Ventriculo-arterial coupling: the comeback?
The study of the left ventricle and the arterial circulation as a coupled system is an old “hot topic” that is back into the spotlight. It refers to concepts that are more commonly used by engineers than by clinicians. A source of energy and the load attached to it are considered “ideally coupled” when a maximum of energy is transferred from the source to the load with minimal losses in friction and heat. For the systemic circulation, this means that most of the energy produced by the left ventricle is converted into forward flow and mean arterial pressure to perfuse the body organs, and that a minimum amount of energy is wasted to overcome viscoelastic forces and other phenomena related to the pulsatile character of the left ventricular ejection. The best matching between a source of energy and the load attached to it is governed by the physical properties of the two units. For example, a battery connected to an electrical circuit will achieve maximum output power when its internal impedance equals the input impedance of the load (1). For the cardiovascular system, several approaches have been described to obtain insights into the efficiency of the energy transfer from the LV to the systemic circulation.
The first approach uses a detailed analysis of instantaneous pressure-flow relationship to obtain a global description of the arterial load (the aortic input impedance spectrum) and to calculate its efficiency in converting left ventricular hydraulic power into forward flow. Indeed, the product of pressure by flow has dimensions of power Eq. [1]:
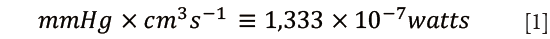
The instantaneous product of pressure (P) by flow (Q) represents the total hydraulic power (Wtot), or total energy transferred from the left ventricle to the systemic circulation and is calculated as Eq. [2]:
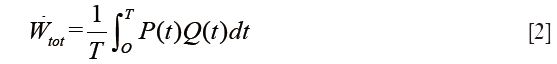
The product of mean pressure (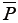 ) by mean flow (
) by mean flow (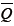 ), or steady power (Wstd), corresponds to the part of that energy that is converted into forward flow, useful for organ perfusion Eq. [3]:
), or steady power (Wstd), corresponds to the part of that energy that is converted into forward flow, useful for organ perfusion Eq. [3]:

The difference between total and steady powers represents the energy that is wasted in pulsatile phenomena and is called oscillatory power (Wosc) Eq. [4]:

The fraction of energy wasted (%Wosc) quantifies the efficiency of energy dissipation in the systemic circulation and is therefore an index of coupling Eq. [5]:

Usually %Wosc is small, attesting for the high efficiency of ventriculo-arterial coupling. But even in situation of altered arterial mechanical properties, such as systemic hypertension, it remains small (<15% of Wtot) and is minimally affected by various antihypertensive treatments (2). However, in a rabbit model of septic shock (another situation of altered arterial mechanical properties), we were able to demonstrate a significant increase in %Wosc independent of fluid resuscitation as a result of endotoxin injection (3). This was explained in part by the increase in aortic characteristic impedance resulting from a loss in aortic endothelium-dependent vasodilatation and from aortic wall edema. This experiment was an early evidence for altered ventriculo-arterial coupling in the setting of septic shock.
Another way to look at the coupling between the left ventricle and the arteries is the representation described by the group of H Suga and K Sagawa, who used the pressure-volume relationships to describe the mechanical properties of the LV and the arteries. The left ventricular contractility can be characterized by the slope of the end-systolic pressure-volume relation called end-systolic elastance (Ees) (4). The slope of the vascular end-systolic pressure-volume relationship represents the effective elastance of the arterial system (Ea) (5). By analogy to the coupling between cardiovascular function and venous return curves, a given hemodynamic situation may be seen as the result of the interaction between ventricular and arterial mechanical characteristics (i.e., Ees and Ea, respectively). Sunagawa et al. have presented a simple framework that illustrates this concept (5). Arterial elastance (Ea) can be included in the ventricular end-systolic pressure-volume diagram, so that its origin on the volume axis lies at end-diastolic volume. The intersection of −Ea and Ees yields a unique pair of values for stroke volume and end-systolic pressure corresponding to that given hemodynamic situation (Figure 1). The pressure-volume diagram of the left ventricle also contains information about ventricular energetics (7). The area contained within the loop itself has dimensions of work (joules) and represents the external stroke work (SW) of the left ventricle. The triangular area between Ees and the PV loop represents the elastic potential energy (PE) of the LV (Figure 1). The sum of PE and SW represents the total energy dissipated during the cardiac cycle (7) and has been shown to correlate with myocardial oxygen consumption (8,9). Therefore, the metabolic efficiency of myocardial contraction may be evaluated as the ratio of SW/(SW + PE) (6,7). According to basic geometrical rules, the SW area will be maximized if the slopes of Ees and Ea are equal, meaning that the mechanical output of the cardiovascular system is maximum in that situation. On the other hand, the ratio between SW and (SW + PE) is maximum when the value of Ea is half of that of Ees, a situation that corresponds to optimum metabolic efficiency (6,10).
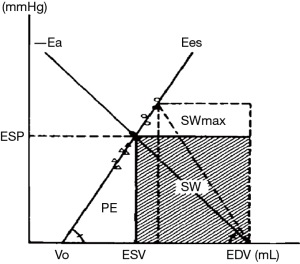
Is this relevant for daily clinical practice? Yes! Even if we are not always aware of it, we use an index of coupling routinely. The ejection fraction (EF) of the left ventricle is in fact an index of ventriculo-arterial coupling (rather than an index of LV systolic function). Indeed, it can be demonstrated that EF can be expressed as a function of Ees and Ea Eq. [6] (10,11):

In the situation of optimal metabolic efficiency, when Ees = 2× Ea, EF is roughly equal to 66% (EF =1/1.5=0.66). Whereas in the situation of maximal SW (when Ees = Ea), EF is roughly equal to 50%. The “normal” value for EF is usually between 60% and 70%, suggesting that the normal cardiovascular system operates in a way that optimizes metabolic efficiency rather than maximizing external work (6,10,11). A low EF reflects the inability of the cardio-vascular system to match the mechanical properties of the heart and the arteries in order to operate at the lowest energetic cost. This might explain in part why an abnormal EF appears as a potent predictor of cardiovascular mortality.
Even though the heart itself has received much more attention than its vascular load, the latter plays a major role in determining the performance of the coupled ventriculo-arterial system. The recently published work by Morelli et al. focuses on arterial elastance in septic shock patients and the effects of esmolol (12). The authors observed a decrease in arterial elastance, concomitant to the decrease in heart rate. Since arterial elastance (Ea) was calculated as Eq. [7]:
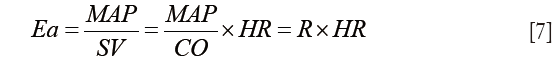
it was expected that reducing heart rate would reduce Ea. Whether or not this was associated with improved VA coupling remains to be demonstrated as left ventricular Ees was not estimated in this work. However, by reducing heart rate one certainly reduces myocardial oxygen consumption and improves LV filling by increasing the duration of diastole. As a result, stroke volume increased and cardiac output was maintained. The reduction in mean arterial pressure could be the result of esmolol administration or due to the tapering in norepinephrine dose. Left ventricular EF remained unaltered, suggesting that the cardiovascular system was operating under conditions of acceptable ventriculo-arterial matching.
Acknowledgements
None.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Zhongheng Zhang (Department of Critical Care Medicine, Jinhua Municipal Central Hospital, Jinhua Hospital of Zhejiang University, Jinhua, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yin FC. Ventricular/Vascular Coupling—Clinical, Physiological, and Engineering Aspects. New York: Springer-Verlag New York, 1987.
- Cholley BP, Shroff SG, Sandelski J, et al. Differential effects of chronic oral antihypertensive therapies on systemic arterial circulation and ventricular energetics in African-American patients. Circulation 1995;91:1052-62. [Crossref] [PubMed]
- Cholley BP, Lang RM, Berger DS, et al. Alterations in systemic arterial mechanical properties during septic shock: role of fluid resuscitation. Am J Physiol 1995;269:H375-84. [PubMed]
- Suga H, Sagawa K. Instantaneous pressure-volume relationships and their ratio in the excised, supported canine left ventricle. Circ Res 1974;35:117-26. [Crossref] [PubMed]
- Sunagawa K, Maughan WL, Burkhoff D, et al. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol 1983;245:H773-80. [PubMed]
- Asanoi H, Sasayama S, Kameyama T. Ventriculoarterial coupling in normal and failing heart in humans. Circ Res 1989;65:483-93. [Crossref] [PubMed]
- Suga H. Ventricular energetics. Physiol Rev 1990;70:247-77. [PubMed]
- Suga H, Hayashi T, Shirahata M. Ventricular systolic pressure-volume area as predictor of cardiac oxygen consumption. Am J Physiol 1981;240:H39-44. [PubMed]
- Nozawa T, Yasumura Y, Futaki S, et al. Relation between oxygen consumption and pressure-volume area of in situ dog heart. Am J Physiol 1987;253:H31-40. [PubMed]
- Hayashida K, Sunagawa K, Noma M, et al. Mechanical matching of the left ventricle with the arterial system in exercising dogs. Circ Res 1992;71:481-9. [Crossref] [PubMed]
- Robotham JL, Takata M, Berman M, et al. Ejection fraction revisited. Anesthesiology 1991;74:172-83. [Crossref] [PubMed]
- Morelli A, Singer M, Ranieri VM, et al. Heart rate reduction with esmolol is associated with improved arterial elastance in patients with septic shock: a prospective observational study. Intensive Care Med 2016. [Epub ahead of print]. [Crossref] [PubMed]


