
Trends and predictors of functional class after high-risk left ventricular assist device implantation at a destination therapy center
Highlight box
Key findings
• In our series, the best predictor of a sustainable improvement in functional status is an ischemic etiology of heart failure (HF).
What is known and what is new?
• Left ventricular assist device (LVAD) implantation is associated with improved survival, less is known about what factors predict functional status improvements.
• We demonstrated trends for improved New York Heart Association functional class in patients >70 years of age and those with a high acuity Interagency Registry for Mechanically Assisted Circulatory Support profile. Patients with an ischemic etiology of their HF showed significant improvements of functional status.
What is the implication, and what should change now?
• These findings may better help identify patients who will benefit the most from LVAD implantation.
Introduction
Patients with advanced heart failure (HF) have significantly increased morbidity and mortality despite optimized medical therapy (1,2). Additionally, these patients require frequent hospitalizations and have a high amount of associated medical spending (3,4). Further, non-pharmacologic methods are limited to transplant and destination left ventricular assist device (LVAD) therapy (3).
Early experiences with LVAD therapy demonstrated improved survival compared to optimal medical management (OMM) (5). As LVAD technology improved subsequent increases in implantation rates and patient survival were observed (6-8). Further, LVAD implantation has been associated with improved patient-reported quality of life amongst multiple studies (6,9). These improvements are coupled with data regarding improved functional capabilities as well. Multiple assessments of physical functioning following LVAD implantation have demonstrated significant improvements in function following implantation (10-12). These improvements have correlated to subsequent improvements in New York Heart Association (NYHA) functional status (12).
Despite demonstrations of improvements in functional status following LVAD implantation, this remains a poorly understood topic, particularly in the high-risk non-transplant, destination therapy (DT) cohort. For the purpose of this study, we defined the high-risk cohort as patients over the age of 70 years, ischemic etiology of HF, and high acuity INTERMACS profile. To understand what patient factors may impact patient functional status following LVAD implantation we undertook a retrospective review of all consecutive patients who underwent LVAD implantation at our DT center. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-514/rc).
Methods
We conducted a retrospective review of all LVAD implantations performed at Baylor Scott and White, The Heart Hospital, Plano from 2017 to 2023. This study was approved by the Baylor Scott and White Institutional Review Board (IRB#: 014-209) and informed consent was waived due to retrospective nature of the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All primary implantations were included. LVAD exchanges were excluded. The HeartMate 3 LVAD system (Abbott Laboratories, Chicago, IL, USA) was the only device implanted during the study period.
The primary outcome of interest was NYHA functional class. NYHA class was assessed at four time points—preimplantation, 6, 12, and 24 months. An improvement in functional class was defined as a decrease in NYHA class of one or more. Patients who died or who have not yet survived to a given time point were excluded from the analysis at that time point. To better understand who most benefits from LVAD implantation, we attempted to measures sustained functional improvement. For the purposes of our analysis, a sustained functional improvement was defined as being alive at 1-year with NYHA class I or II symptoms. This metric was assessed by logistic regression modeling.
Preoperative patient demographics, laboratory values, measures of patient acuity, operative data, and outcomes were extracted from the medical record. NYHA functional class was extracted from the most recent inpatient or outpatient HF note prior to implantation as appropriate. Follow-up assessments at 6, 12, and 24 months were extracted from the HF clinic visit most proximate to these time points.
Statistical analysis
Normally distributed continuous variables were compared using the student’s t-test. Non-normally distributed variables were compared using the Kruskal-Wallis test. Categorical variables were analyzed using the Chi-squared test. Survival was assessed using the Kaplan-Meier method and comparisons were performed using the log-rank test. A multivariable logistic regression model was constructed to identify predictors of a sustained functional improvement. To construct our model, independent covariates with P<0.20 on univariate analysis were incorporated into the model in forward and backward nested fashion using the likelihood ratio test to construct the model with the maximum explanatory power.
For all statistical tests, two-tailed P<0.05 was considered statistically significant. Continuous normally distributed variables are presented as means with standard deviations. Non-normally distributed variables are presented as medians with interquartile ranges. Categorial variables are presented as whole numbers with percentages. Statistical analysis was performed using Stata/SE 18.0/BE.
Results
From 2017 to 2023, 151 primary LVAD implantations were performed at our institution. The average age was 63.69±11.44 years, 123 patients (81.46%) were male, and 110 (72.85%) were White (Table 1). Most patients had an elevated creatinine with a mean of 1.45±0.54 mg/dL, a plurality was INTERMACS profile 1 (64, 42.38%), and many required preoperative Impella support (58, 38.41%). Operative complexity was high, with 46 (30.46%) requiring a reoperative sternotomy and 67 (44.37%) requiring a concomitant cardiac procedure including 15 aortic valve repair/replacements, two mitral valve replacements, and 45 tricuspid valve repairs (Table 2).
Table 1
| Variables | Value |
|---|---|
| Demographics | |
| Age (years) | 63.69±11.44 |
| Male | 123 (81.46) |
| White | 110 (72.85) |
| Black | 26 (17.22) |
| Hispanic | 12 (7.95) |
| BMI (kg/m2) | 28.28±6.06 |
| Preoperative laboratory values | |
| Creatinine (mg/dL) | 1.45±0.54 |
| eGFR (mL/min/1.73 m2) | 58.75±23.11 |
| Total bilirubin (mg/dL) | 0.9 (0.6–1.5) |
| Hemoglobin (g/dL) | 10.85±3.55 |
| Albumin (g/L) | 2.95±0.52 |
| Preoperative measures of acuity | |
| INTERMACS profile 1 | 64 (42.38) |
| INTERMACS profile 2 | 47 (31.13) |
| INTERMACS profile 3 | 31 (20.53) |
| INTERMACS profile 4 | 9 (5.96) |
| Preoperative Impella support | 58 (38.41) |
| Preoperative ECMO support | 4 (2.65) |
Data are presented as mean ± standard deviation, n (%), or median (interquartile range). BMI, body mass index; eGFR, estimated glomerular filtration rate; ECMO, extracorporeal membrane oxygenation.
Table 2
| Operative data | Value |
|---|---|
| Reoperative sternotomy | 46 (30.46) |
| Concomitant valve procedure | 67 (44.37) |
| Cardiopulmonary bypass time (min) | 109.67±35.57 |
| Aortic cross clamp time (min) | 27.10±17.41 |
Data are presented as n (%) or mean ± standard deviation. LVAD, left ventricular assist device.
Operative mortality in this cohort was 7.95%. Thirty-eight (25.17%) patients suffered from major bleeding, 6 (3.97%) sustained an ischemic stroke, and 31 (20.53%) had right HF requiring temporary right ventricular assist device (RVAD) placement. Additionally, 27 (17.88%) required a postoperative tracheostomy for respiratory failure and 23 (15.23%) required temporary dialysis. With a median follow-up of 526 [222–946] days, 110 (72.85%) patients are alive. Thirty-day, 1-year, and 2-year survival are 96.69%, 85.89%, and 75.50% respectively (Figure 1).
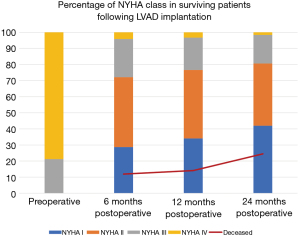
All patients pre-implant were functional class 4 at time of implantation or were inotrope dependent for class 4 symptoms. In terms of functional class, at 6-month follow-up, 35 (28.69%) patients were functional class I, 53 (43.44%) were functional class II, 29 (23.77%) were functional class III, and 5 (4.10%) were functional class IV (Figure 2). In patients with available 6-month follow-up data, an improved functional class was observed in 95.9% (118/123) and 84.3% (118/140) when accounting for operative mortality. At 12-month follow-up, 32 (34.04%) were functional class I, 40 (42.55%) were functional class II, 19 (20.21%) were functional class III, and 3 (3.19%) were functional class IV, suggesting a sustained functional class improvement in 86 (56.95%) patients and 90.43% improvement in functional class for surviving patients. Finally, at 24 months, 26 (41.94%) patients were class I, 24 (38.71%) were class II, 11 (17.74%) were class III, and only 1 (1.61%) was class IV. Overall, at 2-year, 92.98% of surviving patients had improved functional class compared to preoperative baseline. The distribution of NYHA class over time with mortality rate as a competing event is shown in Figure 2.
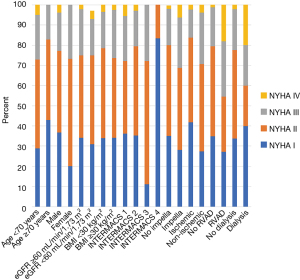
Focusing on NYHA at 1-year follow-up, NYHA strata were evaluated for various groups of patients including age, sex, renal function, body mass index (BMI), INTERMACS profile, HF etiology, need for preoperative Impella support, need for postoperative RVAD support, and need for postoperative dialysis. None of these binary divisions was statistically associated with differences in NYHA functional class at 12 months (Figure 3). However, there were several trends, including a trend toward improved functional class in patients over the age of 70 years, a trend toward improved functional class in patients with lower acuity INTERMACS profiles (P=0.15), a trend toward better functional status in patients with ischemic cardiomyopathy (P=0.09), and a trend toward poorer functional class in those who required RVAD support postoperatively (P=0.06). Interestingly, patients requiring post-implantation dialysis had similar functional class as those who did not (P=0.44).
In assessing for a sustained functional improvement, 72 (76.60%) were NYHA class I or II at 1 year follow-up while 22 (23.40%) were NYHA class III or IV. The remainder of the patients either had inadequate follow-up for assessment either because implant was too recent (n=37) or because of patient death (n=20). After adjusting for covariates, the multivariable logistic regression model suggested that an ischemic etiology for HF was strongly associated with a sustained functional class improvement at 1-year [odds ratio: 5.53 (95% confidence interval: 1.06–28.88), P=0.04] (Table 3).
Table 3
| Variables | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Ischemic etiology | 5.53 | 1.06–28.88 | 0.04 |
| Creatinine | 3.96 | 0.75–20.82 | 0.10 |
| Need for Impella | 0.35 | 0.05–2.38 | 0.28 |
| Weight | 0.97 | 0.94–1.02 | 0.31 |
Discussion
Compared to OMM, LVAD DT has been associated with an improved survival (5). As the technology has improved, we have seen both improved survival and increased rates of implantation (6-8). Further improvements in patient quality of life, physical functioning, and NYHA status have been noted (6,9-12). Despite these improvements, there is little information in what factors may predict improvement in functional status following LVAD implantation. We performed a single-center, retrospective analysis of all consecutive LVAD implantations with a special focus on clinical outcomes and possible predictors of improvements in the high-risk cohort. Importantly this study noted: acceptable 1- and 2-year survival, sustained 1-year functional improvements in 76.60% of patients, in patients with 2-year of follow-up data available 80.65% were NYHA I/II, and several trends for improved functional status. Notably, postoperative dialysis dependence did not negatively impact functional class. Additionally, patients with an ischemic etiology for their HF experienced the most sustainable improvement in functional class.
In an effort to improve patient outcomes multiple studies have attempted to determine predictors of poor outcomes following LVAD implantation (13,14). There is little consistency on factors which have a poor prognosis, however increasing age, advanced chronic kidney disease, and coagulopathy have been implicated in poor results (13,14).
Twelve months following LVAD implantation we observed good durable improvements in NYHA functional status overall. Notably in patients who survived 1-year following the procedure, 34.04% were class I and 42.55% were class II. This result compares favorably to the initial trials demonstrating efficacy of destination LVAD therapy, which reported a median of NYHA II at 1-year postoperatively (5). Our results are further comparable to more recent data as well, McDiarmid et al. reported a mean NYHA class of 1.7±0.8 (15). Further, our observed 1-year survival is improved compared to these studies 85.89% vs. 52% vs. 78.95% in two similar studies (5,15). Taken together these results demonstrate not only improvements in patient survival following LVAD, but also functional improvements as the technology have improved.
It is important to note 23.41% of surviving patients were determined to be class III or IV at 1 year. While this may seem to be a high percentage, this is in line with modern data demonstrating around 20% of patients demonstrate NYHA III/IV symptoms up to 2 years following implantation (16). Further, in our patients with 24 months of follow-up, 19.35% of patients still had class III/IV symptoms. Importantly compared to previous data, our trend for functional class follows a similar improvement. While our data revealed a lower percentage of patients with class I or II symptoms, our results at 12 and 24 months are comparable. These findings suggest durable functional improvements following a progressive improvement over the first year (16). It is important to note the mortality rate within this cohort increased over time which may have impacted our observations of functional improvement.
While we did not observe any statistically significant implicating variables in predicting postoperative outcomes, we did observe interesting trends. Previous studies have determined increasing age to be associated with worse survival, however we observed a trend to improved functional status in those >70 years of age (13,14). This is important to note as the results imply that while this is a higher risk population the improvement in functional status may be worth the risk. A similar trend was noted in patients with an ischemic etiology of HF. Again, this has been associated with worse survival, however our results suggest this population experiences good symptom relief (13,14). Not surprisingly patients who required RVAD trended toward a worse functional class. Right ventricular failure is a common complication following LVAD requiring a complex management strategy (17,18). Importantly RVF following LVAD carries a poor overall prognosis (19). Interestingly this was the only trend we observed which was concordant with predictors of poor survival. These findings suggest that these high-risk patients may experience the best functional benefit if they survive.
Significantly, postoperative renal failure requiring dialysis did not appear to impact functional status. Post-implantation dialysis is known to be associated with decreased survival (20). Additionally, the logistics of thrice weekly dialysis may negatively impact quality of life. However, these findings suggest that despite these limitations, patients requiring post-LVAD dialysis experience similar functional improvements as other patients. These findings may impact preoperative patient selection.
LVAD implantation has been demonstrated to improve survival and patient functional status. Little information is known regarding the predictors of functional status following LVAD implantation. On univariate analysis, we did note several interesting trends for improved functional class. Patients >70 years of age, those with higher acuity INTERMACS profile, and those with ischemic cardiomyopathy trended towards improved functional class, while patients requiring RVAD support trended towards a worse prognosis. On multivariable analysis, an ischemic etiology of HF was predictive of a sustained functional improvement. These findings may better help identify patients who will benefit the most from LVAD implantation. Prior studies have shown similar survival between ischemic and non-ischemic cardiomyopathy patients following LVAD implantation, however there remains little information on what impact if any cardiomyopathy etiology has on functional status (21-23). While we are unable to note the exact causes of why our patients with ischemic cardiomyopathy experienced improved functional class, but this may reflect improved right ventricular function through left ventricular unloading or these patients may have experienced a higher degree of reverse remodeling (24). However, it is important to note this finding may be an incidentally statistically significant finding and may have limited clinical or real-world implications. Future studies will be important to add context to our findings. Prospective trials that assess both functional status along with patient-reported quality of life and objective functional testing will be important to better understanding our findings.
This study is limited by its retrospective nature and thus inherently prone for selection bias. Further, assessment of functional status was performed by multiple physicians leading to possible biases. Our patient population is also heterogenous limiting the overall generalizability of this study.
Conclusions
Although improvements in patient survival and quality of life have been demonstrated, little is known about what patient factors may predict improved patient quality of life following LVAD implantation. We demonstrated trends for improved NYHA functional class in patients >70 years of age and those with a high acuity INTERMACS profile. On multivariate analysis, patients with an ischemic etiology of their HF showed significant improvements of functional status. Interestingly patients requiring dialysis postoperatively had similar reported quality of life as those who did not require dialysis. These findings and a better understanding of functional outcomes may help improve patient selection.
Acknowledgments
We would like to acknowledge the philanthropic gift of the family of Satish and Yasmin Gupta to Baylor Scott and White—The Heart Hospital Plano; the philanthropic gift of the Roberts Foundation to Baylor Scott and White—The Heart Hospital Plano; and The Baylor Scott and White Foundation. The ASAIO 2024 Annual Conference for accepting a portion of this data for presentation.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-514/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-514/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-514/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-514/coif). A.A. and T.J.G. are part of the Abiomed speaker’s bureau. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Baylor Scott and White Institutional Review Board (IRB#: 014-209) and informed consent was waived due to retrospective nature of the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shah KS, Xu H, Matsouaka RA, et al. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J Am Coll Cardiol 2017;70:2476-86. [Crossref] [PubMed]
- Miller LW, Rogers JG. Evolution of Left Ventricular Assist Device Therapy for Advanced Heart Failure: A Review. JAMA Cardiol 2018;3:650-8. [Crossref] [PubMed]
- Kazi DS, Mark DB. The economics of heart failure. Heart Fail Clin 2013;9:93-106. [Crossref] [PubMed]
- Chen J, Normand SL, Wang Y, et al. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA 2011;306:1669-78. [Crossref] [PubMed]
- Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435-43. [Crossref] [PubMed]
- Pinney SP, Anyanwu AC, Lala A, et al. Left Ventricular Assist Devices for Lifelong Support. J Am Coll Cardiol 2017;69:2845-61. Erratum in: J Am Coll Cardiol 2017;70:1308. [Crossref] [PubMed]
- Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant 2017;36:1080-6. [Crossref] [PubMed]
- Jorde UP, Saeed O, Koehl D, et al. The Society of Thoracic Surgeons Intermacs 2023 Annual Report: Focus on Magnetically Levitated Devices. Ann Thorac Surg 2024;117:33-44. [Crossref] [PubMed]
- Maciver J, Ross HJ. Quality of life and left ventricular assist device support. Circulation 2012;126:866-74. [Crossref] [PubMed]
- Pagani FD, Miller LW, Russell SD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol 2009;54:312-21. [Crossref] [PubMed]
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007;357:885-96. [Crossref] [PubMed]
- Allen JG, Weiss ES, Schaffer JM, et al. Quality of life and functional status in patients surviving 12 months after left ventricular assist device implantation. J Heart Lung Transplant 2010;29:278-85. [Crossref] [PubMed]
- Lietz K, Long JW, Kfoury AG, et al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation 2007;116:497-505. [Crossref] [PubMed]
- Cowger J, Sundareswaran K, Rogers JG, et al. Predicting survival in patients receiving continuous flow left ventricular assist devices: the HeartMate II risk score. J Am Coll Cardiol 2013;61:313-21. [Crossref] [PubMed]
- McDiarmid A, Gordon B, Wrightson N, et al. Hemodynamic, echocardiographic, and exercise-related effects of the HeartWare left ventricular assist device in advanced heart failure. Congest Heart Fail 2013;19:11-5. [Crossref] [PubMed]
- Rogers JG, Aaronson KD, Boyle AJ, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol 2010;55:1826-34. [Crossref] [PubMed]
- Coromilas EJ, Takeda K, Ando M, et al. Comparison of Percutaneous and Surgical Right Ventricular Assist Device Support After Durable Left Ventricular Assist Device Insertion. J Card Fail 2019;25:105-13. [Crossref] [PubMed]
- Kapur NK, Esposito ML, Bader Y, et al. Mechanical Circulatory Support Devices for Acute Right Ventricular Failure. Circulation 2017;136:314-26. [Crossref] [PubMed]
- Lo Coco V, De Piero ME, Massimi G, et al. Right ventricular failure after left ventricular assist device implantation: a review of the literature. J Thorac Dis 2021;13:1256-69. [Crossref] [PubMed]
- George TJ, Kabra N, DiMaio JM, et al. Elderly Patients With Higher Acuity Have Similar Left Ventricular Assist Device Outcomes as Younger Patients at a Nontransplant Center. Am J Cardiol 2023;189:93-7. [Crossref] [PubMed]
- Chou BP, Critsinelis A, Lamba HK, et al. Continuous-Flow Left Ventricular Assist Device Support in Patients with Ischemic Versus Nonischemic Cardiomyopathy. Tex Heart Inst J 2021;48:e207241. [Crossref] [PubMed]
- Tsiouris A, Borgi J, Karam J, et al. Ischemic versus nonischemic dilated cardiomyopathy: the implications of heart failure etiology on left ventricular assist device outcomes. ASAIO J 2013;59:130-5. [Crossref] [PubMed]
- Abubakar H, Subahi A, Adegbala O, et al. Comparison of In-Hospital Outcomes of Patients With-Versus-Without Ischemic Cardiomyopathy Undergoing Left Ventricular Assist Device Placement. Am J Cardiol 2019;123:414-8. [Crossref] [PubMed]
- Drakos SG, Kfoury AG, Selzman CH, et al. Left ventricular assist device unloading effects on myocardial structure and function: current status of the field and call for action. Curr Opin Cardiol 2011;26:245-55. [Crossref] [PubMed]



