Patient evaluation for rapid pleurodesis of malignant pleural effusions
Introduction
An estimated 150,000 people experience malignant pleural effusions (MPEs) yearly in the United States (1). MPEs cause discomfort secondary to dyspnea, chest pain, and cough (2,3). Treatment options include recurrent thoracenteses, chemical pleurodesis, and placement of an indwelling tunneled pleural catheter (TPC). Each modality has both advantages and disadvantages for different patient groups. Repeat thoracenteses are generally not recommended as nearly all MPEs will recur within four weeks even on treatment for their malignancy (4). A disadvantage of pleurodesis is that it often necessitates a 5- to 7-day hospitalization (5,6). While placement of TPCs alone have been found to cause spontaneous pleurodesis after a median of 56 days (7,8), there is an inherent infectious risk with TPCs as well as the need for assistance with home drainage (9-11).
A rapid pleurodesis procedure, using the combination of talc pleurodesis with TPC insertion at the same procedure takes advantages of both management strategies and minimizes their disadvantages. The combined rapid pleurodesis procedure with thoracoscopic delivered talc and TPC placement has previously been demonstrated in a pilot study to decrease hospital length of stay, and duration of TPC use measured by time to pleurodesis while significantly improving dyspnea and quality of life in patients with MPE (12). Similarly, the procedure requires one overnight admission and daily TPC drainage (12). Post-operative pain control may be achieved with oral opioids as an outpatient (13). The aim of the study was to evaluate institutional procedural effectiveness with the previously described rapid pleurodesis protocol to optimize future patient selection.
Methods
This study was approved by the Johns Hopkins institutional review board (IRB00033619). A retrospective chart review of patients who had undergone the rapid pleurodesis protocol between February 1, 2011 and June 30, 2014 at Johns Hopkins University and University of Utah Medical Center was performed. Medical thoracoscopy with rapid pleurodesis protocol was performed as previously described by Reddy et al. (12). Records from all patients who were referred to the pleural disease services for palliative intervention for recurrent MPE and underwent the rapid pleurodesis procedure were reviewed chest imaging studies and pleural manometry findings were reviewed and patients with evidence of a non-expandable lung were excluded.
All procedures were performed by an interventional pulmonologist with experience in thoracoscopy/TPC placement. A single 8 or 10 mm port was utilized. Pleural fluid was evacuated and the space visually inspected. Pleural biopsies were performed at the discretion of the interventional pulmonologist. TPC (PleurX; Care Fusion; McGaw Park, Illinois or Rocket; Rocket Medical; Hingham, MA) placement was then performed under direct visualization followed by insufflation of 4 to 5 grams of commercially available sterile talc (Figure 1). Depending on physician preference, a 19–24 French chest tube was placed through the site of the port to ensure continued drainage of the pleural space. The chest tube was placed to −20 cm H2O suction. The TPC was placed to −20 cm H2O suction rather than subsequent chest tube placement in one patient. Per protocol, an overnight admission with continuous chest tube drainage and pain management was required. Patients were then discharged with the TPC in place and instructions for daily home drainage. Once catheter output was deemed to be minimal, which was consistently less than 150 mL per day at home, patients contacted the physician to arrange for TPC removal. Pleural ultrasound to evaluate for residual effusion and/or radiographic imaging prior to TPC removal was at the discretion of the physician.
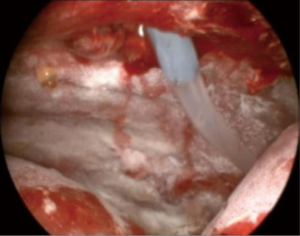
Electronic medical records were evaluated for patient demographics, procedural method, immediate and long-term complications, radiographic and symptomatic improvement, recurrence of pleural effusions with need for subsequent management, and death. The primary outcome was successful pleurodesis as defined by minimal pleural fluid output and subsequent ability to remove the TPC.
Statistical analysis
We performed a simple regression analysis of patient who underwent the rapid pleurodesis protocol to separate by malignancy. Mean, median, standard deviation, and interquartile ranges were calculated for continuous variables. Categorical variables were expressed as percentages (Excel, Microsoft Corporation, Redmond, WA, USA). Wilcoxon-Mann-Whitney test for non-normally distributed continuous variables was performed with SAS software, version 9.3 (SAS Institute, Cary, NC, USA). P value less than 0.05 was deemed significant.
Results
Twenty-nine patients underwent medical thoracoscopy with concomitant pleurodesis of recurrent symptomatic MPEs. Demographic variables are noted in Table 1. The mean age was 62.4 [standard deviation (SD) 9.6] years and 34% were male. Breast cancer was the most common malignancy, present in 10 (34%) patients. Primary lung cancer was the second most common malignancy, present in 7 (24%) patients. Sixty-six percent of patients were actively or recently receiving concomitant chemotherapy at the time of the procedure.
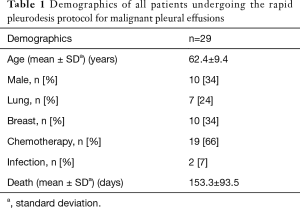
Full table
Insufflation of talc was performed in all patients. Chest tubes placed during the procedure were removed after a median of 25.5 [interquartile range (IQR) 23–29] hours. Median chest tube size was 20F. Median hospitalization was 2 (IQR 1–3) days. Patients with lung cancer had a shorter hospitalization (Table 2). One patient was hospitalized for 16 days due to post-procedural acute lung injury, likely related to talc.
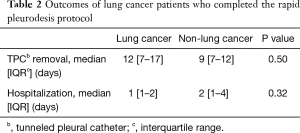
Full table
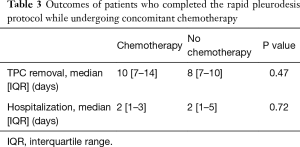
Median time to TPC drainage less than 150 mL per day was 7 (IQR 6–10) days. Once patients informed the physician of decreased drainage and presented for evaluation, TPCs were removed at a median of 10 (IQR 7–14) days. Patients with lung cancer maintained the TPC a median of 3 days longer than patients with other malignancies (Figure 2). Median duration to TPC removal amongst lung and breast cancer patients was 11 (IQR 8–16) days. Patients actively or recently receiving chemotherapy at the time of the procedure had the TPC removed at a median of 10 (IQR 7–14) days (Table 3). Two (7%) patients, one with lymphoma and one with breast cancer, had persistent pleural fluid drainage prohibiting TPC removal. TPCs were present and functional in both patients at the time of death after 88–89 days. Due to the continued presence of the TPC, the two patients were not included in the calculation of time to TPC removal.
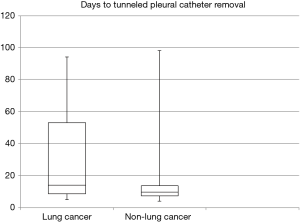
Full table
Fourteen (48%) patients were alive as of the last known hospital record. Median time to death was 102 days in the remaining 15 patients. The primary cause of death was progression of malignancy. No deaths were attributed to the procedure.
No complications were noted during the procedure. A total of 5 (17%) patients experienced any post-procedural complication. Three patients experienced two or more complications. Complications included an infection related to the TPC in 2 of 29 patients (7%). One patient necessitated hospital readmission due to pneumonitis and the other was treated for empyema as an outpatient. Both patients improved with antibiotic therapy. Three patients (10%) experienced post-procedural fever, one of which was also noted to clog the TPC and one whom also had acute lung injury from the talc. The TPC clogged in two patients after a median of 11 (IQR 10–13) days as demonstrated by the continued presence of pleural fluid on ultrasound. It was cleared with tissue plasminogen activator (tPA) in one patient and saline in a non-protocol based manner that did not require removal or replacement. One of the patients in whom the catheter clogged also experienced the previously mentioned empyema. Four (14%) patients experienced recurrent pleural effusions after removal of the TPC, 2 of which necessitated a repeat thoracentesis and 2 repeat TPC placement.
Discussion
Simultaneous insufflation of talc into the pleural cavity via thoracoscopy with concomitant placement of a TPC has previously been described in a pilot study to be feasible and effective (12). Shorter durations of hospitalization and faster pleurodesis allowing for quicker removal of TPCs were described, which were an improvement over the previously published findings for talc pleurodesis and placement of indwelling TPCs alone (7,8,12). Our retrospective review of the institutional experience at two academic centers corroborates these findings and provides data on procedural effectiveness to optimize patient selection. It also highlights minor differences in outcomes in subjects with primary lung carcinoma and those receiving chemotherapy.
Our review indicates a successful pleurodesis rate of 93% as determined by ability to remove the indwelling TPC. Pleural effusions recurred in four patients, decreasing the overall success rate to 79%. Despite the recurrence in a small proportion of patients, the rapid pleurodesis procedure remains an improvement over the 4-week response rates of 48–80% seen with pleurodesis with tube thoracostomy alone (12-15). Trapped lung should be excluded prior to the procedure as those patients would benefit from TPC alone.
In our study, MPEs were most commonly caused by primary lung or metastatic breast cancer in 61% of patients, which is similar to previously published reports (4). A median hospitalization of 2 days with the rapid pleurodesis protocol is shorter than the 6 to 7 days needed for chemical pleurodesis (5,6,12). The median duration to TPC removal of 10 days in our study is slightly longer than that of 7.54 days in the pilot study but is significantly shorter than in studies where a TPC was used alone (range 50–60 days) (5,8). This may be due to patient and/or physician scheduling conflicts, delaying removal. However, it may also be indicative of pleurodesis occurring after a longer time period, as demonstrated amongst lung or breast cancer patients where median time to TPC removal was 11 days. Despite a longer time to pleurodesis in our study, talc insufflation via thoracoscopy has previously been demonstrated to be highly successful amongst lung or breast cancer patients (16). Evaluation of a subset of lung and breast cancer patients as well as those undergoing chemotherapy at the time of the procedure are unique to this study. Similar to lung or breast cancer, patients actively or recently undergoing chemotherapy also had prolonged time to removal of the TPC (Table 3). While the exact mechanism is unknown, administration of anti-inflammatory medications, such as corticosteroids, with chemotherapy may be a contributing factor delaying pleurodesis.
Our study utilized a median chest tube size of 20F to drain the pleural space and allow lung expansion without any significant episodes of tube occlusion from talc or fibrin debris. This suggests that smaller diameter chest tubes were effective in our cohort. The standard chest tube used in the pilot study was slightly larger at 24F. Smaller chest tube size has previously been shown to be effective in managing pleural effusions, empyema, pneumothoraces, and for instillation of talc (17-19). In a recent study there were higher rates of pleurodesis failure with 12F Chest tubes compared to 24F (20). Future placement of a chest tube during the procedure may not be necessary as post-procedural drainage of the pleural space has been accomplished with the TPC alone which is 15.5F in size (21).
Severe post-procedural complications were minimal, but should not be taken lightly. The most severe was 1 episode of post-procedural acute lung injury, attributable to talc. Talc particle size was unknown, but sterile talc commercially available in the United States was used. Complications following intrapleural administration of talc are not uncommon. Fever, chest pain, and less commonly lung injury have been noted (20,22,23). Severe delayed complications were uncommon as evidenced by only 1 infectious complication necessitating repeat hospitalization. As the infectious complications were noted in two patients, both of whom recently received chemotherapy within the past month, continued immunosuppression may have been a contributing factor. Severe TPC infections from other multi-center studies have described severe complications including death (7.7–11.5% severe infection, 0–2.2% mortality) (18,24).
Consideration should be given to the potential added risk of the addition of a TPC even if it is for 1–2 weeks.
The major limitations of this study are the intrinsic issues with a retrospective study. In addition, studies examining MPEs often have a poor completion rate due to the limited life expectancy and palliative management of the MPE. Given that only 29 procedures were performed over 40 months due to patient preference, it would take additional centers and a longer duration to accrue a significant sample size. This should be considered given the potential benefits. In addition, the heterogenous population with MPEs makes control matching difficult in a case control format. As further data supporting the rapid pleurodesis protocol become available, we expect randomized trials to provide additional insight. Other pertinent factors such as procedural cost should be considered but were beyond the scope of this study. Other investigators have examined cost and found variability primarily due to the length of TPC usage and its associated cost of supplies (25).
Conclusions
The rapid pleurodesis protocol should be considered a viable treatment option for carefully selected patients with symptomatic recurrent MPEs undergoing chemical pleurodesis.
Acknowledgements
None.
Footnote
Conflicts of Interest: D Feller-Kopman and C Reddy are consultants for Carefusion (McGaw Park, Illinois). The other authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Johns Hopkins institutional review board (IRB00033619).
References
- Light RW. Pleural diseases. Philadelphia: Lippincott Williams & Wilkins, 2001.
- Martínez-Moragón E, Aparicio J, Sanchis J, et al. Malignant pleural effusion: prognostic factors for survival and response to chemical pleurodesis in a series of 120 cases. Respiration 1998;65:108-13. [Crossref] [PubMed]
- Chernow B, Sahn SA. Carcinomatous involvement of the pleura: an analysis of 96 patients. Am J Med 1977;63:695-702. [Crossref] [PubMed]
- Antunes G, Neville E, Duffy J, et al. BTS guidelines for the management of malignant pleural effusions. Thorax 2003;58 Suppl 2:ii29-38. [Crossref] [PubMed]
- Putnam JB Jr, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer 1999;86:1992-9. [Crossref] [PubMed]
- Putnam JB Jr, Walsh GL, Swisher SG, et al. Outpatient management of malignant pleural effusion by a chronic indwelling pleural catheter. Ann Thorac Surg 2000;69:369-75. [Crossref] [PubMed]
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006;129:362-8. [Crossref] [PubMed]
- Shaw P, Agarwal R. Pleurodesis for malignant pleural effusions. Cochrane Database Syst Rev 2004.CD002916. [PubMed]
- Musani AI, Haas AR, Seijo L, et al. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration 2004;71:559-66. [Crossref] [PubMed]
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011;26:70-6. [Crossref] [PubMed]
- Fysh ET, Tremblay A, Feller-Kopman D, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest 2013;144:1597-602. [Crossref] [PubMed]
- Reddy C, Ernst A, Lamb C, et al. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011;139:1419-23. [Crossref] [PubMed]
- Spiegler PA, Hurewitz AN, Groth ML. Rapid pleurodesis for malignant pleural effusions. Chest 2003;123:1895-8. [Crossref] [PubMed]
- Villanueva AG, Gray AW Jr, Shahian DM, et al. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax 1994;49:23-5. [Crossref] [PubMed]
- Yildirim E, Dural K, Yazkan R, et al. Rapid pleurodesis in symptomatic malignant pleural effusion. Eur J Cardiothorac Surg 2005;27:19-22. [Crossref] [PubMed]
- Dresler CM, Olak J, Herndon JE 2nd, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005;127:909-15. [Crossref] [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518-26. [Crossref] [PubMed]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [Crossref] [PubMed]
- Fysh ET, Smith NA, Lee YC. Optimal chest drain size: the rise of the small-bore pleural catheter. Semin Respir Crit Care Med 2010;31:760-8. [Crossref] [PubMed]
- Rahman NM, Pepperell J, Rehal S, et al. Effect of Opioids vs NSAIDs and Larger vs Smaller Chest Tube Size on Pain Control and Pleurodesis Efficacy Among Patients With Malignant Pleural Effusion: The TIME1 Randomized Clinical Trial. JAMA 2015;314:2641-53. [Crossref] [PubMed]
- Folch E, Santacruz JF. Rapid pleurodesis: an outpatient alternative. Chest 2011;140:1665-6. [Crossref] [PubMed]
- Stefani A, Natali P, Casali C, et al. Talc poudrage versus talc slurry in the treatment of malignant pleural effusion. A prospective comparative study. Eur J Cardiothorac Surg 2006;30:827-32. [Crossref] [PubMed]
- Gonzalez AV, Bezwada V, Beamis JF Jr, et al. Lung injury following thoracoscopic talc insufflation: experience of a single North American center. Chest 2010;137:1375-81. [Crossref] [PubMed]
- Gilbert CR, Lee HJ, Skalski JH, et al. The Use of Indwelling Tunneled Pleural Catheters for Recurrent Pleural Effusions in Patients With Hematologic Malignancies: A Multicenter Study. Chest 2015;148:752-8. [Crossref] [PubMed]
- Shafiq M, Frick KD, Lee H, et al. Management of Malignant Pleural Effusion: A Cost-Utility Analysis. J Bronchology Interv Pulmonol 2015;22:215-25. [Crossref] [PubMed]


