. Guide sheath versus non-guide sheath method for endobronchial ultrasound-guided biopsy of peripheral pulmonary lesions: a multicentre randomised trial. Eur Respir J 2022;59:2101678. [Crossref] [PubMed]
- McInnes MDF, Moher D, Thombs BD, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018;319:388-96. [Crossref] [PubMed]
- Cochrane handbook for systematic reviews of interventions[M/OL]. Available online: https://training.cochrane.org/handbook
- Palys KE, Berger VW. A note on the jadad score as an efficient tool for measuring trial quality. J Gastrointest Surg 2013;17:1170-1. [Crossref] [PubMed]
- Zhu J, Gu Y. Diagnosis of peripheral pulmonary lesions using endobronchial ultrasonography with a guide sheath and computed tomography guided transthoracic needle aspiration. Clin Respir J 2019;13:765-72. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Dinnes J, Deeks J, Kirby J, et al. A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy. Health Technol Assess 2005;9:1-113. iii. [Crossref] [PubMed]
- Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010;1:97-111. [Crossref] [PubMed]
- Hunter JP, Saratzis A, Sutton AJ, et al. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol 2014;67:897-903. [Crossref] [PubMed]
- Huang CT, Chang LY, Chen CY, et al. Endobronchial ultrasound-guided transbronchial biopsy with or without a guide sheath for peripheral pulmonary malignancy. ERJ Open Res 2021;7:00267-2021. [Crossref] [PubMed]
- Ito T, Matsumoto M, Kujime M, et al. Efficacy of endobronchial ultrasound-guided transbronchial biopsy without guide sheath for small peripheral pulmonary lesions (≤15 mm): A retrospective cohort study. Clin Respir J 2021;15:622-7. [Crossref] [PubMed]
- Oki M, Saka H, Kitagawa C, et al. Randomized study of endobronchial ultrasound-guided transbronchial biopsy: thin bronchoscopic method versus guide sheath method. J Thorac Oncol 2012;7:535-41. [Crossref] [PubMed]
- Oki M, Saka H, Ando M, et al. Ultrathin Bronchoscopy with Multimodal Devices for Peripheral Pulmonary Lesions. A Randomized Trial. Am J Respir Crit Care Med 2015;192:468-76. [Crossref] [PubMed]
- Sumi T, Ikeda T, Sawai T, et al. Comparison of ultrathin bronchoscopy with conventional bronchoscopy for the diagnosis of peripheral lung lesions without virtual bronchial navigation. Respir Investig 2020;58:376-80. [Crossref] [PubMed]
- Yatani A, Katsurada N, Fukui T, et al. Diagnostic yield and the number of tumor cells of ultrathin bronchoscopy for peripheral lung lesions: A comparison with thin bronchoscopy. PLoS One 2023;18:e0290609. [Crossref] [PubMed]
- Lee KM, Lee G, Kim A, et al. Clinical outcomes of radial probe endobronchial ultrasound using a guide sheath for diagnosis of peripheral lung lesions in patients with pulmonary emphysema. Respir Res 2019;20:177. [Crossref] [PubMed]
- Sainz Zuñiga PV, Vakil E, Molina S, et al. Sensitivity of Radial Endobronchial Ultrasound-Guided Bronchoscopy for Lung Cancer in Patients With Peripheral Pulmonary Lesions: An Updated Meta-analysis. Chest 2020;157:994-1011. [Crossref] [PubMed]
- Feng X, Zhang Q, Luo F, et al. Study design for a multicenter, randomized controlled trial evaluating the diagnostic value of ultrathin bronchoscope compared to thin bronchoscope without fluoroscopy for peripheral pulmonary lesions. J Thorac Dis 2022;14:1663-73. [Crossref] [PubMed]
- Takashima Y, Oki M. Endobronchial ultrasound with a guide sheath during bronchoscopy for peripheral pulmonary lesions. Expert Rev Respir Med 2023;17:929-36. [Crossref] [PubMed]






