Video-assisted thoracoscopic lobectomy: which is the learning curve of an experienced consultant?
Introduction
Anatomic pulmonary lobectomy with systematic node dissection is the gold standard of surgical treatment for early stage lung cancer; anatomic lobectomy is also a common way to treat various malformations, or infectious and inflammatory diseases. During the past 2 decades, video-assisted thoracoscopic surgery (VATS) techniques have progressively emerged and tend to substitute to formal thoracotomy. There is increasing evidence that VATS offers several advantages compared to thoracotomy. Owing to a decreased operative trauma, post-operative pain is reduced, postoperative pulmonary function is less debilitated and recovers faster. As a consequence, the global postoperative morbidity, and in particular pulmonary complications, is reduced. Most reports conclude to a shorter drainage time and a shorter hospital stay, and to an improved postoperative quality of life. Biologic investigation demonstrated a lower serum level of inflammatory cytokines (1). On socioeconomical grounds, VATS appears to be less expensive than thoracotomy (2-6). The oncologic safety of VATS has been proven by meta-analysis: global survival and disease-free survival are at least equal (5-7). Putting together all these data, it appears unethical to proceed with thoracotomy in a patient who would be eligible for a VATS lobectomy.
Despite the proven superiority of VATS lobectomy, and a general trend to substitute thoracotomy by a minimally invasive approach, there is still a considerable amount of patients, eligible for a VATS approach, which undergoes a classic thoracotomy. According to STS database, 35% of lobectomies was performed by VATS during the most recent period (8). Another report of Danish cancer registry, refers that 53% of patients with clinical stage I NSCLC where operated by VATS, and the remaining part being managed by thoracotomy (9). In the real life, VATS lobectomy still represents a real challenge for many surgeons, especially those having been trained before the exponential development of VATS. For these experienced colleagues, VATS approach seems to be complex, lengthy, and potentially hazardous. With the thread of legal consequences, many of them prefer thoracotomy for intraoperative safety reasons. Further, time consuming all-day practice discourages from the effort to learn a new technique, despite the many educational events organized by scientific societies or industry. Finally, a low volume of activity is felt to be a hurdle across the learning curve.
The necessary time and number of procedures to acquire competence in this innovative approach for a young surgeon in training has been discussed in different reports (10-14). Learning tools such as observation of live surgery, surgical videos, and different types of simulation consisting of black-box training, wet lab or 3-D tailor made virtual lung are effective in the learning process, but time consuming and essentially designed for surgical trainees (15-18).
Finally, there is little information available on the learning process of an experienced consultant thoracic surgeon. This study analyses the initial VATS activity of a single confirmed consultant thoracic surgeon who started a VATS lobectomy program in January 2012, after more than 25 years of experience with open procedures. The aim of this study was to describe the individual learning curve and to identify how many procedures where required to obtain competence and efficiency.
Methods
Patients
We have evaluated the initial experience (January 2012 to September 2014) with VATS lobectomy of a consultant experienced in open surgery and in minor VATS procedures, who performed 145 anatomic VATS resections. After exclusion of bi-lobectomies (2 operations), segmentectomies (12 operations) and lobectomies for infectious diseases (12 operations), we have focused on 119 patients. These represented 110 cases of lung cancer, while 9 procedures were made for non-infectious benign pathologies.
Preoperative data included: age, gender, co-morbidity (obesity, hypertension, diabetes, smoking, COPD). Operative data included: operative procedure and perioperative parameters (operative time, extended systematic nodal dissection or simple sampling, numbers of harvested mediastinal lymph-nodes, presence of pleural adhesions, presence of incomplete fissures), conversion rate, pathologic staging. Post-operative data included: complications (lung atelectasis requiring bronchoscopy, pneumonia, prolonged air leaks, ARDS and arrhythmia), duration of postoperative air leaks and postoperative drainage, and length of hospital stay. We have chronologically divided the patients into 4 groups, the first three with 30 consecutive patients each, and the last group of 29 patients.
Selection of the patients
At the begin of his activity, the surgeon aimed at easy cases, which were mainly selected on CT scan features indicating complete fissures and absence of pleural obliteration. With increasing experience, the surgeon became less regarding; patients were selected by tumour size less than 5 cm and clinical stage 1, or benign disease requiring lobectomy.
Surgical technique
All operations were performed under general anaesthesia, and single lung ventilation was obtained by double-lumen intubation. The patients were placed in the lateral decubitus position with a flexion of the operating table at the level of the scapula. A 4 cm utility-incision was placed in the 5th intercostal space at the level of the sub mammary line and protected with an Alexis retractor (Applied Medical Resources Corporation, California USA) was placed. After careful pleural inspection with a 30°, 10 mm thoracoscope (Karl Storz GmbH & Co. KG, Tuttlingen Germany), an additional 12 mm port was placed the 8th intercostal space, at the level of the medial axillary line, and utilized as camera port. A third access was created by insertion of a 12 mm port the 5th intercostal space, close to the tip of the scapula. The dissection of adhesions, pulmonary ligament and fissures, and mediastinal nodes was performed with a HARMONIC Ultrasonic ACE device (Ethicon, USA). Fissures, bronchus and vascular structures were divided with an endoscopic stapling device (endo GIA Ultra Universal Stapler, Covidien, MA, USA).
Lymphadenectomy was performed either before or after lobectomy, depending on ease of exposure. In the first few cases, a lobe-oriented dissection was performed. With increasing ease, the surgeon moved to a complete node dissection. On the right side, the pulmonary ligament was excised, the subcarinal nodes were dissected off the esophagus, pericardium and bronchial tree, and the paratracheal nodes were dissected en bloc while preserving the azygos vein. On the left side, pulmonary ligament, paraaortic and subaortic nodes were dissected in a standard fashion. Access to the subcarinal nodes from the left differed between upper and lower lobectomy: during lower lobectomy, the lower border of the left bronchus was followed up to the bifurcation; during upper lobectomy, access was gained over the stump of the lower pulmonary vein.
In case of conversion, the anterior incision was extended to a lateral thoracotomy.
Training of the surgeon
The surgeon took into account the guidelines for developing a VATS program (10). He had a 25-year experience with open lobectomy, and had switched to an anterolateral approach 15 years before starting VATS and was familiar with the anterior hilar approach. He had acquired basic skills for endoscopic surgery by performing 120 laparoscopic fundoplications. This allowed him to start immediately in the setting described above.
The familiarity with VATS instrumentarium, 2-D view and the port placement was made by the expertise of the surgeon with minor VATS procedures like pleural biopsies, cyst resections and wedge resections, already performed in the past. During the year before starting the program, surgeon attended several VATS courses and he visited clinics with a high volume in VATS lobectomy for observing and learning the different aspects of the procedure.
Statistical analysis
Continuous variables are reported as mean and standard deviation; categorical variables are reported as frequency and proportion.
The statistical analysis has referred to a Bayesian statistical inference model. We have calculated inference coefficients of each group, which we compared individually. By definition, there is a very strong probability of having a difference between 2 groups if the probability is higher than 95% (probability that the group is superior) or less than 5% (probability that the group is inferior). There is a strong probability of having difference between the groups in case of a 95%<Pr<80% (probability of superiority) or 5%<Pr<20% (probability of inferiority). A Pr<80% or >20% indicates a weak probability of difference.
The study was approved by institutional ethics board of University of Strasbourg.
Results
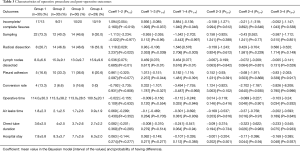
The demographic and staging features of each group are presented in Table 1. There was a weak probability of difference between the 4 groups regarding age, gender and comorbidity, except for COPD (Pr1>3=0.06, Pr1>4=0.08) and smoking (Pr2>3=0.001, Pr3>4=1).

Full table
Operative procedures and pathology are shown in Table 2. There were 110 lung cancers and 9 benign pathologies (6 lung sequestrations and 3 adenomas).

Full table
Operative outcomes are displayed in Table 3. In group 1 (first 30 operations) there was a very strong probability of having less incomplete fissures than in the other three (Pr1>2=0.019, Pr1>3=0.037, Pr1>4=0.046); the probability of difference between group 2, 3 and 4 respectively however was weak.
Full table
A simple sampling was performed in 22 cases in the group 1, in 12 cases in group 2, in 14 cases in group 3 and in 9 cases in group 4. Accordingly, in first group there was a very strong probability to have less sampling than in the other three (Pr1>2=0.977, Pr1>3=0.96, Pr1>4=0.997) and, conversely, to have less radical mediastinal lymph node dissection compared to the others three groups (Pr1>2=0.022, Pr1>3=0.039, Pr1>4=0.003).
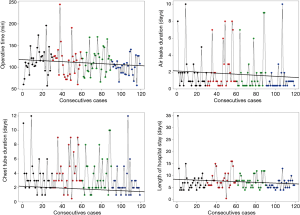
The fact that the number of lymph nodes harvested increases gradually with experience and stabilizes after the first period is credited a very strong probability (Pr1>2≤0.001, Pr1>3≤0.001, Pr1>4≤0.001), as shown in Table 3 and in the Figure 1.

The presence of pleural adhesions increases over the time and there is a strong probability of difference between group 1, 2 and 3 (Pr1>2=0.077, Pr1>3=0.044).
The Bayesian model concludes that the conversion rate decreases over time (group 1 =13.3%, group 2 =6.6%, group 3 =16.6%, group 4 =0%); there is a very strong probability of difference in favour of group 4 (Pr1>4=0.992, Pr3>4=0.996, Pr2>4=0.995).
Regarding decreasing duration of the operation (Pr1>4=0.946, Pr2>4=0.901, Pr3>4=0.932), air leak(Pr1>4=0.9369, Pr2>4=0.97) and chest tube drainage (Pr1>4=0.94, Pr2>4=0.94, Pr3>4=0.937), as well as length of hospital stay (Pr2>4=0.94, Pr3>4=0.937), there is a very strong probability of difference in favour of group 4, i.e., after the initial 90 operations (Table 3 and Figure 2).
In addition, the overall operating time of benign and malign group is similar (115 vs. 110 min; P=0.778). In the first and in the third group, only one lobectomy on thirty, was performed for benign diseases. In the second group (4 lobectomies performed for benign diseases) and in the fourth (3 lobectomies performed for benign disease) there was no significant difference concerning operating time (2nd group: 109 vs. 138 min, P=0.576; 4th group: 101 vs. 92 min, P=0.429).
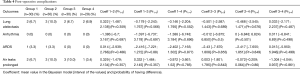
We did not find any very strong probability of difference when considering post-operative complications (Table 4).
Full table
Discussion
There is a bulk of data describing and defining the learning curve of surgical trainees without significant experience with open lobectomy, starting to learn VATS lobectomy from scratch. Konge et al. showed that the learning curve can be overcome with good results, even if the beginner surgeon has limited prior experience in open surgery (19).
In this study, we focused on the opposite situation, i.e., an experienced thoracic surgeon starting to introduce the VATS approach into his practice. We anticipated that an in-depth knowledge of surgical anatomy and anatomical variants joined to an exposure to minor VATS procedures might shorten the learning curve with reference to the up to 100 procedures and even more recommended for surgical trainees (10).
In the past, many studies have analysed the learning curve for different surgical techniques (12,19-24). Generally speaking, a learning curve is considered to have been overcome when the monitored outcome indicators reach a steady state (20). Hence, the conclusions about the learning curve depend from an adequate selection of outcome indicators.
In this study, we have deliberately considered a large panel of pre, intra and post-operative parameters, and tested which of them can improve over time, and how many procedures are required to reach a steady state.
The fact that clinical results improve in correlation with increasing numbers of VATS-procedures performed is underscored by several studies. Toker et al. suggested that a surgeon can have a high success rate in VATS thymectomy (98%) after 60 procedures (22). Osugi et al. found that an improvement plateau for VATS esophagectomy was reached after 34 cases (23). Focusing on VATS lobectomy, Hansen estimated that 47 VATS lobectomies are required for a training consultant in order to achieve similar results as a confirmed VATS expert (11). Zhao et al. observed that the surgeon becomes more proficient and able to perform the procedure with decreased blood loss and operative time after crossing a plateau of 30 VATS lobectomies (20). Arad et al. reveal the necessity of performing 30–60 VATS lobectomies in order to achieve constant results that are coherent with those reported from other medical centres worldwide (13). Li and Ferguson affirm that between 100 and 200 cases are required to achieve efficiency and consistency in this procedure for trainees (24).
Each of these reports evaluates some parameters like operative time, intraoperative blood loss or intra or postoperative complications (11-13,24). In our study, we have evaluated all these operative outcomes but, in addition, we have screened staging, individual patient selection in terms of complexity of the procedure defined by incomplete fissure and/or presence of pleural adhesions, and oncologic radicality, defined by extent of node dissection and number of nodes harvested.
We noticed that selection of “easy” patients is more obvious for group 1, which were the 30 first VATS lobectomies in the surgeon’s practice. Comparing group 1 to the 3 other groups, there were half as much patients with pleural adhesions. Incomplete fissures were seen in 1/3 of patients in group 1, and in 2/3 of patients in the subsequent groups. It appears that after these 30 initial VATS lobectomies, the surgeon started to feel more comfortable and confident with the VATS approach and became less selective.
Similarly, we noticed a set-off after 30 lobectomies with reference to oncologic radicality. In the first period, the majority of patients underwent (73%) a simple sampling, relying on PET staging completed with EBUS or mediastinoscopy in PET positive patients. A systematic mediastinal lymph node dissection has been performed in 27% only. In the subsequent groups, radical dissection increased gradually from 54% to 60%. Concurrently, the mean number of lymph nodes harvested almost doubled from group 1 to groups 2, 3 and 4 (Figure 1).
Actually, concerning operating time, the matching of benign and malign diseases could have a relevant impact on treatment results (i.e., young patients, no COPD, no comorbidities). The initial short operating time for a beginner may be explained by the a 25-year experience with open lobectomy in a high volume unit, and existing skills for endoscopic surgery owing to a previous experience with laparoscopic fundoplication. There are no significant differences in each group for the duration of the intervention. So we can infer that, the matching of benign and malign disease hasn’t a real relevant impact on the operating time. Actually, in malignant cases, the lobectomy is relatively easy and expeditive, and additive time is consumed for node dissection. An additive time of similar range is required in benign cases, where lobectomy is more complicated.
Based on these data, we can infer that an initial experience of 30 VATS-lobectomies led our consultant to the level of competence: the surgeon accepts more complex procedures and reaches a steady state in terms of oncologic radicality, materialized by the extent of node dissection.
The Bayesian inference model obviated a second set-off after 90 lobectomies, where indicators such as operative time, duration of air leaks and chest tube drainage, and length of hospital stay decreased at a level of very strong probability. Concurrently, the conversion rate was lowest in the final group. These indicators describe the efficiency of the surgeon, which requires considerably more experience than competence (Table 3, Figure 2).
In summary, our data lead to the conclusion that the learning curve of VATS-lobectomy is bimodal; the consultant on scrutiny needed about 30 lobectomies to acquire competence, and about 90 to reach the level of maximal efficiency. Comparable data have been reported (12,13,20).
We need to keep in mind that many factors interfere and may modulate the learning curve for VATS lobectomy. At first, the experience of the surgeon with minor VATS procedures such as lung biopsy, pleurectomy or sympathectomy is determinant. As opposed to open surgery, the view on the operative field in VATS is limited to 2 dimensions. Further, the tactile feed-back is decreased. VATS requires a specific instrumentation and manipulation is hampered by the fulcrum effect. Experience with minor VATS procedures is essential to acquire basic skills with accurate insertion of instruments, stapling, suturing or dissection.
The OR team around surgeon represents another capital factor. When building up a VATS program, nurses, assistants and anaesthesiologists must be trained on the specificities of this novel approach and the dependant technology. Training should also encompass scenarios with complications related to failing single lung ventilation, intraoperative bleeding, and other.
Needless to stress that the excellence of equipment such as video cameras, large size HD screens, staplers and energy devices, dedicated instrumentarium contribute to the success of the program.
An expert group has stated the steps of learning VATS-lobectomy. The surgeon should first see some procedures performed by an expert and attend a training symposium. Lack of experience with minor VATS.
Procedures can be compensated with training sessions on a simulator. Assistants and nursing staff should participate in this training. The first lobectomies should ideally be performed with the help of an experienced proctor; this is easy to concretize in a larger training institution, but may be impossible in small, low volume units. The first patients should be carefully selected as easy cases (25).
Conclusions
Analysis of the initial experience of a senior consultant demonstrated a bimodal learning curve. The level of competence, where quality of surgery became reproducible, was met after 30 lobectomies. The level of efficiency, where duration of operation, duration of hospital stay and conversion rate decreased, was reached after 90 operations. We conclude that learning VATS lobectomy is possible at an advanced stage of career in a reasonable time and without undue risk for the patient. We encourage reluctant quinquagenarian surgeons to engage themselves into VATS lobectomy!
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This is an observational study, without any risk for the patient, approved by University of Strasbourg. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [Crossref] [PubMed]
- Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Ohbuchi T, Morikawa T, Takeuchi E, et al. Lobectomy: video-assisted thoracic surgery versus posterolateral thoracotomy. Jpn J Thorac Cardiovasc Surg 1998;46:519-22. [Crossref] [PubMed]
- Li Z, Liu H, Li L. Video-assisted thoracoscopic surgery versus open lobectomy for stage I lung cancer: A meta-analysis of long-term outcomes. Exp Ther Med 2012;3:886-892. [PubMed]
- Taioli E, Lee DS, Lesser M, et al. Long-term survival in video-assisted thoracoscopic lobectomy vs open lobectomy in lung-cancer patients: a meta-analysis. Eur J Cardiothorac Surg 2013;44:591-7. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Licht PB, Jørgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg 2013;96:943-9. [Crossref] [PubMed]
- Petersen RH, Hansen HJ. Learning curve associated with VATS lobectomy. Ann Cardiothorac Surg 2012;1:47-50. [PubMed]
- Petersen RH, Hansen HJ. Learning thoracoscopic lobectomy. Eur J Cardiothorac Surg 2010;37:516-20. [Crossref] [PubMed]
- Ra Yong Joon, Ahn Hyo Yeong, Kim Min Su, et al. Learning curve of a young surgeon's video-assisted thoracic surgery lobectomy during his first year experience in newly established institution. Korean J Thorac Cardiovasc Surg 2012;45:166-70. [Crossref] [PubMed]
- Arad T, Levi-Faber D, Nir RR, et al. The learning curve of video-assisted thoracoscopic surgery (VATS) for lung lobectomy--a single Israeli center experience. Harefuah 2012;151:261-5, 320. [PubMed]
- Belgers EH, Siebenga J, Bosch AM, et al. Complete video-assisted thoracoscopic surgery lobectomy and its learning curve. A single center study introducing the technique in The Netherlands. Interact Cardiovasc Thorac Surg 2010;10:176-80. [Crossref] [PubMed]
- Jensen K, Ringsted C, Hansen HJ, et al. Simulation-based training for thoracoscopic lobectomy: a randomized controlled trial: virtual-reality versus black-box simulation. Surg Endosc 2014;28:1821-9. [Crossref] [PubMed]
- Ferguson J, Walker W. Developing a VATS lobectomy programme--can VATS lobectomy be taught? Eur J Cardiothorac Surg 2006;29:806-9. [Crossref] [PubMed]
- Larsen CR, Soerensen JL, Grantcharov TP, et al. Effect of virtual reality training on laparoscopic surgery: randomised controlled trial. BMJ 2009;338:b1802. [Crossref] [PubMed]
- Akiba T, Morikawa T, Ohki T. Simulation of thoracoscopic surgery using 3-dimensional tailor-made virtual lung. J Thorac Cardiovasc Surg 2012;143:1232-4. [Crossref] [PubMed]
- Konge L, Petersen RH, Hansen HJ, et al. No extensive experience in open procedures is needed to learn lobectomy by video-assisted thoracic surgery. Interact Cardiovasc Thorac Surg 2012;15:961-5. [Crossref] [PubMed]
- Zhao H, Bu L, Yang F, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer: the learning curve. World J Surg 2010;34:2368-72. [Crossref] [PubMed]
- Meyer M, Gharagozloo F, Tempesta B, et al. The learning curve of robotic lobectomy. Int J Med Robot 2012;8:448-52. [Crossref] [PubMed]
- Toker A, Tanju S, Ziyade S, et al. Learning curve in videothoracoscopic thymectomy: how many operations and in which situations? Eur J Cardiothorac Surg 2008;34:155-8. [Crossref] [PubMed]
- Osugi H, Takemura M, Higashino M, et al. Learning curve of video-assisted thoracoscopic esophagectomy and extensive lymphadenectomy for squamous cell cancer of the thoracic esophagus and results. Surg Endosc 2003;17:515-9. [Crossref] [PubMed]
- Li X, Wang J, Ferguson MK. Competence versus mastery: the time course for developing proficiency in video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2014;147:1150-4. [Crossref] [PubMed]
- Yan TD, Cao C, D'Amico TA, et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg 2014;45:633-9. [Crossref] [PubMed]


