
Efficacy and safety of mesenchymal stem cell therapy for acute respiratory distress syndrome—a systematic review and meta-analysis
Highlight box
Key findings
• Mesenchymal stem cells (MSCs) do not elevate the risk of adverse reactions in acute respiratory distress syndrome (ARDS) patients.
• Significant reductions in mortality of ARDS can be seen during MSC treatments.
• MSCs can also improve patients’ clinical symptoms to a certain extent.
• MSC therapy effectively regulates the uncontrolled inflammatory response.
What is known and what is new?
• MSC therapy for ARDS represents a burgeoning treatment approach, supported by numerous preclinical studies confirming its efficacy.
• Our study comprehensively analyzed the safety and effectiveness of MSC treatment from the clinical trial. We found that MSCs are reliably safe and can significantly improve certain clinical manifestations, reduce inflammatory reactions, and lower mortality rates. Notably, the improvement in clinical symptoms and anti-inflammatory effect has never been reported in previous similar studies.
What is the implication, and what should change now?
• It suggested that MSCs is an effective method to treat ARDS. However, these findings necessitate further validation through high-quality randomized controlled trials.
Introduction
Acute respiratory distress syndrome (ARDS) is a prevalent clinical syndrome characterized by diffuse pulmonary inflammation and edema, leading to acute respiratory failure. A primary cause of ARDS is the systemic inflammatory response triggered by endotoxins or injurious factors (1-3). This response increases endothelial and epithelial permeability, resulting in alveolar edema and worsening respiratory failure, ultimately leading to ARDS (4). The LUNG-SAFE study indicates that ARDS patients constitute 10.4% of intensive care unit (ICU) admissions in 50 countries, with a high mortality rate ranging from 34.9% to 46.1% (5). Current conventional treatments for ARDS primarily focus on respiratory support, including lung-protective ventilation strategies, prone positioning, extracorporeal membrane oxygenation (ECMO), and others (6). Therefore, there is an urgent need for research into new therapeutic approaches targeting the pathogenesis of ARDS.
As a result of the coronavirus disease 2019 (COVID-19) outbreak, the uncontrolled inflammatory response in ARDS is being increasingly scrutinized. Regulating the pro-inflammatory and anti-inflammatory balance in ARDS has become crucial. Mesenchymal stem cells (MSCs) are proposed as a potential therapeutic modality for ARDS due to their ability to modulate this balance.
MSCs possess characteristics of plastic adhesion and multipotent differentiation potential, making them advanced cell therapy products. MSCs can be isolated from various sources, including bone marrow, adipose tissue, perinatal tissues, dermal tissues, dental tissues, and peripheral blood (7). The potential of MSCs as a treatment for ARDS has been demonstrated in numerous animal experiments.
Firstly, MSCs can modulate the balance of the inflammatory environment by directly secreting soluble factors that regulate immune cells, suppressing pro-inflammatory cytokines, and upregulating anti-inflammatory cytokines (8,9). Secondly, in a mouse lipopolysaccharide (LPS) model, MSCs can reduce tissue damage in the ARDS model, mitigating alveolar hemorrhage, edema, membrane formation, and collagen deposition while restoring the function of endothelial and epithelial cells (10,11). Lastly, MSCs can secrete antimicrobial peptides (AMPs) with direct antibacterial effects to enhance bacterial clearance (9,10,12).
Despite some clinical trials assessing the safety and efficacy of MSC therapy for ARDS, uncertainties persist due to generally small sample sizes and many are associated with COVID-19 (13-29). Therefore, we conducted a comprehensive meta-analysis of clinical trials involving MSC treatment for ARDS patients up to June 23, 2024. We evaluated adverse events, mortality, PaO2/FiO2 ratio, ICU length of stay, ventilation-free days, and changes in pro-inflammatory and anti-inflammatory cytokines to thoroughly assess the safety and effectiveness of MSC therapy for ARDS. We present this article in accordance with PRISMA reporting checklist (30) (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-281/rc).
Methods
Protocol and registration
The study protocol was registered at the International Prospective Register of Systematic Reviews (CRD42023427079).
Eligibility criteria
Our inclusion criteria were as follows: (I) study type: all published randomized controlled trials (RCTs) that evaluated the safety and/or efficacy of MSCs. (II) Study subjects: individuals aged 18 years and above, conclusively diagnosed with ARDS based on the Berlin definition. (III) Intervention measures: the intervention involved the application of MSCs. (IV) Outcome measures: the included outcome measures comprised adverse events, mortality, ICU length of stay, ventilation-free days, and changes in pro-inflammatory and anti-inflammatory cytokines.
The exclusion criteria were as follows: (I) conference records and abstracts; (II) case series studies; (III) animal experiments; (IV) clinical protocols; (V) data that could not be extracted.
Data collection
Between January 23, 2024 and June 23, 2024, we systematically searched for relevant studies in three databases: PubMed, Embase, and Cochrane, encompassing publications up to June 23, 2024.
Search strategy
The search keywords included ‘ARDS’, ‘ALI’, ‘Acute Respiratory Distress Syndrome’, ’shock Lung’, ‘acute lung injury’, ‘Respiratory Distress Syndromes’, ‘MSCs’, ‘Mesenchymal Stem Cell’, ‘Mesenchymal Stromal Cell’, and ‘Mesenchymal Progenitor Cell’. The detailed search strategies for the three English databases can be found in the Appendix 1.
Study selection
Two researchers independently conducted title and abstract screening in the databases to identify literature for full-text assessment. If the information in the titles and abstracts met the inclusion criteria, both researchers retrieved the full text for independent screening. In cases of disagreement, consensus was reached through discussion, or a third party was consulted for resolution.
Data extraction
Two independent data extractors collected pertinent information according to our predefined data extraction table. In instances of discordance, the two extractors engaged in discussion to achieve consensus. The extracted data encompassed: (I) first author, publication year; (II) study type; (III) number of included patients; (IV) details regarding MSC and control group sources, doses, administration routes, and timing; (V) adverse events, mortality, ICU length of stay, ventilation-free days, and changes in pro-inflammatory and anti-inflammatory cytokines.
Analysis of results
The primary outcomes of the study focused on adverse events and all-cause mortality. Secondary outcomes included the changes in the PaO2/FiO2 ratio, ICU length of stay, ventilation-free days, as well as changes of levels of pro-inflammatory and anti-inflammatory cytokines.
Risk of bias assessment
For RCTs, we employed the Cochrane Risk of Bias tool for evaluation. This tool assesses six key aspects: (I) sequence generation, (II) allocation concealment, (III) blinding of participants and outcome assessors, (IV) incomplete outcome data, (V) selective outcome reporting, and (VI) other sources of bias. We utilized the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) approach to ascertain the certainty of the impact of MSCs on adverse event rates and mortality.
Statistical analysis
We conducted a meta-analysis of the safety and efficacy of MSCs in treating ARDS using RevMan 5.4 and Stata 15.0 software. For dichotomous variables, we calculated the relative risk (RR) for each relevant outcome between the experimental and control groups. For continuous variables, we computed the mean difference (MD) or standardized mean difference (SMD) between the experimental and control groups. Heterogeneity among studies was assessed using the I2 test. The random-effects model was utilized in all our analyses. Funnel plots and the trim-and-fill method were employed for publication bias analysis. Sensitivity analysis was performed to assess the stability of the results. Additionally, subgroup analysis was conducted based on different sources of MSCs. All statistical tests were two-tailed, and a P value less than 0.05 was considered statistically significant.
Results
Literature screening process
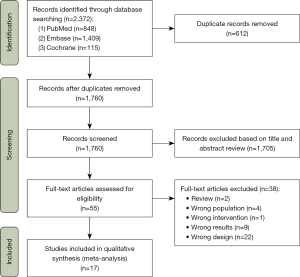
Following the designated search strategy, we identified 2,372 records in the databases. After eliminating 612 duplicates, 1,760 remaining records underwent title and abstract screening. During this initial screening, 1,705 records were preliminarily excluded. Subsequently, full-text screening was conducted, resulting in the exclusion of thirty-eight articles for the following reasons: (I) 2 articles were reviews; (II) 4 articles had subjects that did not meet the inclusion criteria; (III) 1 article had inappropriate intervention measures; (IV) 9 articles reported outcomes that did not meet the requirements; (V) 22 articles had flawed experimental designs. Ultimately, 17 articles met the criteria and were included in the meta-analysis (Figure 1).
Characteristics of included studies
Tables 1,2 provide a comprehensive overview of the primary features of the 17 included studies and the demographic details of the patients. A total of 796 patients were enrolled in these studies, with 410 in the MSC group and 386 in the control group; of these, males comprised 62.75%. Regarding the severity of ARDS, two studies delineated the distribution among mild, moderate, and severe ARDS patients, while five studies included individuals with PaO2/FiO2 <200 mmHg. Concerning the etiology of ARDS, 15 studies reported COVID-19 as the inducing factor, while the remaining two did not specify the cause. Characteristics of MSCs in all studies matched the International Society for Cell & Gene Therapy (ISCT) criteria. The MSC products employed for treatment originated from diverse sources, including adipose tissue, bone marrow, umbilical cord, and placenta. Dosages ranged from 1×106/kg to 10×106/kg, with intravenous injection as the common administration route. Meanwhile, concomitant treatments of the included studies were reported in the Table S1.
Table 1
| References | Year | Design | Sample size (MSC/COT) | Age (MSC/COT), years | Gender (male ratio) | Group (dose, treatment duration) | Outcomes | |
|---|---|---|---|---|---|---|---|---|
| MSC | COT | |||||||
| Monsel et al. (23) | 2022 | RCT | 45 (21/24) | 64.00±10.40/63.20±11.40 | 17 /21 vs. 20/24 | UC-MSCs, 3×106 cells/kg body weight, IV | 150 mL NS | PaO2/FiO2, biomarkers of endothelial, alveolar epithelial injury and inflammatory response, SARS-CoV-2 N-antigenemia and viral RNA levels, HLA and DSAs directed against UC-MSCs |
| Bowdish et al. (15) | 2023 | RCT | 222 (112/110) | 61.80±13.00/59.60±13.80 | 79/112 vs. 75/110 | BM-MSCs, 2×106 MSC/kg of body weight, IV | Placebo | All-cause mortality, days alive off mechanical ventilation within 60 days, resolution and/or improvement of ARDS, and clinical improvement, total and ICU LOS, the total number of days in hospital, adverse events |
| Rebelatto et al. (25) | 2022 | RCT | 17 (11/6) | 53±15.3/61.7±9.7 | 8/ 11 vs. 4/6 | UC-MSCs, 5×106 cells/kg body weight, IV | Placebo | Adverse events, patient recovery demonstrated through viral load, blood tests and plasma levels of inflammatory cytokines, PBMC assessment of T cell populations, PASC reduction, CT scan |
| Lanzoni et al. (20) | 2021 | RCT | 24 (12/12) | 58.58±15.93/58.83±11.61 | 5/12 vs. 8/12 | UC-MSCs, 100±20×106 UC-MSCs 2 IV dose | 50 mL vehicle solution | Adverse events, survival at day 28, time to recovery, viral load, inflammatory cytokines, chemokines, growth factors |
| Zheng et al. (29) | 2014 | RCT | 12 (6/6) | 66.7±20.4/69.8±9.1 | 6/6 vs. 5/6 | AD-MSCs, 1×106 cells/kg of body weight, one IV dose COT:NS | NS | Adverse events, oxygenation index, length of hospital stay, ventilator-free days, ICU-free days at day 28, SP-D, IL-6 or IL-8 levels in serum |
| Aghayan et al. (14) | 2022 | RCT | 20 (10/10) | 62.30/58.40 | 6/10 vs. 8/10 | PL-MSCs, 1×106 cells/kg body weight, IV | Placebo | Adverse events, vital signs, mortality, the duration of hospitalization, biochemistry, hematology parameters, CD4+ and CD8+ T-cells |
| Dilogo et al. (16) | 2021 | RCT | 40 (20/20) | NR | 15/20 vs. 15/20 | UC-MSCs, 1×106 cells/kg body weight, one IV dose | 100 mL NS | Mortality rate, length of ventilator usage, length of stay in the ICU, improvement in the routine laboratory value, improvement in biomarker laboratory value of cytokines and lymphocyte subpopulation, adverse events and serious adverse events |
| Matthay et al. (22) | 2019 | RCT | 60 (40/20) | 55.00±17.00/55.00±20.00 | 23 /40 vs. 10/20 | BM-MSCs, 10×106 cells/kg body weight, one IV dose | Placebo | Adverse events, all-cause mortality, ventilator-free days to day 28, duration of ventilation in patients alive, intensive-care-free days, days free from organ failure, SOFA score, oxygenation index, the lung injury score, angiopoietin 2, IL-6 and IL-8, RAGE |
| Kaffash Farkhad et al. (19) | 2022 | RCT | 20 (10/10) | 62.00±2.42/61.30±5.34 | 7/10 vs. 6/10 | UC-MSCs, 1×106 cells/kg body weight, IV | Placebo | Mortality, PaO2/FiO2, lung imaging, infammatory biomarkers such as IL-1 beta, IL-6, TNF-α |
| Gorman et al. (18) | 2023 | RCT | 59 (30/29) | 58.40±9.20/58.40±12.5 | 24/30 vs. 20/29 | UC-MSCs, 400×106 cells/person, IV | Placebo | Adverse events, oxygenation index, indices of pulmonary and nonpulmonary organ dysfunction, PaO2/FiO2 ratio, SOFA, extubation, reintubation, ventilator-free days, lengths of ICU, hospital stays, mortality, RNA sequencing |
| Pochon et al. (24) | 2023 | RCT | 30 (15/15) | 58.45±13.90/65.64±7.36 | 13/15 vs. 7/15 | UC-MSCs, 1×106 cells/kg, IV | Placebo | The percentage of patients with a PaO2/FiO2 >200 mmHg, PaO2/FiO2, ventilator free days, SOFA, ICU length of stay, respiratory morbidity, RT-PCR SARS-CoV-2 positivity, adverse events |
| Zarrabi et al. (28) | 2023 | RCT | 35 (11/24) | 50.00±12.48/47.75±12.72 | 10/11 vs. 16/24 | MSCs derived from perinatal tissue, 100×106 cells/person, IV | Placebo | Adverse events, CBC, ABG, biochemistry analysis, inflammatory parameters |
| Adas et al. (13) | 2021 | RCT | 20 (10/10) | NR | NR | WJ-MSCs, 3×106 cells/kg body weight, IV | Placebo | Adverse events, mortality, inflammatory parameters |
| Shi et al. (26) | 2021 | RCT | 100 (65/35) | 60.72±9.14/59.94±7.79 | 37/65 vs. 19/35 | UC-MSC, 4.0×107 cells/person, IV | Placebo | Adverse event, chest CT, lung volume |
| Shu et al. (27) | 2020 | RCT | 41 (12/29) | 61.00±17.87/57.86±15.79 | 8/12 vs. 16/29 | UC-MSCs, 2×106 cells/kg, IV | Placebo | The incidence of progression, the time to a clinical improvement, seven-category ordinal scale, hospital stay, oxygenation index, hematological inflammatory factors, imaging |
| Martínez-Muñoz et al. (21) | 2024 | RCT | 20 (10/10) | 61.34±25.80/61.81±24.94 | 5/10 vs. 8/10 | BM-MSCs, 1×106 MSC/kg, IV | Placebo | PaO2/FiO2, mortality, clinical status, adverse events, inflammatory parameters |
| Fathi-Kazerooni et al. (17) | 2022 | RCT | 30 (15/15) | 46.43±11.91/53.67±10.30 | 9/15 vs. 10/15 | MSCs derived from the menstrual blood, 5 mL, IV | Placebo | Adverse events, mortality, chest CT, time to recovery, inflammatory parameters |
Data types: sample size/gender: number; age: mean, mean ± standard deviation. MSC, mesenchymal stem cell; COT, control group; RCT, randomized controlled trial; UC-MSC, umbilical cord mesenchymal stem cell; IV, intravenous infusion; NS, normal saline; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; N-antigenemia, nucleocapsid antigenemia; HLA, human leukocyte antigen; DSA, donor-specific antibodies; BM-MSC, bone-marrow-derived mesenchymal stem cell; ARDS, acute respiratory distress syndrome; ICU, intensive care unit; LOS, length of stay; PBMC, peripheral blood mononuclear cell; PASC, post-acute sequelae of SARS-CoV-2 infection; CT, computed tomography; AD-MSC, adipose-derived mesenchymal stem cell; SP-D, surfactant protein D; IL, interleukin; PL-MSC, placental mesenchymal stem cells; SOFA, sequential organ failure assessment; RAGE, receptor for advanced glycation end-products; TNF, tumor necrosis factor; RT-PCR, reverse transcription polymerase chain reaction; CBC, complete blood count; ABG, arterial blood gas; NR, not reported.
Table 2
| References | BMI [MSC/COT], kg/m2 | SOFA [MSC/COT] | Comorbidities (MSC/COT) | Severity of ARDS | PaO2/FiO2 [MSC/COT] |
|---|---|---|---|---|---|
| Monsel et al. (23) | 28.6 [3.5]/28 [5.5] | 5.5 [2.7]/5.9 [2.7] | HT: 11/10, DM: NR | NR | 156.2 [68.2]/171.2 [72.9] |
| Bowdish et al. (15) | NR | 6.6 [2.1]/6.7[1.9] | HT: 65/63, DM: 46/42 | Moderate 79/76, severe 33/34 | NR |
| Rebelatto et al. (25) | NR | NR | HT: 6/3, DM: 4/3 | Mild 4/5, moderate 6/0, severe 1/1 | NR |
| Lanzoni et al. (20) | 34.5 [4.5]/29.6 [3.5] | NR | HT: 7/9, DM: 5/6 | Mild-to-moderate 3/3, moderate-to-severe 9/9 | 118.1 [80.5]/114.7 [81.3] |
| Zheng et al. (29) | NR | NR | HT: 3/3, DM: 2/1 | NR | 122.4 [42.0]/103.5 [32.2] |
| Aghayan et al. (14) | NR | NR | HT: 4/4, DM: 3/4 | NR | NR |
| Dilogo et al. (16) | NR | NR | HT: 6/10, DM: 8/12 | NR | NR |
| Matthay et al. (22) | NR | 8.1 [3.3]/6.9 [2.7] | NR | NR | 135.8 [32.3]/143.3 [39] |
| Kaffash Farkhad et al. (19) | NR | NR | HT: 1/3, DM: 2/1 | NR | NR |
| Gorman et al. (18) | NR | 7.7 [3.4]/7.9 [3.1] | NR | NR | 15.2 [4.2]/16.1 [5.4] |
| Pochon et al. (24) | 30.7 [6.5]/34.0 [3.3] | 4.4 [2.5]/5.4 [4.1] | HT: 5/10, DM: 3/4 | Moderate-to-severe 15/15 | 138 [49]/137 [36] |
| Zarrabi et al. (28) | NR | NR | NR | NR | NR |
| Adas et al. (13) | NR | NR | NR | NR | NR |
| Shi et al. (26) | 24.71 [3.19]/25.01 [3.02] | NR | HT: 17/10, DM: 12/5 | NR | NR |
| Shu et al. (27) | NR | NR | HT: 3/6, DM: 3/5 | NR | NR |
| Martínez-Muñoz et al. (21) | 29.0 [3.1]/32.0 [4.5] | NR | HT: NR, DM: 4/2 | NR | 99.5 [42.1]/91.0 [37.6] |
| Fathi-Kazerooni et al. (17) | NR | NR | HT: 4/5, DM: 3/4 | NR | NR |
Data types: BMI, SOFA, PaO2/FiO2: mean [standard deviation]; comorbidities, severity of ARDS: number. BMI, body mass index; MSC, mesenchymal stem cell; COT, control group; SOFA, sequential organ failure assessment; ARDS, acute respiratory distress syndrome; HT, hypertension; DM, diabetes mellitus; NR, not reported.
Primary results
The primary outcome for assessing the safety of MSC therapy is the number of adverse events. Among the 17 included studies, 12 reported adverse event numbers. Of these, 5 studies documented adverse events related to MSC infusion, all of which were mild and self-limiting (16,18,22,24,29). Additionally, 9 studies reported severe adverse events, but these were deemed unrelated to MSC infusion (13,15,16,18,20,22,24,26,29). The consolidated findings revealed no significant difference in adverse event numbers between the MSC and control groups, suggesting that MSC infusion does not lead to an increase in adverse events [RR =1.04; 95% confidence interval (CI): 0.90, 1.19; P=0.59; I2=0%] (Figure 2). The evidence quality was considered good, with no evident heterogeneity observed and no notable publication bias detected (Figure S1).
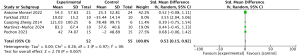
For all-cause mortality, data from all 16 studies were available. Combining the results across all studies, it indicates that MSCs can significantly reduce mortality (RR =0.79; 95% CI: 0.64, 0.97; P=0.02; I2=0%) (Figure 3). The evidence exhibits low heterogeneity, and no evident publication bias was observed (Figure S2). Sensitivity analysis indicated a robust stability of the results (Figures S3,S4).
Secondary outcomes
Change in the PaO2/FiO2 ratio
Five studies examined changes in the PaO2/FiO2 ratio, and our analysis identified significant heterogeneity in one of these studies (19,21,23,24,29). Upon exclusion of this outlier, it was revealed that MSC treatment had an obvious positive effect on improving the PaO2/FiO2 ratio (SMD =0.53; 95% CI: 0.15, 0.92; P=0.007; I2=0%) (Figure 4, Figure S5). This suggests that MSCs may enhance clinical outcomes to some extent. Additionally, no significant publication bias was detected, and the quality of the data remained stable (Figures S6,S7).
ICU length of stay
Five studies provided data on the ICU length of stay (15,16,18,21,24). The results of the analysis indicate that, in comparison to the control group, the MSC group shows a trend towards a shorter ICU stay, although it lacked statistical significance (MD =−1.77; 95% CI: −6.97, 3.43; P=0.50; I2=63%) (Figure S8). This observation may be influenced by the small sample size in the included experiments and baseline imbalances. The data did not reveal any significant bias (Figure S9).
Ventilation-free days
The duration without mechanical ventilation supporting to some extent reflected the extent of respiratory function recovery in ARDS patients. Six studies analyzed the days without mechanical ventilation support (15,18,22-24,29). Unfortunately, similar to the ICU length of stay, MSC treatment had the potential to increase ventilation-free days, but the change was not statistically significant (MD =−1.29; 95% CI: −4.09, 1.51; P=0.37; I2=0%) (Figure S10). The data quality was high, and there is low heterogeneity (Figure S11).
Analysis of pro-inflammatory and anti-inflammatory cytokines
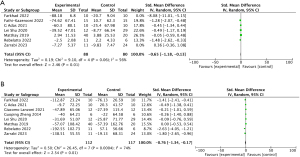
All 17 studies reported data on pro-inflammatory and anti-inflammatory cytokines. Among them, seven studies provided values for C-reactive protein (CRP) (13,17,19,22,25,27,28). The analysis of ∆CRP (the difference in CRP levels between baseline and the endpoint) revealed a significant difference in CRP levels between the MSC and control groups whether or not heterogeneity is excluded (SMD =−0.65; 95% CI: −1.18, −0.13; P=0.01; I2=56%) (Figure 5A, Figure S12). Eight studies reported changes in interleukin-6 (IL-6) levels (13,19,20,22,25,27-29), and pooled analysis of ∆IL-6 showed a significant reduction in IL-6 levels in the MSC group (SMD =−0.76; 95% CI: −1.34, −0.17; P=0.01; I2=74%) (Figure 5B). Other pro-inflammatory factors such as tumor necrosis factor alpha (TNF-α) (SMD =−1.5; 95% CI: −3.39, 0.40; P=0.12; I2=92%) showed no significant differences between the MSC and control groups (Figure S13).
Concerning anti-inflammatory factors, we performed a statistical analysis of ∆IL-10 levels. After excluding an article with significant heterogeneity, the results revealed no statistically significant difference in IL-10 levels between the MSC group and the control group (SMD =−0.46; 95% CI: −1.51, 0.58; P=0.38; I2=77%) (Figure S14).
The results presented above exhibit significant heterogeneity. Galbraith plot for heterogeneity in ∆IL-6 (Figure S15) revealed that 3 articles exhibited high heterogeneity in ∆IL-6, collectively representing 37.5% of the total studies included in the meta-analysis. Simple exclusion of these studies was deemed inappropriate. Subsequently, we conducted a trim-and-fill analysis, which did not alter the results. Moreover, sensitivity analyses for it affirmed the stability and reliability of the findings (Figures S16,S17). Correlation analysis of CRP level also showed similar results (Figures S18,S19). The limited number of studies and inconsistency in endpoint times may contribute to the observed heterogeneity.
Subgroup analysis results
We performed subgroup analyses for all-cause mortality rates and the number of adverse events based on the different sources of MSCs. The studies included 3 using MSCs from bone marrow (15,21,22), 1 from placental origin (14), 1 from adipose tissue (29), 10 from umbilical cord (13,16,18-20,23-27), 1 from human menstrual blood (17), and 1 from perinatal tissue (without clarification if it was from placenta or umbilical cord) (28).
Mortality was statistically significant for UC-MSCs but not for bone marrow origin MSCs, likely due to the small number of studies (Figure S20). No significant difference was observed between subgroups in the number of adverse events (Figure S21).
Risk of bias assessment
We employed the ROB2 tool to evaluate the risk of bias in RCTs. Most domains received a low-risk rating, with some uncertainties primarily arising from unspecified randomization methods (Figure S22).
Discussion
Our study reveals that, firstly, in terms of safety, MSCs exhibit similarity to the standard treatment group, suggesting that the use of MSCs does not elevate the risk of adverse reactions. Secondly, significant reductions in mortality can be seen during MSC treatments. Lastly, MSC therapy not only effectively regulates the uncontrolled inflammatory response but also improves patients’ clinical symptoms to a certain extent.
Among the 17 included studies, the analysis of adverse reactions indicates the reliability of MSC treatment’s safety. Adverse reactions induced by MSCs were mostly non-severe, such as diarrhea and rash, with the majority of patients recovering within 1–2 days, as reported in most studies. Such results are consistent with the findings of Wilson et al. (31).
According to previous studies, the over-activated immune state in ARDS patients, characterized by a severe imbalance between anti-inflammatory and pro-inflammatory factors, is a significant cause of their mortality (3). In contrast, MSCs have been shown to inhibit pro-inflammatory factors and increase the capacity of anti-inflammatory factors (32,33). Based on this mechanism, MSC treatment has the potential to reduce mortality in ARDS patients. A meta-analysis of animal experiments on MSC treatment of ARDS by McIntyre et al. found that MSC substantially reduced mortality in animal models of ARDS (34). This finding is consistent with the results of our meta-analysis of clinical studies. Furthermore, Chen’s cohort study also supports our conclusion (35). Our study, which used mortality as the primary measure of the effectiveness of MSC treatment, found that MSC significantly reduced mortality in ARDS patients. Only the studies by Matthay and Rebelatto showed opposite results (22,25), which were related to the imbalance of clinical baseline characteristics between the experimental and control groups.
Alveolar injury caused by a storm of inflammatory factors is a significant determinant of respiratory function and prognosis in patients with ARDS. Improvement in clinical symptoms in ARDS depends on the recovery of alveolar epithelial function. Animal experiments have demonstrated that MSC reduces inflammatory lung injury and promotes the recovery of alveolar epithelial function (36). In the case report series by Atluri et al., MSC effectively relieved patients’ clinical symptoms and improved their oxygenation index, consistent with our findings (37). However, these results still need to be supported by more large-scale clinical trials.
In our statistical analysis of anti-inflammatory and pro-inflammatory cytokines, MSCs were found to significantly reduce CRP and IL-6 levels, especially the IL-6 levels, aligning with the findings of Jackson et al. Their study demonstrated that in an ARDS model, MSC administration led to a substantial reduction in IL-6 levels (38-43). IL-6 plays a pivotal role in the inflammatory response to ARDS. A marked increase in IL-6 can trigger various immune cells to migrate from the circulation to specific organs, resulting in immune hyperactivity and invasion of lung tissue (44). Evidence suggests that ARDS patient survival rates are lower when the baseline level of IL-6 is higher, and the substantial reduction in IL-6 levels induced by MSCs partially reflects the potency of the anti-inflammatory impact of MSCs (45). While a trend of reduction in pro-inflammatory factors such as TNF-α was observed, significance was not evident.
In terms of anti-inflammatory factors, Rebelatto’s study shows that the ∆IL-10 levels can also be substantially reduced (25). However, in our study, we found that the MSC group did not show a significant increase in the levels of IL-10. Therefore, more experiments are needed to prove that MSC can promote the production of anti-inflammatory factors.
We observed significant heterogeneity in the statistical results of these biomarkers. After analyzing the sources of heterogeneity, we found that half of the studies contributed to the observed heterogeneity, suggesting the need for more large-scale RCTs to systematically investigate changes in the levels of inflammatory factors and standardize endpoint time points to reduce heterogeneity. However, through the application of trim-and-fill analysis and sensitivity analysis, we bolstered the stability of the results, indicating that the findings are robust and reliable. Thus, to some extent, it can be inferred that MSCs may mitigate inflammatory responses and modulate the inflammatory storm in ARDS.
Although MSC therapy is a promising treatment, it is crucial to use it wisely. We analyzed different sources of MSC as one of the factors that may affect its effectiveness (46-48). In our subgroup analysis of different MSC sources, we found that only the umbilical cord source demonstrated a statistically significant reduction in mortality. This may also be related to the lack of experimental data for the bone marrow source. Additionally, varying MSC doses and the microenvironment of ARDS patients may influence the efficacy of MSC, which warrants further investigation in future studies.
There are some limitations in this study. Firstly, the absence of large-scale RCTs and the relatively small sample size may introduce selection bias. Secondly, the measurement of mortality and laboratory indicators at non-uniform time points introduces variability. Thirdly, there is an imbalance in the male-to-female ratio, with a higher proportion of males. Lastly, the majority of experiments focused on ARDS caused by COVID-19, and there is a lack of data from studies on ARDS unrelated to COVID-19.
Conclusions
In summary, the safety of MSC therapy is deemed reliable. MSCs have the ability to reduce mortality and improve clinical symptoms to some extent. Furthermore, MSCs may offer certain benefits in alleviating the inflammatory response in ARDS. However, these findings necessitate further validation through high-quality RCTs.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-281/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-281/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-281/coif). All authors report that this study was supported by National Key R&D Program of China (No. 2022YFC2504405), the Clinical Science and Technology Specific Projects of Jiangsu Province (BE2020786), the National Natural Science Foundation of China (Nos. 81870066 and 82270083), the Second Level Talents of the “333 High Level Talents Training Project” in the sixth phase in Jiangsu (LGY2022025), and Jiangsu Provincial Medical Key Laboratory (ZDXYS202205). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers 2019;5:18. [Crossref] [PubMed]
- ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [Crossref] [PubMed]
- Sweeney RM, McAuley DF. Acute respiratory distress syndrome. Lancet 2016;388:2416-30. [Crossref] [PubMed]
- Huppert LA, Matthay MA, Ware LB. Pathogenesis of Acute Respiratory Distress Syndrome. Semin Respir Crit Care Med 2019;40:31-9. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Gorman EA, O'Kane CM, McAuley DF. Acute respiratory distress syndrome in adults: diagnosis, outcomes, long-term sequelae, and management. Lancet 2022;400:1157-70. [Crossref] [PubMed]
- Gorman E, Millar J, McAuley D, et al. Mesenchymal stromal cells for acute respiratory distress syndrome (ARDS), sepsis, and COVID-19 infection: optimizing the therapeutic potential. Expert Rev Respir Med 2021;15:301-24. [Crossref] [PubMed]
- Gupta N, Krasnodembskaya A, Kapetanaki M, et al. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 2012;67:533-9. [Crossref] [PubMed]
- Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 2009;15:42-9. [Crossref] [PubMed]
- Devaney J, Horie S, Masterson C, et al. Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax 2015;70:625-35. [Crossref] [PubMed]
- Yang Y, Hu S, Xu X, et al. The Vascular Endothelial Growth Factors-Expressing Character of Mesenchymal Stem Cells Plays a Positive Role in Treatment of Acute Lung Injury In Vivo. Mediators Inflamm 2016;2016:2347938. [Crossref] [PubMed]
- Krasnodembskaya A, Samarani G, Song Y, et al. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol 2012;302:L1003-13. [Crossref] [PubMed]
- Adas G, Cukurova Z, Yasar KK, et al. The Systematic Effect of Mesenchymal Stem Cell Therapy in Critical COVID-19 Patients: A Prospective Double Controlled Trial. Cell Transplant 2021;30:9636897211024942. [Crossref] [PubMed]
- Aghayan HR, Salimian F, Abedini A, et al. Human placenta-derived mesenchymal stem cells transplantation in patients with acute respiratory distress syndrome (ARDS) caused by COVID-19 (phase I clinical trial): safety profile assessment. Stem Cell Res Ther 2022;13:365. [Crossref] [PubMed]
- Bowdish ME, Barkauskas CE, Overbey JR, et al. A Randomized Trial of Mesenchymal Stromal Cells for Moderate to Severe Acute Respiratory Distress Syndrome from COVID-19. Am J Respir Crit Care Med 2023;207:261-70. [Crossref] [PubMed]
- Dilogo IH, Aditianingsih D, Sugiarto A, et al. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: A randomized controlled trial. Stem Cells Transl Med 2021;10:1279-87. [Crossref] [PubMed]
- Fathi-Kazerooni M, Fattah-Ghazi S, Darzi M, et al. Safety and efficacy study of allogeneic human menstrual blood stromal cells secretome to treat severe COVID-19 patients: clinical trial phase I & II. Stem Cell Res Ther 2022;13:96. [Crossref] [PubMed]
- Gorman EA, Rynne J, Gardiner HJ, et al. Repair of Acute Respiratory Distress Syndrome in COVID-19 by Stromal Cells (REALIST-COVID Trial): A Multicenter, Randomized, Controlled Clinical Trial. Am J Respir Crit Care Med 2023;208:256-69. [Crossref] [PubMed]
- Kaffash Farkhad N, Sedaghat A, Reihani H, et al. Mesenchymal stromal cell therapy for COVID-19-induced ARDS patients: a successful phase 1, control-placebo group, clinical trial. Stem Cell Res Ther 2022;13:283. [Crossref] [PubMed]
- Lanzoni G, Linetsky E, Correa D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med 2021;10:660-73. [Crossref] [PubMed]
- Martínez-Muñoz ME, Payares-Herrera C, Lipperheide I, et al. Mesenchymal stromal cell therapy for COVID-19 acute respiratory distress syndrome: a double-blind randomised controlled trial. Bone Marrow Transplant 2024;59:777-84. [Crossref] [PubMed]
- Matthay MA, Calfee CS, Zhuo H, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med 2019;7:154-62. [Crossref] [PubMed]
- Monsel A, Hauw-Berlemont C, Mebarki M, et al. Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial. Crit Care 2022;26:48. [Crossref] [PubMed]
- Pochon C, Laroye C, Kimmoun A, et al. Efficacy of Wharton Jelly Mesenchymal Stromal Cells infusions in moderate to severe SARS-Cov-2 related acute respiratory distress syndrome: a phase 2a double-blind randomized controlled trial. Front Med (Lausanne) 2023;10:1224865. [Crossref] [PubMed]
- Rebelatto CLK, Senegaglia AC, Franck CL, et al. Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: a randomized clinical trial. Stem Cell Res Ther 2022;13:122. [Crossref] [PubMed]
- Shi L, Huang H, Lu X, et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther 2021;6:58. [Crossref] [PubMed]
- Shu L, Niu C, Li R, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther 2020;11:361. [Crossref] [PubMed]
- Zarrabi M, Shahrbaf MA, Nouri M, et al. Allogenic mesenchymal stromal cells and their extracellular vesicles in COVID-19 induced ARDS: a randomized controlled trial. Stem Cell Res Ther 2023;14:169. [Crossref] [PubMed]
- Zheng G, Huang L, Tong H, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res 2014;15:39. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [Crossref] [PubMed]
- Wilson JG, Liu KD, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med 2015;3:24-32. [Crossref] [PubMed]
- Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal Stem Cell Therapy for COVID-19: Present or Future. Stem Cell Rev Rep 2020;16:427-33. [Crossref] [PubMed]
- Sengupta V, Sengupta S, Lazo A, et al. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev 2020;29:747-54. [Crossref] [PubMed]
- McIntyre LA, Moher D, Fergusson DA, et al. Efficacy of Mesenchymal Stromal Cell Therapy for Acute Lung Injury in Preclinical Animal Models: A Systematic Review. PLoS One 2016;11:e0147170. [Crossref] [PubMed]
- Chen MC, Lai KS, Chien KL, et al. pcMSC Modulates Immune Dysregulation in Patients With COVID-19-Induced Refractory Acute Lung Injury. Front Immunol 2022;13:871828. [Crossref] [PubMed]
- Zhou Y, Yamamoto Y, Xiao Z, et al. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J Clin Med 2019;8:1025. [Crossref] [PubMed]
- Atluri S, Manchikanti L, Hirsch JA. Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use. Pain Physician 2020;23:E71-83.
- Hayes M, Masterson C, Devaney J, et al. Therapeutic efficacy of human mesenchymal stromal cells in the repair of established ventilator-induced lung injury in the rat. Anesthesiology 2015;122:363-73. [Crossref] [PubMed]
- Jackson MV, Morrison TJ, Doherty DF, et al. Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells 2016;34:2210-23. [Crossref] [PubMed]
- Pedrazza L, Cunha AA, Luft C, et al. Mesenchymal stem cells improves survival in LPS-induced acute lung injury acting through inhibition of NETs formation. J Cell Physiol 2017;232:3552-64. [Crossref] [PubMed]
- Perlee D, de Vos AF, Scicluna BP, et al. Human Adipose-Derived Mesenchymal Stem Cells Modify Lung Immunity and Improve Antibacterial Defense in Pneumosepsis Caused by Klebsiella pneumoniae. Stem Cells Transl Med 2019;8:785-96. [Crossref] [PubMed]
- Rocheteau P, Chatre L, Briand D, et al. Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat Commun 2015;6:10145. [Crossref] [PubMed]
- Zhu H, Xiong Y, Xia Y, et al. Therapeutic Effects of Human Umbilical Cord-Derived Mesenchymal Stem Cells in Acute Lung Injury Mice. Sci Rep 2017;7:39889. [Crossref] [PubMed]
- Shimizu M. Clinical Features of Cytokine Storm Syndrome. In: Cron R, Behrens E. editors. Cytokine Storm Syndrome. Cham Springer 2019:31-41.
- Meduri GU, Headley S, Kohler G, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 1995;107:1062-73. [Crossref] [PubMed]
- Bárcia RN, Santos JM, Filipe M, et al. What Makes Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells Superior Immunomodulators When Compared to Bone Marrow Derived Mesenchymal Stromal Cells? Stem Cells Int 2015;2015:583984. [Crossref] [PubMed]
- Cóndor JM, Rodrigues CE. Treatment With Human Wharton's Jelly-Derived Mesenchymal Stem Cells Attenuates Sepsis-Induced Kidney Injury, Liver Injury, and Endothelial Dysfunction. Stem Cells Transl Med 2016;5:1048-57. [Crossref] [PubMed]
- Li X, Bai J, Ji X, et al. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med 2014;34:695-704. [Crossref] [PubMed]




