
Prognostic impact based on tumor diameter of pathological N1 lymph node metastases for non-small cell lung cancer
Highlight box
Key findings
• For lung adenocarcinoma, there is a significant difference in prognosis when the cutoff value for tumor diameter of N1 lymph node metastases is set at 4 mm. By applying this, it becomes possible to subdivide the N1 classification.
• Tumor diameters of N1 lymph node metastasis differ between adenocarcinoma and squamous cell carcinoma.
What is known and what is new?
• The pathological N (pN) classification of lung cancer is based on the location of lymph node metastases, and the relationship between the tumor diameter of lymph node metastases and prognosis is still under investigation. Although research has been conducted on micrometastasis, there has been little research on macrometastasis.
• A new finding in this study is that there is a significant difference in survival rate for adenocarcinoma when the cutoff value is 4 mm, and that this criterion cannot be applied to squamous cell carcinoma. Additionally, while it is rare for other cancers to have multiple histological types arising from one organ, lung cancer encompasses multiple histological types, complicating the determination of the pN factor based on the tumor diameter of lymph node metastases.
What is the implication, and what should change now?
• Applying this research, it will be possible to further refine the subdivision of pN factors and stage classification, and it will be possible to consider in more detail the indications for postoperative adjuvant chemotherapy and the follow-up period. We believe that this may lead to an improved prognosis for lung cancer.
Introduction
Lung cancer has a poor prognosis compared with other types of cancer, and according to a report from the American Cancer Society, the 5-year overall survival (OS) rate is 26.7% (1). Even when curative surgery is performed, the prognosis is often unfavorable. The 5-year OS for lung cancer is worse compared to other cancers (2). Although uniform treatment strategies have been recommended for patients with advanced-stage disease (3), recurrence patterns and prognosis can vary even between patients with the same stage of disease. To improve the prognosis of patients who undergo complete resection but have regional lymph node metastasis, a new classification system is needed to identify those patients at high risk of recurrence so that postoperative adjuvant chemotherapy can be administered more appropriately.
For lung cancer, determination of degree of lymph node metastasis (N) using the pathological tumor-node-metastasis (TNM) classification, which was formulated and revised by the Union for International Cancer Control (UICC), is determined by the region of lymph node metastasis, such as the ipsilateral hilum, mediastinum, or contralateral side (4). It does not involve evaluation based on the maximum diameter of the lymph node metastasis or the number of metastatic lymph nodes.
Calculation of the pathological N (pN) parameter when using the TNM classification varies depending on the site of cancer origin. The American Joint Committee on Cancer (AJCC) Cancer Staging Manual defines the pN parameter based not only on the region of lymph node involvement but also on factors such as the maximum diameter and number of metastatic lymph nodes. For example, breast cancer lymph node metastases are categorized as follows: <0.2 mm, isolated tumor cells (ITCs); 0.2–2 mm, micrometastasis; and >2 mm, macrometastasis, and these categories are reflected in the N parameter (5). For other cancers, such as cancers of the head and neck (6), oral cavity (7), pharynx (8), skin (9), and testicles (10), the pN parameter considers lymph node metastasis diameter. For example, two cutoff values for head and neck tumors have been set: 3 and 6 cm (6).
In patients with lung cancer, controversial aspects about the association between lymph node metastasis and OS include the comparison between a single lymph node station and multiple stations, as well as the significance of skip metastases (11-15). Although some research about micrometastasis in lung cancer has been conducted, few data about macrometastasis are available (16). Skip metastasis has been analyzed for pN2 (11-15), so there are few comparative studies for pN1.
In this study, we measured the tumor diameter of lymph node metastases in patients with adenocarcinoma and squamous cell carcinoma who underwent curative surgical resection and compared the relationship between the tumor diameter of lymph node metastases and OS. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-792/rc).
Methods
Study design and participants
This study is a retrospective study. This study focused on cases where surgery was indicated and performed. From January 1, 2013 to December 31, 2020, there were 635 patients with lung cancer for whom radical surgery (lobectomy + regional lymph node dissection) was performed in the Division of Chest Surgery, Department of Surgery, Toho University Medical Center Omori Hospital. For regional lymph node dissection, we specifically included patients who underwent lobectomy and mediastinal lymph node dissection (ND 2a-1 or ND 2a-2). Lymph node metastasis was confirmed in 132 patients. Among them, 69 patients had pN1 disease. One of these patients was excluded due to direct invasion into the lung, making it difficult to measure the diameter of the metastatic tumor. As a result, 68 patients were evaluated, including 35 cases of adenocarcinoma and 26 cases of squamous cell carcinoma (Figure 1A). We aimed to analyze adenocarcinoma and squamous cell carcinoma, so the five cases of adenosquamous carcinoma and the two cases of large cell neuroendocrine carcinoma were not included in the analysis. We measured the maximum diameter of lymph node metastases in these subjects (Figure 1B). We measured the maximum diameter of lymph node metastases by marking them on tissue glass slides and measuring them with a ruler. This method is aimed at applying our analysis to routine pathological diagnostic practices. If there was a metastatic deposit in a lymph node or multiple lymph node metastases, we measured and analyzed the largest metastasis by its longest diameter. Lymph nodes associated with the lungs often fragment more frequently during retrieval than lymph nodes from other organs. When lymph node tissue was fragmented in the specimen, we did not measure areas with unclear continuity. We evaluated the maximum diameter of metastases only within the range where continuity could be confirmed. The average observation period for the study participants was 47 months.

First, a receiver operating characteristic (ROC) curve was drawn for pN1 adenocarcinoma cases based on the occurrence or nonoccurrence of death during the observation period. Using the cutoff value of the ROC curve as a reference, a Kaplan-Meier curve was drawn for the occurrence or nonoccurrence of death. The outcome of this analysis is OS. Based on these data, we evaluated what tumor diameter of lymph node metastasis was significantly associated with OS. We also performed Cox regression analysis to examine how much tumor diameter of lymph node metastasis contributed to OS. Next, we determined whether a significant association between OS and tumor diameter of lymph node metastasis was observed in patients with pN1 squamous cell carcinoma. In this study, a potential source of bias is that only patients eligible for surgery were included as participants.
This analysis was performed using existing samples collected during surgery. The residual formalin-fixed paraffin-embedded (FFPE) specimens that were used for pathological diagnosis were used for this analysis. Information on the use of existing samples is posted on the Toho University Medical Center Omori Hospital Department of Pathology website and opt-outs are available. This study was approved by the Ethics Committee of Toho University Medical Center Omori Hospital (Nos. M21206 [2021] and M24015 [2024]). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Histopathological analysis
All surgical specimens were fixed in 10% neutral buffered formalin solution by injection through the bronchial tree and then embedded in paraffin. The diameters of lymph node metastases were measured using the glass slide used for final diagnosis. The slides were examined by two pathologists (Y.K. and N.T.).
Immunohistochemistry (IHC)
Serial sections 3-µm thick were cut from paraffin blocks of FFPE tissues and stained with the following primary antibodies and dilutions: TTF-1 (clone SPT24, 1:200; Novocastra, Newcastle, UK) and Ki-67 (clone MIB-1, 1:200; Dako, Glostrup, Denmark). Following heat-induced epitope retrieval, IHC was performed using an automated immunostainer (Ventana Medical Systems, Tucson, AZ, USA) according to the manufacturer’s protocol.
The Ki-67 labeling index determines the percentage of Ki-67-positive cells in a field of 2,000 cells (17). The Ki-67 labeling index was evaluated in the primary tumor site, not in the lymph node metastases. The Ki-67 labeling index is interpreted in various ways, with cutoff values such as 10% and 20%, with no clear consensus. We elected to use a cutoff value of 10% (18,19).
Statistical analysis
Data are presented as mean ± standard deviation (SD) or median with interquartile range. Categorical variables were expressed as percentages of the sample. To compare groups, we used the Student’s t-test for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables. Statistical significance was defined as a P value <0.05.
Survival curves were generated using the Kaplan-Meier method, and univariate comparisons were made using the log-rank test. We utilized a Cox proportional hazards model to identify factors significantly associated with survival. All statistical analyses were conducted using JMP software version 17.0, provided by SAS Institute, Inc., located in Cary, NC, USA. Cox hazard regression models were employed to calculate adjusted 95% confidence intervals (CIs).
Results
We compared the characteristics of 35 adenocarcinoma patients and 26 squamous cell carcinoma patients and found significant differences in two aspects: tumor diameter of lymph node metastasis and smoking rate (Table 1). Squamous cell carcinoma cases had a significantly larger maximum lymph node metastasis diameter compared with adenocarcinoma cases (P=0.03). In addition, patients with squamous cell carcinoma more commonly had a history of smoking (96.2%) compared with patients with adenocarcinoma (74.3%) (P=0.01). For the remaining patient characteristics evaluated, no significant differences were observed between patients with adenocarcinoma and those with squamous cell carcinoma (Table 1).
Table 1
| Categories | Adenocarcinoma (n=35) |
Squamous cell carcinoma (n=26) | P value |
|---|---|---|---|
| Age, years | 69.6±8.6 | 70.2±7.7 | 0.81 |
| Sex | 0.34 | ||
| Male | 23 (65.7) | 20 (76.9) | |
| Female | 12 (34.3) | 6 (23.1) | |
| Smoking history | 0.01* | ||
| Yes | 26 (74.3) | 25 (96.2) | |
| No | 9 (25.7) | 1 (3.8) | |
| cStage | 0.24 | ||
| IA3 | 2 (5.7) | 0 | |
| IB | 1 (2.9) | 0 | |
| IIA | 14 (40.0) | 7 (26.9) | |
| IIB | 8 (22.9) | 10 (38.5) | |
| IIIA | 10 (28.6) | 9 (34.6) | |
| pStage | 0.92 | ||
| IIB | 26 (74.3) | 19 (73.1) | |
| IIIA | 9 (25.7) | 7 (26.9) | |
| Maximum size of metastatic lymph node, mm | 3.9±2.5 | 6.2±5.2 | 0.03* |
| Number of metastatic lymph nodes | 1.5±0.9 | 1.9±1.2 | 0.13 |
| Station | 0.31 | ||
| Single | 27 (77.1) | 17 (65.4) | |
| Multiple | 8 (22.9) | 9 (34.6) | |
| Postoperative adjuvant chemotherapy | 0.77 | ||
| Yes | 12 (34.3) | 8 (30.8) | |
| No | 23 (65.7) | 18 (69.2) | |
| Recurrence | 0.34 | ||
| Yes | 15 (42.9) | 14 (53.8) | |
| No | 20 (57.1) | 12 (46.2) | |
| Death | 0.72 | ||
| Yes | 8 (22.9) | 7 (26.9) | |
| No | 27 (77.1) | 19 (73.1) | |
Data are presented as n (%) or mean ± SD. *, P<0.05. cStage, clinical stage; pStage, pathological stage; SD, standard deviation.
The 5-year OS rate for patients with adenocarcinoma was 75.2%, compared with 71.7% for patients with squamous cell carcinoma; the difference between groups was not significant (P=0.86) (Figure 2A).
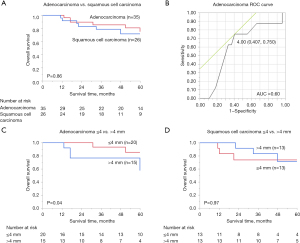
In 35 patients with adenocarcinoma, to determine the cutoff value for tumor diameter for lymph node metastasis, univariate analysis was performed on the occurrence or nonoccurrence of death during the study. The mean tumor diameter of lymph node metastasis between patients who survived and those who died was 3.69±2.57 and 4.56±2.25 mm, respectively; this difference was not statistically significant (P=0.40). Drawing an ROC curve suggested that 4 mm, which had a maximum sensitivity (1 − specificity) of 0.343, might be a suitable cutoff value (Figure 2B).
Among patients with adenocarcinoma, no significant differences in patient demographics or histopathological characteristics were noted between the 20 patients in the ≤4 mm group and the 15 patients in the >4 mm group (Table 2). However, a significant difference in Ki-67 labeling index was observed, with 60.0% and 93.3% of cases having a Ki-67 labeling index >10% in the ≤4 and >4 mm groups, respectively (P=0.02) (Table 2).
Table 2
| Categories | Size of lymph node metastases | P value | |
|---|---|---|---|
| ≤4 mm (n=20) | >4 mm (n=15) | ||
| Age, years | 69.0±7.8 | 70.5±9.7 | 0.62 |
| Sex | 0.41 | ||
| Male | 12 (60.0) | 11 (73.3) | |
| Female | 8 (40.0) | 4 (26.7) | |
| Smoking history | 0.14 | ||
| Yes | 13 (65.0) | 13 (86.7) | |
| No | 7 (35.0) | 2 (13.3) | |
| cStage | 0.60 | ||
| IA3 | 1 (5.0) | 1 (6.7) | |
| IB | 1 (5.0) | 0 | |
| IIA | 9 (45.0) | 5 (33.3) | |
| IIB | 3 (15.0) | 5 (33.3) | |
| IIIA | 6 (30.0) | 4 (26.7) | |
| pStage | 0.20 | ||
| IIB | 16 (80.0) | 10 (66.7) | |
| IIIA | 4 (20.0) | 5 (33.3) | |
| Number of metastatic lymph nodes | 1.5±0.7 | 1.5±1.1 | 0.78 |
| Station | 0.20 | ||
| Single | 17 (85.0) | 10 (66.7) | |
| Multiple | 3 (15.0) | 5 (33.3) | |
| Postoperative adjuvant chemotherapy | 0.92 | ||
| Yes | 7 (35.0) | 5 (33.3) | |
| No | 13 (65.0) | 10 (66.7) | |
| Recurrence | 0.32 | ||
| Yes | 10 (50.0) | 5 (33.3) | |
| No | 10 (50.0) | 10 (66.7) | |
| Death | 0.20 | ||
| Yes | 3 (15.0) | 5 (33.3) | |
| No | 17 (85.0) | 10 (66.7) | |
| Solid | 0.69 | ||
| Yes | 12 (60.0) | 10 (66.7) | |
| No | 8 (40.0) | 5 (33.3) | |
| Micropapillary | 0.14 | ||
| Yes | 5 (25.0) | 1 (6.7) | |
| No | 15 (75.0) | 14 (93.3) | |
| Cribriform | 0.83 | ||
| Yes | 6 (30.0) | 5 (33.3) | |
| No | 14 (70.0) | 10 (66.7) | |
| Morule-like | 0.39 | ||
| Yes | 1 (5.0) | 2 (13.3) | |
| No | 19 (95.0) | 13 (86.7) | |
| STAS | 0.35 | ||
| Yes | 5 (25.0) | 6 (40.0) | |
| No | 15 (75.0) | 9 (60.0) | |
| TTF-1 | 0.06 | ||
| Positive | 17 (85.0) | 14 (93.3) | |
| Negative | 3 (15.0) | 1 (6.7) | |
| Ki-67 labeling index | 0.02* | ||
| ≤10% | 8 (40.0) | 1 (6.7) | |
| >10% | 12 (60.0) | 14 (93.3) | |
Data are presented as n (%) or mean ± SD. *, P<0.05. cStage, clinical stage; pStage, pathological stage; STAS, spread through air spaces; TTF-1, thyroid transcription factor-1; SD, standard deviation.
Using 4 mm as the cutoff value, Kaplan-Meier OS curves were drawn for the ≤4 and >4 mm groups, log-rank testing was performed, and a significant difference was observed (P=0.04). The 5-year OS rate was 85.6% for ≤4 mm and 57.7% for >4 mm (Figure 2C). Univariate analyses using the Cox proportional hazards model revealed significant differences in metastatic lymph node diameter (≤4 vs. >4 mm) (P=0.04), sex (P=0.04), and pathological T (pT) value (pT1 and 2 vs. pT3 and 4) (P=0.03) (Table 3).
Table 3
| Categories | Univariate analysis | ||
|---|---|---|---|
| HR | 95% CI | P value | |
| Age, years | 0.60 | ||
| ≤75 | 1 | Reference | |
| >75 | 1.61 | 0.29–8.94 | |
| Sex | 0.04* | ||
| Female | 1 | Reference | |
| Male | 6.28 | 0.75–52.37 | |
| Tumor size of metastatic lymph node | 0.04* | ||
| ≤4 mm | 1 | Reference | |
| >4 mm | 5.21 | 0.96–28.29 | |
| pT | 0.03* | ||
| 1 or 2 | 1 | Reference | |
| 3 or 4 | 7.52 | 1.24–45.60 | |
| Postoperative adjuvant chemotherapy | 0.62 | ||
| No | 1 | Reference | |
| Yes | 0.69 | 0.16–2.97 | |
*, P<0.05. HR, hazard ratio; CI, confidence interval; pT, pathological T.
Although we tried to perform similar analyses for patients with squamous cell carcinoma, a cutoff value that differentiated prognoses in these patients could not be identified. When we evaluated whether a 4 mm cutoff value could be applied to squamous cell carcinoma cases, no significant differences in age (P=0.77), sex (P=0.35), cStage (P=0.05), pStage (P=0.18), smoking history (P=0.23), presence or absence of postoperative chemotherapy (P=0.39), recurrence rate (P>0.99), or mortality rate (P=0.66) were observed (Table 4). The mean number of metastatic lymph nodes in the ≤4 mm group was 1.4±0.7, while that in the >4 mm group was 2.4±1.4. The P value for this comparison was 0.03, indicating a statistically significant difference between groups (Table 4). Using 4 mm as a cutoff value, Kaplan-Meier OS curves were created for the ≤4 and >4 mm groups, and log-rank testing was conducted (Figure 2D). The 5-year OS rate was 74.1% in the ≤4 mm group and 71.4% in the >4 mm group; the difference between groups was not statistically significant (P=0.97) (Figure 2D).
Table 4
| Categories | Size of lymph node metastases | P value | |
|---|---|---|---|
| ≤4 mm (n=13) | >4 mm (n=13) | ||
| Age, years | 70.6±6.6 | 69.7±8.8 | 0.77 |
| Sex | 0.35 | ||
| Male | 9 (69.2) | 11 (84.6) | |
| Female | 4 (30.8) | 2 (15.4) | |
| Smoking history | 0.23 | ||
| Yes | 13 (100.0) | 12 (92.3) | |
| No | 0 | 1 (7.7) | |
| cStage | 0.05 | ||
| IIA | 4 (30.8) | 3 (23.1) | |
| IIB | 3 (23.1) | 7 (53.8) | |
| IIIA | 6 (46.2) | 3 (23.1) | |
| pStage | 0.18 | ||
| IIB | 8 (61.5) | 11 (84.6) | |
| IIIA | 5 (38.5) | 2 (15.4) | |
| Station | 0.21 | ||
| Single | 10 (76.9) | 7 (53.8) | |
| Multiple | 3 (23.1) | 6 (46.2) | |
| Number of metastatic lymph nodes | 1.4±0.7 | 2.4±1.4 | 0.03* |
| Postoperative adjuvant chemotherapy | 0.39 | ||
| Yes | 5 (38.5) | 3 (23.1) | |
| No | 8 (61.5) | 10 (76.9) | |
| Recurrence | >0.99 | ||
| Yes | 7 (53.8) | 7 (53.8) | |
| No | 6 (46.2) | 6 (46.2) | |
| Death | 0.66 | ||
| Yes | 3 (23.1) | 4 (30.8) | |
| No | 10 (76.9) | 9 (69.2) | |
Data are presented as n (%) or mean ± SD. *, P<0.05. cStage, clinical stage; pStage, pathological stage; SD, standard deviation.
Discussion
This study revealed that the tumor diameter of pathological N1 lymph node metastases influences the prognosis of patients with lung adenocarcinoma. This may be useful for identifying patients at high risk for postoperative recurrence. This study represents the first attempt to compare the maximum diameter of N1 lymph node metastasis between primary lung adenocarcinoma and squamous cell carcinoma.
In this study, a significant difference in OS was observed among patients with adenocarcinoma with tumor diameters of pathological N1 lymph node metastases of ≤4 and >4 mm, despite no clinically significant differences in patient characteristics, except for Ki-67 labeling index. Previous studies have indicated that in non-small cell lung cancer (NSCLC), a high Ki-67 labeling index is associated with a decreased survival rate, and some reports have shown that >10% of cases with a high Ki-67 labeling index have a high recurrence rate (20,21). Together with these reports, the present results suggest there may be an association between tumor diameter of lymph node metastases, the Ki-67 labeling index, and OS. OS was thought to be shorter in patients with lymph node metastasis tumor diameter >4 mm because the Ki-67 labeling index was >10% in many of these cases. The Ki-67 labeling index may be useful for the subcategorization of the pN factor. In contrast, given the lack of significant differences between the ≤4 and >4 mm groups, it is improbable that the station(s) or number of metastatic lymph nodes contributed to the present results.
If pathological N1 adenocarcinoma can be subdivided into pN1 (≤4 mm) and pN1 (>4 mm) disease, it may be possible to select appropriate adjuvant chemotherapy and identify cases for rigorous follow-up. This could potentially lead to improved prognoses for adenocarcinoma patients with pN1 disease. In the future, it will be necessary to analyze factors associated with tumor diameter of lymph node metastases, including OS, on a larger scale in patients with pN1 adenocarcinoma.
This analysis was performed at a single institution. The advantage of single-center research is that it fosters collaboration between surgeons and pathologists, and in this study, the identification of lymph nodes was performed using the same criteria. It has been reported that the determination of N1 and N2 disease varies among surgeons (22). To facilitate future multicenter studies, it will be essential to establish precise criteria.
While this study focused on comparing the two major histological types of lung cancer, adenocarcinoma and squamous cell carcinoma, future research may explore a wider range of histological types. Such studies could potentially lead to the development of a histology-specific pN classification for lung cancer, providing more tailored and accurate prognostic information and treatment guidance for patients with different subtypes of the disease. These findings suggest that it is feasible to subdivide the pN parameter using a cutoff value of 4 mm for adenocarcinoma. However, it appears challenging to subdivide pN based on tumor diameter of lymph node metastases in squamous cell carcinoma.
Previous reports have indicated variations in the rates of lymph node metastasis between squamous cell carcinoma and adenocarcinoma of the lung. Some studies have suggested a greater number of positive lymph nodes and a higher rate of lymph node metastasis in patients with adenocarcinoma (23,24). Furthermore, studies comparing the two histological types have identified distinct characteristics, such as adenocarcinoma being associated with female sex, no smoking history, and smaller tumor size, while squamous cell carcinoma is associated with smoking and older age (25). These differences in patient characteristics may contribute to variations in OS, with some reports suggesting a better prognosis for patients with adenocarcinoma, even for the same stage of disease. Clinical differences between adenocarcinoma and squamous cell carcinoma exist, suggesting that similar stratification of the pN parameter may not be feasible as demonstrated herein. Additionally, in this analysis, the difference in the average tumor size of lymph node metastases between adenocarcinoma and squamous cell carcinoma may also explain the reason why they cannot be compared using the same criteria.
As outlined above, adenocarcinoma and squamous cell carcinoma exhibit distinct characteristics, which may translate into differences in aggressiveness, patterns of lymph node metastasis, and overall prognosis. In this study, we observed differences in the mean maximum diameter of lymph node metastasis between adenocarcinoma and squamous cell carcinoma. However, it is important to note that while a cutoff value of 4 mm was used for adenocarcinoma to show a significant difference in OS, the same value could not be applied to squamous cell carcinoma. This suggests that comparing tumor diameters of lymph node metastasis using the same index may not be feasible for these two histological types; this may be the reason why tumor diameter of lymph node metastases has not been adopted for staging of lung cancer.
Carcinomas in other organs often have more finely subdivided pN categories than lung cancer. However, lung cancer encompasses multiple histological types, making it more diverse than some other organ-specific cancers. Given the differences in behavior and characteristics among these histological types, it can indeed be challenging to compare and categorize them using the same scale for pN. With respect to breast cancer, patients with macrometastases have been reported to have a higher postsurgical recurrence rate than those with micrometastases. Within the first 6 years after surgery, the disease-free survival (DFS) rates are comparable between patients without lymph node metastases and those with micrometastases. However, at the 12-year mark, the recurrence rates have been reported to be equivalent between patients with micrometastases and macrometastases (26). In head and neck squamous cell carcinoma (HNSCC), some studies have found that a cutoff value of 10 mm is an independent predictor of DFS and OS (27).
In this analysis, we attempted to subdivide the pN category in pT1 lung adenocarcinoma and squamous cell carcinoma based on the tumor diameter of lymph node metastases. A significant difference in OS was observed for adenocarcinoma using a cutoff value of 4 mm; this may have been due to a high Ki-67 labeling index. We believe that subdividing pN using this method will lead to identification of cases at high risk for recurrence after surgery. In contrast, it was not possible to use 4 mm as a cutoff value for squamous cell carcinoma. We believe this is due to the unique clinicopathological characteristics of squamous cell carcinoma, such as the average diameter of lymph node metastases being larger than those of adenocarcinoma. Additionally, while only one histological type of cancer often occurs in a single organ, lung cancer presents with multiple histological types, which may complicate this analysis.
Limitations
A limitation of this study is the small number of cases, and the study did not consider lymph node station or the number of metastatic lymph nodes. In addition, postoperative treatment also differed. This study is a retrospective analysis; therefore, OS was used as the predictive evaluation criterion. This choice is because the timing and methods for imaging examinations to evaluate recurrence were not standardized. As a result, we considered that DFS could not be accurately calculated. In contrast, OS depends on the survival status of the subjects, making it a more accurate measure than DFS. In the future, it will be necessary to increase the number of cases, standardize the timing and methods of recurrence evaluation, and conduct a prospective study. On the other hand, the number of metastatic lymph nodes, differences in lymph node stations, and the presence or absence of adjuvant chemotherapy could be potential confounding factors for OS.
Conclusions
This study represents the first attempt to compare the maximum diameter of N1 lymph node metastasis between primary lung adenocarcinoma and squamous cell carcinoma cases. The present findings suggest it is feasible to subdivide the pN parameter using a cutoff value of 4 mm for adenocarcinoma. However, it appears challenging to categorize pN based on tumor diameter of lymph node metastases in squamous cell carcinoma. While other organs typically exhibit few variations in histological types among cancers originating from the same organ, lung cancer manifests as many clinically distinct histological types. We believe this is why it is challenging to subdivide pN according to tumor diameter in lymph node metastases of lung cancer.
Acknowledgments
We acknowledge Mr. Kazuki Amemiya (Department of Surgical Pathology, Toho University School of Medicine) for his devoted technical support.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-792/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-792/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-792/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-792/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Information on the use of existing samples is posted on the Toho University Medical Center Omori Hospital Department of Pathology website and opt-outs are available. This study was approved by the Ethics Committee of Toho University Medical Center Omori Hospital (Nos. M21206 [2021] and M24015 [2024]). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute; 2024 Apr 17. [cited 2024 Jun 20]. Available online: https://seer.cancer.gov/statistics-network/explorer/
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12-49. Erratum in: CA Cancer J Clin 2024;74:203. [Crossref] [PubMed]
- Douillard JY, Tribodet H, Aubert D, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J Thorac Oncol 2010;5:220-8. [Crossref] [PubMed]
- Amin MB, Edge SB, Greene FL, et al. 25 Lung, AJCC Cancer Staging Manual, 8th ed., Springer, New York; 2017:251-78.
- Amin MB, Edge SB, Greene FL, et al. 32 Breast, AJCC Cancer Staging Manual, 8th ed., Springer, New York; 2017:345-76.
- Amin MB, Edge SB, Greene FL, et al. PART II Head and neck, AJCC Cancer Staging Manual, 8th ed., Springer, New York; 2017:21-5.
- Amin MB, Edge SB, Greene FL, et al. 3 Lip and Oral Cavity, AJCC Cancer Staging Manual, 8th ed., Springer, New York; 2017:29-40.
- Amin MB, Edge SB, Greene FL, et al. 4 Pharynx, AJCC Cancer Staging Manual, 8th ed., Springer, New York; 2017:41-56.
- Amin MB, Edge SB, Greene FL, et al. 29 Cutaneous Squamous Cell Carcinoma and Other Cutaneous Carcinomas, AJCC Cancer Staging Manual, 8th ed., Springer, New York; 2017:299-344.
- Amin MB, Edge SB, Greene FL, et al. 42 Testis, AJCC Cancer Staging Manual, 8th ed., Springer, New York; 2017:469-78.
- Katsumata S, Aokage K, Ishii G, et al. Prognostic Impact of the Number of Metastatic Lymph Nodes on the Eighth Edition of the TNM Classification of NSCLC. J Thorac Oncol 2019;14:1408-18.
- Riquet M, Manac'h D, Saab M, et al. Factors determining survival in resected N2 lung cancer. Eur J Cardiothorac Surg 1995;9:300-4. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675-84.
- Park S, Cho S, Yum SW, et al. Comprehensive analysis of metastatic N1 lymph nodes in completely resected non-small-cell lung cancer. Interact Cardiovasc Thorac Surg 2015;21:624-9. [Crossref] [PubMed]
- Yamada E, Ishii G, Aramaki N, et al. Tumor-size-based morphological features of metastatic lymph node tumors from primary lung adenocarcinoma. Pathol Int 2014;64:591-600. [Crossref] [PubMed]
- Okudela K, Woo T, Saigusa Y, et al. A method to obtain reproducible Ki-67 indices in lung adenocarcinoma. Histopathology 2021;78:414-23. [Crossref] [PubMed]
- Jakobsen JN, Sørensen JB. Clinical impact of ki-67 labeling index in non-small cell lung cancer. Lung Cancer 2013;79:1-7. [Crossref] [PubMed]
- Wang S, Xie S, Han Y, et al. Role of skip N2 lymph node metastasis for patients with the stage III-N2 lung adenocarcinoma: a propensity score matching analysis. BMC Pulm Med 2023;23:147. [Crossref] [PubMed]
- Xu J, Liu P, Da J, et al. Prognostic value of Ki-67 in stage I non-small-cell lung cancer: A meta-analysis involving 1931 patients. Pathol Res Pract 2019;215:855-60. [Crossref] [PubMed]
- Woo T, Okudela K, Yazawa T, et al. Prognostic value of KRAS mutations and Ki-67 expression in stage I lung adenocarcinomas. Lung Cancer 2009;65:355-62. [Crossref] [PubMed]
- Watanabe S, Ladas G, Goldstraw P. Inter-observer variability in systematic nodal dissection: comparison of European and Japanese nodal designation. Ann Thorac Surg 2002;73:245-8; discussion 248-9. [Crossref] [PubMed]
- Zhang YK, Chai ZD, Tan LL, et al. Association of lymph node involvement with the prognosis of pathological T1 invasive non-small cell lung cancer. World J Surg Oncol 2017;15:64. [Crossref] [PubMed]
- Deng HY, Zeng M, Li G, et al. Lung Adenocarcinoma has a Higher Risk of Lymph Node Metastasis than Squamous Cell Carcinoma: A Propensity Score-Matched Analysis. World J Surg 2019;43:955-62. [Crossref] [PubMed]
- Fukui T, Taniguchi T, Kawaguchi K, et al. Comparisons of the clinicopathological features and survival outcomes between lung cancer patients with adenocarcinoma and squamous cell carcinoma. Gen Thorac Cardiovasc Surg 2015;63:507-13. [Crossref] [PubMed]
- Rosen PP, Saigo PE, Braun DW, et al. Axillary micro- and macrometastases in breast cancer: prognostic significance of tumor size. Ann Surg 1981;194:585-91. [Crossref] [PubMed]
- Xie W, Lin P, Li Z, et al. The prognostic value of lymphatic metastatic size in head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol 2024;281:387-95. [Crossref] [PubMed]


