
Prediction of hemoptysis in patients with anomalous systemic arterial supply to normal basal segments of the lower lobe using a combination of CT features and clinical materials
Highlight box
Key findings
• This retrospective study focused on a developmental anomaly in which an anomalous systemic arterial supply to the normal basal segments of the lower lobe (ASALL). In this study, a combined risk scoring (CRS) model was constructed using multivariate logistic regression with the combination of radiological and clinical features to predict hemoptysis. The CRS scoring model incorporated four independent predictors [age, sex, ground glass opacity, and combined systemic arterial features (CD-A, >0.522)]. The area under the curve value of the scoring system reached 0.939 according to the receiver operating characteristic curve analysis.
What is known and what is new?
• ASALL is a rare congenital anomaly with the left inferior lobe often affected. Although asymptomatic in most patients, hemoptysis is still a complication which should not be overlooked, and the risk assessment of hemoptysis has not been well investigated.
• This study enrolled 43 eligible ASALL patients and they were classified into two groups: hemoptysis group and non-hemoptysis group, and the differences between these two groups in terms of radiological and clinical features were compared. The independent predictors for hemoptysis were detected and a risk scoring model was identified.
What is the implication, and what should change now?
• This CRS model provides a feasible approach to predict hemoptysis in ASALL patients and help clinicians formulate optimal treatment regimens.
• Patients at high risk of hemoptysis need to take necessary interventions, such as interventional therapy or lobectomy, to cope with the possible occurrence of massive hemoptysis.
Introduction
Anomalous systemic arterial supply to the normal basal segments of the lower lobe (ASALL) is a kind of rare congenital abnormality. This entity typically contains an aberrant artery originating from the descending aorta and feeding the otherwise normal parenchyma of the lower lobe. The involved lung tissue has no anomaly in the tracheobronchial tree structure that differs from the conventional pulmonary sequestration (PS) (1). The basal segments of the left inferior lobe are mostly involved (2,3).
Although the radiological signs of this variant have been reported in brief (4-6) and most patients with this anomaly are asymptomatic, hemoptysis is still a complication that cannot be ignored. The risk assessment of hemoptysis in this variant has not been fully explored.
In our investigation, 43 patients with ASALL were retrospectively analyzed. We assessed the risk predictors for ASALL-induced hemoptysis and established a simple and practical scoring model that can facilitate clinicians in developing optimal treatment plans for this group of patients. We present this article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-738/rc).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Union Hospital Affiliated with Tongji Medical College of Huazhong University of Science and Technology (number/date: UHCT231000/Feb 2024) and individual consent for this retrospective analysis was waived. From January 2013 to December 2022, we retrospectively searched the electronic medical database in Union Hospital, Tongji Medical College, Huazhong University of Science and Technology to collect consecutive patients, who underwent multi-stage high-resolution enhanced lung computed tomography (CT) imaging, with systemic arterial supply to the lung confirmed by radiological data or surgical pathology (n=429). A total of 112 patients were obtained from the inclusion criteria below: (I) the branch from the aorta supplied the basal segments of the lower lobe; (II) no abnormalities were observed in the bronchial trees of the involved lung lobe. The exclusion criteria were as follows: (I) hypertrophied normal systemic arterial supply to the lung, for example, the enlarged bronchial arteries (n=27); (II) transpleural systemic arteries supplying the pulmonary parenchyma with pleural adhesion (n=32); (III) with other significant lung lesions or heart disease, such as severe infections, tumors, bronchiectasis, pulmonary arterial hypertension, etc. (n=10). Ultimately, the remaining 43 eligible patients were added to the study. A flow chart of patients included in the study population is shown in Figure 1. A thoracic radiological colleague (Y.F.Z.) with 5 years of experience was committed to clinical data gathering, case screening and collection, and study coordination.

Pulmonary CT image acquisition and reconstruction
All patients underwent pulmonary multi-phase contrast-enhanced CT examination in the supine position with breath holding on the following scanners: Aquillion ONE CT (Toshiba Healthcare, Tokyo, Japan) or Discovery CT750 HD (GE Healthcare, Boston, USA). The scanning protocol in our institution includes a non-contrast, arterial phase (AP), venous phase (VP), and delayed phase (DP) scan of the lung with the range from apex pulmonis to the adrenal level. After the non-contrast scan was acquired, a non-ionic iodine contrast agent (Iohexol, 370 mg I/mL, MERCK, Darmstadt, Germany) was administrated intravenously through the elbow vein using a high-pressure power injector (BAYER MEDRAD Vistron Plus, Leverkusen, Germany) at a standard of 1.5 mL/kg and a flow rate of 2.0–2.5 mL/s.
The contrast-enhanced CT scan took the form of a threshold trigger that set the trigger point to the descending aorta. The AP scan began when the value of the descending aorta reached 100 HU, and then the consecutive VP and the DP scans were performed at the time intervals of 20 seconds and 45 seconds respectively. The width and level of the lung window were 1,600 and −550 HU, respectively. The mediastinal window had a width of 330 HU and a level of 40 HU. The image reconstruction slice thickness was between 1.5 and 2.0 mm. All CT images were reviewed by two radiologists (H.S. and Q.Z.) with at least 5 years of expertise, who were blind to the clinical data of each patient but aware of the diagnosis of ASALL. When the observations diverged, a final discussion with a third radiologist culminated in a consensus. The diameter of the main pulmonary artery at the bifurcation level and the ratio of the pulmonary artery diameter to the ascending aorta diameter were estimated to detect the possible presence of pulmonary hypertension (7). The measurement index of the aberrant systemic artery was carried out on multiplanar reconstruction (MPR) pictures. To quantify the degree of abnormality [ground glass opacity (GGO), and septal thickening] in the lower lobe involved by the aberrant systemic artery, a semi-quantitative CT score (8) was used, based on the area involved in the affected lower lobe, as follows: 0, noninvolvement; 1, <5% involvement; 2, 5–25% involvement; 3, 26–49% involvement; 4, 50–75% involvement; and 5, >75% involvement, with potential total scores ranging from 0 to 5. The volume CT dose index (CTDIvol) and dose length product (DLP) values were recorded for each patient after the CT examination to determine their effective dose (ED). The ED was obtained by multiplying DLP with conversion coefficient k (9).
Angle, diameter, and distribution of the anomalous systemic artery
By indicating the angle along the path of blood flow on contrast-enhanced CT images at the original point of the abnormal artery, the angle between the aorta (or its branch) and the aberrant systemic artery was calculated. The diameter of the systemic artery was obtained at the site of its origin on MPR pictures by selecting the optimal level of cross-section. The angle and diameter were measured three times consecutively and their respective average values were obtained.
The distribution of abnormal arteries depends on the location of their origin. In this study, we roughly divided three locations: the inferior pulmonary vein opening level (middle thoracic aorta level), the lower segment of the thoracic aorta (inferior thoracic aorta level), and the abdominal aorta or its branches. The anomalous systemic arterial supply to the left pulmonary lower lobe was identified as the left side and vice versa.
Information about hemoptysis and treatment
The clinical information for those individuals with hemoptysis was recorded in detail. The volume of hemoptysis per time in each case, the annual frequency of hemoptysis, and the length of the hemoptysis course were all noted. In addition, all patients with or without hemoptysis received one of three protocols: conservative therapy, pulmonary lobectomy, and interventional embolization for the aberrant systemic artery.
Prediction for hemoptysis in ASALL
The prediction performance of hemoptysis was successively investigated using the abnormal systemic arterial features, radiological characteristics and demographic data, and the combination of radiological and clinical data.
Statistical analysis
Mean ± standard deviation (SD) was used to describe data that conformed to the normal distribution with continuity variables, while median and interquartile range (IQR) were used to show data that were not comparable to the normal distribution. The Mann-Whitney U test or the independent sample t-test was used to compare continuous variables. Categorical variables passed the Fisher Exact test or the Chi-squared test and were shown in frequency and percentage terms. The variable screening was conducted using single-factor analysis, and the predictors were assessed using the Wald test (test level, P<0.1). The likelihood of hemoptysis was evaluated using multiple logistic regression analysis. To determine the appropriate cutoff value, a receiver operating characteristic (ROC) curve analysis was employed. The threshold probability of the prediction model was probed using the decision curve analysis (DCA). The statistical analysis program employed SPSS (version 26.0, IBM Corporation, Armonk, NY, USA) and determined the statistical difference with an asymptotically double-tailed P value of less than 0.05.
Results
Demographic data
Forty-three suitable patients (seventeen women and 26 males), with an average age (± SD) of 43.35±19.49 years, ranging from 12 to 76 years, were subsequently included in this study.
Of the 43 cases, 17 were assigned to the hemoptysis group based on clinical symptoms (hemoptysis or not) and follow-up records, while the remaining 26 patients were assigned to the non-hemoptysis group. The clinical and demographic features in both groups are described detailedly in Table 1. Patients with hemoptysis had a younger average age than the non-hemoptysis group (P<0.001). Male patients made up the majority of the hemoptysis group (n=14, 82.35%; P=0.01), whereas the proportion of female patients in the non-hemoptysis group was a bit higher (n=14, 53.85%). No significant differences were found in any other characteristics, including chest pain and congestive heart failure (CHF). In addition, from the electronic medical record system, 40 of the 43 patients, who were not suspected or diagnosed with pulmonary hypertension, had a systolic and/or mean pulmonary artery pressure (sPAP, and/or mPAP) estimated by right heart catheterization (RHC, n=9) and/or Doppler echocardiography (D-ECHO, n=32). The median time interval between the CT scan and the onset of hemoptysis was 4 days (range, 2–6 days).
Table 1
| Variables | All patients | Hemoptysis | Non-hemoptysis | P value |
|---|---|---|---|---|
| Number | 43 (100.00) | 17 (39.53) | 26 (60.47) | NA |
| Clinical features | ||||
| Age (years) | 43.35±19.49 | 31.29±13.08 | 51.23±19.11 | <0.001 |
| Sex | 0.01 | |||
| Female | 17 (39.53) | 3 (17.65) | 14 (53.85) | |
| Male | 26 (60.47) | 14 (82.35) | 12 (46.15) | |
| Chest pain | 5 (11.63) | 1 (5.88) | 4 (15.38) | 0.63 |
| CHF | 1 (2.33) | 0 | 1 (3.85) | >0.99 |
| Radiological features | ||||
| Diameter (mm) | 6.21±3.05 | 7.68±2.91 | 5.25±2.79 | 0.009 |
| Angle (°) | 28.4 (15.6, 46.6) | 18.3 (11.4, 27.8) | 36.5 (26.2, 99.7) | 0.001 |
| Sides | 0.003 | |||
| Left | 33 (76.74) | 17 (100.00) | 16 (61.54) | |
| Right | 10 (23.26) | 0 | 10 (38.46) | |
| Distribution | 0.007 | |||
| Middle thoracic aorta† | 24 (55.81) | 15 (88.24) | 9 (34.62) | |
| Inferior thoracic aorta | 15 (34.88) | 2 (11.76) | 13 (50.00) | |
| Abdominal aorta | 4 (9.31) | 0 | 4 (15.38) | |
| Thrombosis or calcification | 11 (25.58) | 1 (5.88) | 10 (38.46) | 0.02 |
| GGO | 23 (53.49) | 13 (76.47) | 10 (38.46) | 0.01 |
| Septal thickening | 31 (72.10) | 16 (94.12) | 15 (57.69) | 0.01 |
| Draining veins | 0.003 | |||
| Left inferior vein | 33 (76.74) | 17 (100.00) | 16 (61.54) | |
| Right inferior vein | 10 (23.26) | 0 | 10 (38.46) | |
| Lower interlobar artery | 39 (90.70) | 14 (82.35) | 25 (96.15) | 0.28 |
P values are obtained by comparing the hemoptysis and non-hemoptysis groups with independent sample t-test, Mann-Whitney U-test, Chi-squared test, or Fisher Exact test used. Data are expressed as mean ± SD, n (%), or median (IQR). †, for the significant subgroup comparison (P=0.003) between middle thoracic aorta and inferior thoracic aorta level in the distribution of systemic artery with adjusted threshold to 0.016 by Bonferroni method. CHF, congestive heart failure; GGO, ground glass opacity; NA, not applicable; SD, standard deviation; IQR, interquartile range.
Interobserver agreements
With Cohen’s kappa values ranging from 0.688–0.937, the subjective radiological characteristics of CT images demonstrated good to excellent interobserver consistency (Table S1). With intra-class correlation coefficients (ICCs) values ranging from 0.734–0.989, the aberrant systemic arterial features measurement and CT scores for GGO and septal thickening demonstrated excellent interobserver agreements (Table S2).
Radiological metrics
In comparison to the non-hemoptysis group, the aberrant systemic arteries in the hemoptysis group had a larger diameter (P=0.009). Additionally, the hemoptysis group had a smaller angle between the abnormal artery and aorta than that of the non-hemoptysis group (P=0.001) (Table 1, Figures 2,3). All patients (17/17) in hemoptysis group were involved in the left lower lobe compared with cases (16/26) in the non-hemoptysis group (P=0.003). The distribution of the opening site of the abnormal systemic artery was different between the hemoptysis group and non-hemoptysis group (P=0.007) and the subgroup comparison between the middle thoracic aorta and the inferior thoracic aorta level was significantly different (P=0.003). In addition, the proportion of anomalous arteries with thrombosis or calcification was higher in the non-hemoptysis group (10/26) than in the hemoptysis group (1/17) (P=0.02).


Other radiological indicators of ASALL had been gathered in this investigation in addition to its iconic CT features. The ratio of GGO (76.47% vs. 38.46%, P=0.01) and interlobular septal thickening (94.12% vs. 57.69%, P=0.01) in the hemoptysis group was relatively higher as compared to the non-hemoptysis group. The radiological features (location, distribution, and CT scores) of GGO and interlobular septal thickening were summarized in detail in Table S3. The distribution (peri-systemic artery, peripheral, and diffuse) for GGO (P=0.03) and location (left lower lobe, and right lower lobe) for septal thickening (P=0.02) were significantly different between the hemoptysis group and non-hemoptysis group. CT scores for septal thickening were higher {median [IQR], 3 [3, 3] vs. 2 [2, 3], P=0.02} in the hemoptysis group than that in the non-hemoptysis group.
The left inferior pulmonary vein was severed as the 33 (76.74%) patients’ drainage vein, while the right inferior pulmonary vein was severed as the drainage vein of the remaining 10 (23.26%) cases, all of whom belonged to the non-hemoptysis group (P=0.003). There was no discernible difference in the presence of a lower interlobar artery distal to the origin of the dorsal segment artery between these two groups.
There were no obvious CT results that would have indicated pulmonary hypertension alterations. Each patient in this research had an average (± SD) main pulmonary artery diameter of 24.88±2.97 mm (range, 19.7–29.7 mm), and the mean (± SD) ratio of the pulmonary artery diameter to the ascending aortic diameter was 0.83±0.08 (range, 0.65–0.98).
Radiometric quantities
The average CTDIvol value in each phase of CT scanning was about 6.47±3.11 mGy (range, 1.47–18.92 mGy), while the mean DLP for the complete scanning protocol in each patient was 705.73±326.65 mGy·cm (range, 173.40–1,585.00 mGy·cm). The individual ED was about 11.99±5.55 mSv (range, 2.95–26.95 mSv).
About hemoptysis
Table 2 provides clinical details on hemoptysis in all patients (n=17). This information was obtained from the electronic medical records system (EMRS) in our hospital.
Table 2
| Events | Patients (n=17), n (%) |
|---|---|
| Amount of hemoptysis, per time | |
| <50 mL | 8 (47.1) |
| 50–100 mL | 6 (35.3) |
| >100 mL | 3 (17.6) |
| Frequency of hemoptysis, per year | |
| <3 | 5 (29.4) |
| 3–10 | 8 (47.1) |
| >10 | 4 (23.5) |
| Time of duration, years | |
| <1 | 9 (52.9) |
| 1–10 | 7 (41.2) |
| >10 | 1 (5.9) |
| Treatment | |
| Conservative treatment | 9 (52.9) |
| Interventional embolization | 2 (11.8) |
| Pulmonary lobectomy | 6 (35.3) |
There was a history of considerable hemoptysis (more than 100 mL, per time) in 3 (17.6%) cases, and there were 8 (47.1%) cases in which the volume of hemoptysis each time was less than 50 mL. In terms of the frequency of hemoptysis per year, 8 (47.1%) patients had about 3 to 10 times annually, 5 (29.4%) cases were less than 3 times, and 4 (23.5%) cases were greater than 10 times.
The course of hemoptysis within 1 year occupied more than half of the patients (n=9, 52.9%). Seven (41.2%) cases occurred between 1 and 10 years, and only 1 case (5.9%) was over 10 years.
Treatment for ASALL
Despite the hemoptysis, more than half of the patients (9 cases, 52.9%) in this group chose conservative treatment (Table 2). Six patients (35.3%) underwent surgical resection of the affected pulmonary lobe. In 2 cases (11.8%), the procedure of interventional embolization was performed. Treatment regiments in the non-hemoptysis group were recorded as follows: conservative (n=21, 80.8%), lobectomy (n=2, 7.7%), and interventional embolization (n=3, 11.5%).
Combined systemic arterial features: diameter and angle
Significant systemic indices were screened from Table 1 by single-factor analysis. Using maximally selected test statistics from the Survival Module of ClinicoPath (10,11), we determined the optimal cutoff points of the following two continuity variables for regression analysis, with a diameter of 5.42 mm and an angle of 24.5°.
To assess the effect of combined systemic arterial features (CD-A) on hemoptysis in patients with ASALL, the formula could be used to combine the following dichotomous variables: diameter (≥5.42 vs. <5.42, P=0.02) and angle (≤24.5° vs. >24.5°, P=0.001) using multivariate logistic regression analysis: CD-A = −2.860 + 2.075 × diameter + 2.949 × angle. CD-A showed predictive performance with an area under the curve (AUC) of 0.869 [95% confidence interval (CI): 0.744–0.993] (Figure 4), accuracy of 81.4% (35/43) (95% CI: 63.2–94.5%), sensitivity of 76.5% (13/17) (95% CI: 50.1–93.2%), specificity of 84.6% (22/26) (95% CI: 65.1–95.6%), positive predictive value (PPV) of 76.5% (13/17) (95% CI: 56.0–89.3%), negative predictive value (NPV) of 84.6% (22/26) (95% CI: 69.7–92.9%). The optimal cut-off value of the ROC curve analysis was identified as 0.522.

Combined demographic and radiographic data
By analyzing the cohort in this study and maximizing selection test statistics, we converted the age into a binary variable: ≤40 vs. >40 years.
Combined demographic and radiological features (CD-R; age, P=0.006; sex, P=0.01; GGO, P=0.01) using multivariate logical regression analysis could be achieved through the following formulas: CD-R = −6.205 + 3.478 × age + 3.028 × sex + 3.189 × GGO. CD-R revealed predictive performance with an AUC of 0.890 (95% CI: 0.798–0.983) as shown in Figure 4, accuracy of 79.1% (34/43) (95% CI: 61.2–87.6%), sensitivity of 94.1% (16/17) (95% CI: 71.3–99.9%), specificity of 69.2% (18/26) (95% CI: 48.2–85.7%), PPV of 66.7% (16/24) (95% CI: 52.6–78.3%), and NPV of 94.7% (18/19) (95% CI: 72.5–99.2%). The ROC curve analysis determined that 0.282 was the ideal cut-off value for CD-R.
Combined CD-A, demographic, and radiographic data
A multiple regression analysis combining demographic, radiological characteristics, and dichotomized CD-A (>0.522 vs. ≤0.522) was established by a forward likelihood ratio (LR) test method. Hemoptysis was demonstrated to be independently predicted by age [odds ratio (OR), 28.322, P=0.02], sex (OR, 35.233, P=0.02), GGO (OR, 41.698, P=0.01), and CD-A >0.522 (OR, 21.869, P=0.01). The forest plot of the multivariate logistic regression model is shown in the composite chart in Figure 5.

According to the regression coefficients, a linear regression equation was obtained as follows: 3.344 × age + 3.562 × sex + 3.730 × GGO + 3.085 × (CD-A >0.522).
We created a straightforward combined risk scoring (CRS) model for hemoptysis based on the weights assigned to every variable in the equation (Table 3). The following describes the way the risk rating model was expressed: CRS = 3 × age + 3 × sex + 4 × GGO + 3 × (CD-A >0.522).
Table 3
| Variables | Classification | Score |
|---|---|---|
| Age | >40 years | 0 |
| ≤40 years | 3 | |
| Sex | Female | 0 |
| Male | 3 | |
| GGO | Absent | 0 |
| Present | 4 | |
| CD-A | ≤0.522 | 0 |
| >0.522 | 3 |
CRS, combined risk scoring model; ASALL, anomalous systemic arterial supply to the normal basal segments of the lower lobe; GGO, ground glass opacity; CD-A, combined systemic arterial features.
The performance of this combined scoring system in predicting hemoptysis in patients is depicted in Figure 6A. The cut-off value of the ROC curve analysis was determined as 7. Thus, patients with a CRS score >7 were considered to have a high risk of hemoptysis, and a CRS score ≤7 was considered to be a low-risk score.

The CRS scoring system performed an AUC of 0.939 (95% CI: 0.871–0.989), accuracy of 88.4% (38/43) (95% CI: 67.2–94.3%), sensitivity of 76.5% (13/17) (95% CI: 50.1–93.2%), specificity of 96.2% (25/26) (95% CI: 80.4–99.9%), PPV of 92.9% (13/14) (95% CI: 65.1–98.9%), and NPV of 86.2% (25/29) (95% CI: 72.6–93.7%). The Hanley and McNeil test demonstrated that the CRS scoring system significantly outperformed the CD-A and CD-R in terms of prediction performance (P=0.046 and P=0.02, respectively). The Hosmer and Lemeshow test revealed no statistical significance (P=0.85), indicating that the scoring system had an adequate model fit.
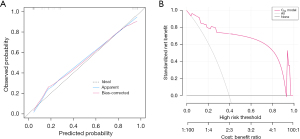
The predicted probability distribution between the hemoptysis group and the non-hemoptysis group with the CRS score model (ranging from 0 to13 points) was expressed by the violin diagram (Figure 6B). It was clear that the non-hemoptysis group’s estimated probability value generally fell less than 0.5, with the median and IQR of 0.024 (0.008–0.353). In contrast, the predicted probability values of the hemoptysis group were more evenly distributed and typically more than 0.5, with median 0.923 (IQR, 0.582–0.996). The calibration plot demonstrated an adequate level of prediction accuracy between the predicted and actual probabilities (Figure 7A). The DCA revealed that when the threshold probability was between 5% and 92% (Figure 7B), the CRS scoring model performed better in predicting hemoptysis in patients with ASALL than assuming high or low risk for all patients.
Discussion
ASALL is considered to be a rare congenital developmental anomaly in which the pulmonary parenchyma fed by the systemic artery has otherwise normal bronchial architecture. This aberrant systemic artery usually originates from the middle and distal thoracic aorta or the proximal abdominal aorta (12). The exact cause of this variance is unknown, but it may be attributed to the permanent existence of the connections between the post-branchial pulmonary plexus and the primitive dorsal aorta during the embryonic period (12,13). The inferior lobe of the lung, especially the basal segment of the left lower lobe, is most often affected (2,14). The pulmonary artery at the basal segments of the involved lower lobe may be normal or dysplastic, so the systemic arterial supply has the potential to function as both a supplement (15) and a substitute (16) for the normal pulmonary artery. The draining vein for ASALL is always the dilated inferior pulmonary vein on the same side (12).
The diverse origin position of the aberrant systemic artery may confine its shape and course. The artery tends to be fairly straight within the pulmonary ligament, but it becomes twisted as it enters the lung tissue. According to histological reviews (5,6,17), this aberrant arterial wall is elastic, resembling the pulmonary artery rather than the muscular systemic artery. Prolonged arterial pressure leads to degenerative conditions in these anomalous systemic arteries, such as thrombosis and calcification (6,18), or atheromatous involvement (19). Eleven participants (25.58%) in this study had degenerative changes in their systemic arteries, and the majority (10 cases, 38.46%) belonged to the non-hemoptysis group. Surgical hazards may arise from aberrant systemic arteries given the potential for massive hemorrhage (17). In addition to serving as a guide for interventional therapy of the condition, the framework based on enhanced CT assessment of the features of the aberrant systemic artery in ASALL can assist in lowering the risk of potential substantial hemorrhage during thoracic surgery. The optimal scanning protocol minimizes the radiation exposure from CT scanning while ensuring image quality.
Although previously classified into the broad spectrum of PS, named Pryce type I sequestration, ASALL is markedly different from conventional PS in many aspects (4,20,21). Together with sequestration, ASALL has a common denominator, namely the anomalous systemic artery supplying the lung tissue. However, the lung tissue involved in sequestration often lacks normal communication with the tracheobronchial tree, and the internal bronchus is not completely developed, so it is prone to repeated infection, and even cystic degeneration (3,22). In comparison, the pulmonary parenchyma affected in ASALL has a normal bronchial tree. Recurrent hemoptysis is more common in ASALL than PS due to the higher arterial pressure of the donor systemic artery and the potential arteriovenous connections of the primitive vascular plexus between the terminal branches of the aberrant systemic artery and the draining pulmonary veins (19,23).
In addition to recurrent hemoptysis, ASALL may also present with non-specific symptoms such as chest pain, cough, and sputum (4,24), but most adults may be asymptomatic, while pediatric patients may encounter a clear continuous heart murmur during the back auscultation (12,14), or CHF after birth (1,25). Bruwer et al. (17) reported that the onset of symptoms of this anomaly was usually within the first two decades of life. Jiang et al. (26) reported thirteen ASALL patients with hemoptysis whose mean age (± SD) was about 32.7±11.2 years. The average age of the hemoptysis patients in the present study was about 31 years old (31.29±13.08 years), but the occurrence of non-specific symptoms such as chest pain was relatively earlier, thus it was roughly similar to the literature reports. At the same time, the risk of hemoptysis was higher in patients equal to or under 40 years old than in those over 40, according to a multiple logistic regression analysis of the CD-R in this study. Age is therefore an important consideration in people with ASALL. The appearance of hemoptysis at an earlier age reminds clinicians to take timely intervention measures to alleviate the occurrence of recurrent hemoptysis. Finally, this study demonstrated that male patients were predominant (about 82.35%) in the hemoptysis group, which is consistent with the previous views reported in the literature (27,28). The male-dominated features imply that hemoptysis may be associated with lifestyle choices, extent of activity, and even genetics, which have not been clarified to date and will be investigated in more detail in future studies.
One of the primary causes of hemoptysis in ASALL patients is the diameter of the aberrant artery. The diameter of the systemic artery was greater in the hemoptysis group than in the non-hemoptysis group in this study, and the overall mean diameter of anomalous vessels was about 6.21 mm (6.21±3.05 mm), which was comparable with the diameter range (2 to 10 mm) mentioned in the literature (17,29). Furthermore, the angle between the systemic artery and the aorta appeared to contribute to the occurrence of hemoptysis, which has rarely been mentioned in previous reports (30,31). In this study, patients with hemoptysis had a median angle of 18.3° (IQR, 11.4–27.8°), compared to those without hemoptysis who had a median angle of 36.5° (IQR, 26.2–99.7°). The cutoff values for diameter and angle were 5.42 mm and 24.5°, respectively. When the above two indicators were combined to predict hemoptysis, the AUC value from the ROC curve analysis was 0.869 (95% CI: 0.744–0.993). The greater systemic arterial diameter and narrower angle between the systemic artery and the aorta may confine the vortical flow close to its opening site and decrease the wall shear stress (31-33), resulting in hemodynamic alterations and raising blood flow in the aberrant systemic artery. This, in turn, may exacerbate the extent of pulmonary congestion. Thus, in ASALL patients, we speculate that the larger the diameter and smaller the angle of the aberrant systemic artery to the aorta, the greater the probability of hemoptysis.
GGO is another important factor of hemoptysis in patients with ASALL. In this study, GGO was relatively more common in the hemoptysis group than in the non-hemoptysis group (P=0.01). GGO is a manifestation of moderate pulmonary congestion caused by high abnormal systemic arterial pressure (1,2). The appearance of GGO is associated with intra-alveolar hemorrhage and pigmented macrophage aggregation (5,19). Persistent regional GGO may contribute to recurrent hemoptysis. Nearly half of the patients (47.1%) in the hemoptysis group recorded in the present study had repeated the condition 3 to 10 times annually and 13 of the 17 hemoptysis patients had GGO signs. In the multiple regression analysis with combined CD-A, demographic, and radiographic data, GGO was an important independent risk factor for predicting hemoptysis. In addition, due to the inclusion of the variable GGO, the CRS scoring model established in this study was superior to CD-A, and the weight value of GGO in the CRS scoring model was higher than that in the CD-R regression formula.
The common causes of smooth thickening of the interlobular septum are venous and lymphatic disorders, particularly pulmonary edema or hemorrhage (34,35). In ASALL, the high abnormal systemic arterial pressure may develop interlobular septal thickening as a result of compensatory thickening of the drained pulmonary venous branches and fluid accumulation (34) in the pulmonary interstitial. The hemoptysis group in this study showed significantly more frequencies and higher CT scores for septal thickening than the non-hemoptysis group. This implied that the extent of the lobular septal thickening was slightly higher in hemoptysis patients. Whereas the follow-up CT scans after interventional embolization of the abnormal systemic arteries show the normalized parenchymal density of the involved lower lobe and decreased diameter of the initially dilated inferior pulmonary veins (18,26), suggesting that the thickened interlobular septa of the involved lower lobe may be reversible. As this parameter was significant in single-factor analysis but not in multivariate logistic regression analysis (Table S4), there may be an indirect association between interlobular septal thickening and hemoptysis.
The CRS scoring model included age, sex, GGO, and a combination of diameter and angle (CD-A >0.522), which were obtained from clinical and radiological data. All four of these variables are quantified by this scoring system to determine a patient’s risk score for hemoptysis. The scoring values in this risk-scoring model ranged from 0 to 13 points. ROC curve analysis revealed a cut-off value of seven points. Therefore, non-conservative treatment such as surgical lobectomy or interventional therapy could be considered when the ASALL score is greater than 7, as this indicates a reasonably high risk of hemoptysis. When the score value is 7 or less, the possibility of hemoptysis is relatively low. This helps physicians establish a more realistic treatment plan by estimating the probability of hemoptysis in ASALL patients. For the patients in this investigation, the scoring model had an accurate classification percentage of roughly 88.4% and showed trustable predictive performance for hemoptysis assessment.
The research has a few limitations. Firstly, only a small number of patients were included in this retrospective analysis due to the very rare nature of ASALL as a developmental anomaly. For the time being, some parameters that did not show a statistically significant difference between the hemoptysis group and the non-hemoptysis group could not fully reflect the absence of difference between these two groups. Larger sample size follow-up investigations are needed. Secondly, some patients may not receive an exact diagnosis or get a diagnosis of lung sequestration due to variations in the recognition of ASALL, thus they were unwittingly left out. Thirdly, GGO may not always remain in the lungs of ASALL patients, and GGO findings may not be visible on CT images during the hemoptysis interval. This may have an impact on the assessment of the risk of hemoptysis in this kind of patient.
Conclusions
ASALL is a rather uncommon abnormality in lung development. Clinical signs and radiological characteristics differ between ASALL patients with and without hemoptysis. The scoring model CRS, based on clinical and radiological features, can well predict the risk of hemoptysis in ASALL patients, which assists clinicians evaluate the condition of these patients and formulate an optimal treatment plan.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-738/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-738/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-738/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-738/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Union Hospital Affiliated with Tongji Medical College of Huazhong University of Science and Technology (number/date: UHCT231000/Feb 2024) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim TS, Lee KS, Im JG, et al. Systemic arterial supply to the normal basal segments of the left lower lobe: radiographic and CT findings in 11 patients. J Thorac Imaging 2002;17:34-9. [Crossref] [PubMed]
- Miyake H, Hori Y, Takeoka H, et al. Systemic arterial supply to normal basal segments of the left lung: characteristic features on chest radiography and CT. AJR Am J Roentgenol 1998;171:387-92. [Crossref] [PubMed]
- Do KH, Goo JM, Im JG, et al. Systemic arterial supply to the lungs in adults: spiral CT findings. Radiographics 2001;21:387-402. [Crossref] [PubMed]
- Qin J, Huang SH, Yan RH, et al. CT findings of anomalous systemic artery to the left lower lobe: comparison with pulmonary sequestration in the left lower lobe. Clin Radiol 2014;69:e485-90. [Crossref] [PubMed]
- Makino T, Hata Y, Otsuka H, et al. Simultaneous resection of bilateral anomalous systemic supply to the basal segments of the lungs: a case report. J Cardiothorac Surg 2015;10:140. [Crossref] [PubMed]
- Utsumi T, Hino H, Kuwauchi S, et al. Anomalous systemic arterial supply to the basal segment of the lung with giant aberrant artery: a case report. Surg Case Rep 2020;6:285. [Crossref] [PubMed]
- Ng CS, Wells AU, Padley SP. A CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameter. J Thorac Imaging 1999;14:270-8. [Crossref] [PubMed]
- Chang YC, Yu CJ, Chang SC, et al. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT. Radiology 2005;236:1067-75. [Crossref] [PubMed]
- European Commission. European guidelines on quality criteria for computed tomography, EUR 16262EN (M/OL). Luxembourg: Office for Official Publications of the European Communities. 2000. Available online: https://www.drs.dk/guidelines/ct/quality/mainindex.htm. Accessed 12 January 2011.
- Assareh H, Smith I, Mengersen K. Change point detection in risk adjusted control charts. Stat Methods Med Res 2015;24:747-68. [Crossref] [PubMed]
- Ellenberger D, Lausen B, Friede T. Exact change point detection with improved power in small-sample binomial sequences. Biom J 2021;63:558-74. [Crossref] [PubMed]
- Currarino G, Willis K, Miller W. Congenital fistula between an aberrant systemic artery and a pulmonary vein without sequestration. A report of three cases. J Pediatr 1975;87:554-7. [Crossref] [PubMed]
- Painter RL, Billig DM, Epstein I. Anomalous systemic arterialization of the lung without sequestration. N Engl J Med 1968;279:866-7. [Crossref] [PubMed]
- Kurosaki Y, Kurosaki A, Irimoto M, et al. Systemic arterial supply to normal basal segments of left lower lobe: CT findings. J Comput Assist Tomogr 1993;17:857-61. [Crossref] [PubMed]
- Cole FH, Alley FH, Jones RS. Aberrant systemic arteries to the lower lung. Surg Gynecol Obstet 1951;93:589-96. [PubMed]
- Hessel EA 2nd, Boyden EA, Stamm SJ, et al. High systemic origin of the sole artery to the basal segments of the left lung: findings, surgical treatment, and embryologic interpretation. Surgery 1970;67:624-32. [PubMed]
- Bruwer A, Clagett OT, McDonald JR. Anomalous arteries to the lung associated with congenital pulmonary abnormality. J Thorac Surg 1950;19:957-72. [Crossref] [PubMed]
- Chabbert V, Doussau-Thuron S, Otal P, et al. Endovascular treatment of aberrant systemic arterial supply to normal basilar segments of the right lower lobe: case report and review of the literature. Cardiovasc Intervent Radiol 2002;25:212-5. [Crossref] [PubMed]
- Campbell DC Jr, Murney JA, Dominy DE. Systemic arterial blood supply to a normal lung. JAMA 1962;182:497-9. [Crossref] [PubMed]
- Pryce DM. Lower accessory pulmonary artery with intralobar sequestration of lung; a report of seven cases. J Pathol Bacteriol 1946;58:457-67. [Crossref] [PubMed]
- Sade RM, Clouse M, Ellis FH Jr. The spectrum of pulmonary sequestration. Ann Thorac Surg 1974;18:644-58. [Crossref] [PubMed]
- Stocker JT. Sequestrations of the lung. Semin Diagn Pathol 1986;3:106-21. [PubMed]
- Varma KK, Clarke CP. Congenital systemic-to-pulmonary arteriovenous fistula: report of a case. Aust N Z J Surg 1971;40:360-2. [Crossref] [PubMed]
- Baek WK, Cho J, Kim JT, et al. Systemic arterial supply to normal basal segments of the left lower lobe along with the pulmonary artery: is lung resection warranted? J Thorac Cardiovasc Surg 2006;131:742-3. [Crossref] [PubMed]
- Ellis K. Fleischner lecture. Developmental abnormalities in the systemic blood supply to the lungs. AJR Am J Roentgenol 1991;156:669-79. [Crossref] [PubMed]
- Jiang S, Yu D, Jie B. Transarterial Embolization of Anomalous Systemic Arterial Supply to Normal Basal Segments of the Lung. Cardiovasc Intervent Radiol 2016;39:1256-65. [Crossref] [PubMed]
- Yamanaka A, Hirai T, Fujimoto T, et al. Anomalous systemic arterial supply to normal basal segments of the left lower lobe. Ann Thorac Surg 1999;68:332-8. [Crossref] [PubMed]
- Iijima Y, Ishikawa M, Iwai S, et al. Role of indocyanine green in anomalous arterial supply to the normal dorsobasal segment of the lung. J Cardiothorac Surg 2022;17:52. [Crossref] [PubMed]
- Inoue S, Fujino K, Tezuka N, et al. A case report of anomalous systemic arterial supply to the left basal lung. Nihon Kyobu Geka Gakkai Zasshi. 1997;45:1195-202. [PubMed]
- Park YJ, Park CW, Park KB, et al. Inference from clinical and fluid dynamic studies about underlying cause of spontaneous isolated superior mesenteric artery dissection. J Vasc Surg 2011;53:80-6. [Crossref] [PubMed]
- Wu Z, Yi J, Xu H, et al. The Significance of the Angle between Superior Mesenteric Artery and Aorta in Spontaneous Isolated Superior Mesenteric Artery Dissection. Ann Vasc Surg 2017;45:117-26. [Crossref] [PubMed]
- Tanweer O, Wilson TA, Metaxa E, et al. A comparative review of the hemodynamics and pathogenesis of cerebral and abdominal aortic aneurysms: lessons to learn from each other. J Cerebrovasc Endovasc Neurosurg 2014;16:335-49. [Crossref] [PubMed]
- Arnaz A, Pişkin Ş, Oğuz GN, et al. Effect of modified Blalock-Taussig shunt anastomosis angle and pulmonary artery diameter on pulmonary flow. Anatol J Cardiol 2018;20:2-8. [Crossref] [PubMed]
- Franquet T, Müller NL, Lee KS, et al. High-resolution CT and pathologic findings of noninfectious pulmonary complications after hematopoietic stem cell transplantation. AJR Am J Roentgenol 2005;184:629-37. [Crossref] [PubMed]
- Webb WR. Thin-section CT of the secondary pulmonary lobule: anatomy and the image--the 2004 Fleischner lecture. Radiology 2006;239:322-38. [Crossref] [PubMed]


