Progastrin-releasing peptide as a diagnostic and therapeutic biomarker of small cell lung cancer
Introduction
Lung cancer is the most common fatal cancer and its prevalence is increasing in Korea (1-3). Tumor markers have been studied in patients with lung cancer as tools to differentiate lung cancer subtypes and improve diagnosis and treatment selection (4,5). Neuron-specific enolase (NSE) and progastrin-releasing peptide (proGRP) are the most beneficial tumor markers in neuroendocrine tumors, such as small cell lung cancer (SCLC) (6,7). Although NSE was a historically recommended tumor marker for SCLC (8). NSE also stains up to 80% of non-small cell lung cancer (NSCLC) in tissue examinations and is elevated in the serum of 20–30% patients with NSCLC (9).
ProGRP is a precursor of a neuropeptide hormone called gastrin-releasing peptide (GRP) and is frequently produced by SCLC cells (10,11). Circulating proGRP levels serve as a reliable marker in patients with SCLC (12-14) and is the most sensitive marker for discriminating SCLC from benign diseases of the lung (15). ProGRP is rarely elevated in patients with other malignancies or in benign conditions except in patients with renal insufficiency, neuroendocrine tumors of the lung, and medullary carcinoma of the thyroid (16,17). And proGRP provides additional information on the pathological characteristics of lung cancer compared to NSE (13,18-20). Several studies have reported serum proGRP is useful to monitor the therapeutic response and detect recurrent SCLC (18,20,21). However, few studies have measured plasma proGRP in patients with SCLC and prospectively evaluated the association between proGRP level and diagnosis or treatment of SCLC.
The objective of this study was to evaluate the usefulness of automated proGRP measurement as a tumor marker for diagnosis and treatment monitoring in patients with SCLC.
Methods
Patients
We collected plasma samples from 452 patients who visited the Lung and Esophageal Cancer Clinic in Chonnam National University Hwasun Hospital for tissue diagnosis under suspicion of lung cancer between January 1, 2011 and December 31, 2013.
The patients were divided by pathological diagnosis. Among the 452 patients with measured proGRP levels, 212 (46.9%) were diagnosed with NSCLC and 105 (23.2%) were diagnosed with SCLC. The remaining 135 patients (29.8%) were not diagnosed with lung cancer and had various diseases, including infectious diseases, such as pulmonary tuberculosis, pneumonia, or another neoplasm (Figure 1). Carcinoembryonic antigen (CEA) level was measured in patients diagnosed with NSCLC. CEA level was compared to the histological type and stage of NSCLC and clinical parameters, such as sex and smoking history.
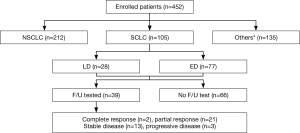
All lung cancer cases were diagnosed histologically and staged with the TNM system. Of the 105 patients with SCLC, 77 (73.3%) had extensive disease and 28 (26.6%) had limited disease. These patients received chemotherapy consisting of a combination of 100 mg/m2 etoposide on days 1, 2, and 3 plus 60 mg/m2 cisplatin on day 1 for 3-week cycles. Both regimens required hydration and administration of antiemetic drugs. Treatment response was assessed every two treatment cycles at follow-up visits until evidence or suspicion of disease progression. Thirty-nine patients were able to recheck proGRP level after chemotherapy. Treatment response was classified based on Response Evaluation Criteria in Solid Tumors version 1.1 (22), and they were divided into responder and non-responder groups. The responder group included patients who showed complete response (CR) or partial response (PR) and the non-responder group included patients who showed progressive disease (PD) or stable disease (SD). This study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (number: CNUHH-2016-013) and written informed consent was waived because of retrospective study design.
Measurement of plasma proGRP level
We used a two-step automated immunoassay and the ARCHITECTⓇ ProGRP assay kit (Abbott Diagnostics; Abbott Park, IL, USA). In the first step, the sample, assay diluent, and the anti-proGRP-coated paramagnetic microparticles were combined. ProGRP in the sample binds to the anti-proGRP-coated microparticles. After a wash step, the anti-proGRP acridinium-labeled conjugate was added to create a reaction mixture. Following another wash, pre-trigger and trigger solutions were added to the reaction mixture. The resulting chemiluminescent reaction was measured as relative light units. A direct relationship existed between the quantity of proGRP in the sample and the relative light units detected. The cutoff plasma proGRP level was 63 pg/mL derived from previous report (23).
Statistical analysis
All data are expressed as median [interquartile range (IQR)] or numbers and percentage. Intergroup comparisons were performed using the Kruskal-Wallis test and Mann-Whitney U-test because statistical distribution is non-normal. The diagnostic accuracy of proGRP was assessed by plotting receiver operating characteristic curves and estimating the AUC to discriminate SCLC from the other conditions. We analyzed change of proGRP according to chemotherapy by paired Wilcoxon signed-rank test. Survival analysis was performed by Kaplan-Meier method and Log-Rank test. Multivariate analysis of survival was performed using Cox’s regression model. Possible predictors found to be significant in univariate analysis were entered into binary logistic regression. Statistical analyses were conducted using IBM SPSS ver. 20.0 software (IBM Co.; Armonk, NY, USA). A P value <0.05 was considered significant.
Results
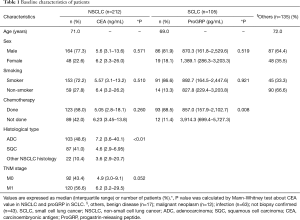
Baseline characteristics of patients
The median age of the 452 patients ranged from 71 (IQR, 62–77) years, and 337 (74.5%) were males. The patients were divided into three groups according to the pathological diagnosis: 212 (46.9%) with NSCLC, 105 (23.2%) with SCLC, and 135 (29.8%) with other conditions (Table 1). CEA level was different only between adenocarcinoma and squamous cell carcinoma group (P<0.01) and proGRP level was different by history of chemotherapy (P=0.008).
Full table
ProGRP as a diagnostic biomarker
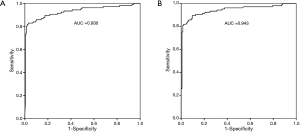
According to the lung cancer histological type, the positive rates of proGRP were 85.7% (90/105) in SCLC, 11.8% (25/212) in NSCLC, and 6.7% (9/135) in other diseases. Sensitivity of proGRP for SCLC diagnosis was 85.7%, and specificity was 90.2%. Positive predictive value and negative predictive value were 72.5% and 95.4%, respectively. The proGRP values of two cases of large cell neuroendocrine carcinoma (100.2 and 2,629.2 pg/mL) were all positive in NSCLC group. The one case of carcinoid was negative (25.2 pg/mL) in others group. The AUC values were 0.93 for distinguishing SCLC from NSCLC, and 0.943 for distinguishing SCLC from the other conditions (Figure 2).
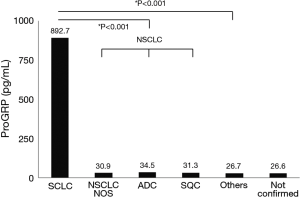
The median (IQR) proGRP level in patients with SCLC was 892.7 (183.7–2,768.7) pg/mL. The median proGRP values were 34.5 (23.7–47.4), 31.3 (22.7–44.8), and 31.0 (24.1–47.0) pg/mL in patients with adenocarcinoma, squamous cell carcinoma, and other NSCLC histology, respectively. ProGRP level was significantly higher in patients with SCLC than that in all patients with NSCLC [32.3 (23.2–46.2) pg/mL, P<0.001] and those with other diseases [26.6 (20.2–39.9) pg/mL, P<0.001] (Figure 3). The median proGRP level was higher in patients with extensive disease [1,055.2 (330.9–3,048.1) pg/mL] than in those with limited disease [253.7 (52.6–1,474.2) pg/mL, P=0.005].
Considering the reference range of creatinine is 0.5–1.3 mg/dL, the patients with high creatinine value were 43 (9.5%) in total population who was composed of 11 in SCLC, 20 in NSCLC, and 12 in others group. The proGRP value of high creatinine group was significantly higher than low group in total population (Z=−3.970, P<0.001), NSCLC group (Z=−3.620, P<0.001), and others group (Z=−3.704, P<0.001). There was no difference of proGRP value according to creatinine level in SCLC group (Z=−1.036, P=0.300). Among the SCLC patients, 11 (10.5%) patients had higher creatinine value. Using glomerular filtration rate (GFR), chronic kidney disease (CKD) stages were classified to stage I (41 patients), II (55 patients), III (7 patients), and stage IV (2 patients). There was no difference of proGRP values according to CKD stage in SCLC group.
ProGRP as a therapeutic biomarker
We analyzed change of proGRP level after chemotherapy among the 39 patients with SCLC in whom proGRP was measured at follow-up by Wilcoxon signed-rank test (Figure 4). In 23 responders, proGRP levels were significantly decreased after chemotherapy (Z=−3.802, P<0.001) (Table 2). However, proGRP level in the 16 non-responders was not different before and after treatment (Z=−0.310, P=0.756). The values were decreased in nine patients and increased in seven patients.


Full table
ProGRP as a prognostic biomarker
In the 105 patients with SCLC, the median overall survival (OS) was 8.4 (range, 0.1–40.4) months. Median OS was significantly shorter in patients with extensive disease (6.0±0.7 months) than in those with limited disease (12.7±4.5 months, P<0.001). But there were no differences between positive proGRP group (7.7±1.1 months) and negative group (12.7±0.7 months; P=0.195), and between above-mean proGRP level group (8.4±1.8 months) and below-mean group (8.0±1.9 months; P=0.275). Among the 39 patients in whom chemotherapy was performed, the median OS was not different between responders (13.3±2.9 months) and non-responders (10.2±2.6 months, P=0.784).
In multivariate analysis, age [adjusted odd ratio (aOR): 1.861, 95% confidence interval (CI): 1.209–2.865, P=0.005], stage (aOR: 1.764, 95% CI: 1.054–2.953, P=0.031) and history of chemotherapy (aOR: 9.848, 95% CI: 4.518–21.467, P<0.001) were independent prognostic factors of survival.
Discussion
This study showed the usefulness of plasma proGRP level as a diagnostic and therapeutic marker in patients with SCLC. Median proGRP level was significantly higher in patients with SCLC than in those with NSCLC or other diseases. At cut-off level of 63 pg/mL, proGRP shows 85.7% sensitivity, 90.2% specificity, 72.5% positive predictive value and 95.4% negative predictive value in patients with SCLC. In addition, median proGRP level was significantly higher in patients with extensive disease than in those with limited disease, suggesting that it may reflect tumor extent. And the median OS of extensive disease was significantly shorter than that of limited disease.
ProGRP is a biologically active protein that stimulates tumor cell proliferation. GRP may function as an autocrine growth factor in SCLC (24,25). It appears that the growth-stimulating properties of proGRP may be responsible for more aggressive tumor behavior and a poor prognosis. This mechanism may explain why proGRP level was higher in patients with extensive disease in our study. In a meta-analysis of 5,146 patients enrolled in 11 clinical trials, sensitivity and specificity of proGRP for diagnosing SCLC was 0.716 (95% CI, 0.688–0.743) and 0.921 (95% CI, 0.909–0.932), respectively (23). Thus, the clinical utility of proGRP as a biomarker to distinguish SCLC from other lung cancers has been established.
In our previous study, plasma proGRP concentration measured by the two-step automated proGRP ARCHITECT chemiluminescent assay was sensitive and specific for discriminating SCLC from nonmalignant conditions or NSCLC (26). Due to the poor stability of proGRP in serum on the ARCHITECT assay, which is believed to be due to thrombin-induced proteolysis, plasma samples are the recommended source material (26,27). A new immunoassay, called the Elecsys ProGRP assay (Roche Diagnostics GmbH, Penzberg, Germany) has been designed to quantitatively determine proGRP levels in human serum and plasma and shows good precision, stability, and specificity (28).
Our results also show the usefulness of plasma proGRP level as a treatment monitoring marker in patients with SCLC. Among the 39 patients with SCLC who were followed, the mean proGRP level of the 23 responders decreased significantly after chemotherapy, whereas that of the 16 non-responders was not different between and after chemotherapy. Several studies using in-house proGRP measurement methods have also reported that changes in proGRP level are more precise than those of NSE as a tool for monitoring therapy and that measuring proGRP more reliably predicts relapses and the prognosis in patients with limited disease SCLC (19,21,22,29,30). And more recently, one study also evaluated change of proGRP level during chemotherapy. According to their result, changes in proGRP level are associated with image-based response, progression free survival and OS (31). However, large-scale studies are necessary to further evaluate the usefulness of proGRP for monitoring treatment and prognosis.
Because it has well known that proGRP value is higher in patient who has impaired renal function, we also performed non-parametric analysis by creatinine and GFR. In spite of small sample size, we found the proGRP value of high creatinine group was significantly higher than low group in total population, NSCLC group, and others group. But there was no difference of proGRP value in SCLC group according to creatinine level and CKD stage.
Several limitations of this study should be mentioned. First, this was a non-randomized prospective study and enrolled a small number of patients from a single institution. Especially there was too small number of patients who were followed to have result regarding the significance of the biomarker for monitoring of treatment response. Particularly valuable would be to show whether prognosis is differed by the proGRP response and whether association exists between proGRP and imaging responses. Second, the upper limit of the proGRP value was set to 5,000 pg/mL, but three patients had higher proGRP levels, so their levels were determined by diluting the samples. And we used nonparametric statistics which would decrease an effect of very high levels.
In conclusion, plasma proGRP level could be a sensitive and specific biomarker for discriminating SCLC from NSCLC or non-malignant disease. It may also be a useful SCLC biomarker for treatment monitoring. The initial proGRP level may represent tumor extent.
Acknowledgements
This work was supported by a clinical research grant from Chonnam National University Hwasun Hospital 2014.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (number: CNUHH-2016-013) and written informed consent was waived because of retrospective study design.
References
- Jung KW, Won YJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat 2015;47:127-41. [Crossref] [PubMed]
- In KH, Kwon YS, Oh IJ, et al. Lung cancer patients who are asymptomatic at diagnosis show favorable prognosis: a korean Lung Cancer Registry Study. Lung Cancer 2009;64:232-7. [Crossref] [PubMed]
- Park JY, Jang SH. Epidemiology of Lung Cancer in Korea: Recent Trends. Tuberc Respir Dis (Seoul) 2016;79:58-69. [Crossref] [PubMed]
- Pass HI, Beer DG, Joseph S, et al. Biomarkers and molecular testing for early detection, diagnosis, and therapeutic prediction of lung cancer. Thorac Surg Clin 2013;23:211-24. [Crossref] [PubMed]
- Rosell R, Bivona TG, Karachaliou N. Genetics and biomarkers in personalisation of lung cancer treatment. Lancet 2013;382:720-31. [Crossref] [PubMed]
- Molina R, Auge JM, Filella X, et al. Pro-gastrin-releasing peptide (proGRP) in patients with benign and malignant diseases: comparison with CEA, SCC, CYFRA 21-1 and NSE in patients with lung cancer. Anticancer Res 2005;25:1773-8. [PubMed]
- Molina R, Holdenrieder S, Auge JM, et al. Diagnostic relevance of circulating biomarkers in patients with lung cancer. Cancer Biomark 2010;6:163-78. [PubMed]
- Tumour markers in lung cancer: EGTM recommendations. European Group on Tumour Markers. Anticancer Res 1999;19:2817-9. [PubMed]
- Slodkowska J, Zych J, Szturmowicz M, et al. Neuroendocrine phenotype of non-small cell lung carcinoma: immunohistological evaluation and biochemical study. Int J Biol Markers 2005;20:217-26. [PubMed]
- Miyake Y, Kodama T, Yamaguchi K. Pro-gastrin-releasing peptide(31-98) is a specific tumor marker in patients with small cell lung carcinoma. Cancer Res 1994;54:2136-40. [PubMed]
- Yamaguchi K, Abe K, Kameya T, et al. Production and molecular size heterogeneity of immunoreactive gastrin-releasing peptide in fetal and adult lungs and primary lung tumors. Cancer Res 1983;43:3932-9. [PubMed]
- Takada M, Kusunoki Y, Masuda N, et al. Pro-gastrin-releasing peptide (31-98) as a tumour marker of small-cell lung cancer: comparative evaluation with neuron-specific enolase. Br J Cancer 1996;73:1227-32. [Crossref] [PubMed]
- Molina R, Filella X, Augé JM. ProGRP: a new biomarker for small cell lung cancer. Clin Biochem 2004;37:505-11. [Crossref] [PubMed]
- Stieber P, Dienemann H, Schalhorn A, et al. Pro-gastrin-releasing peptide (ProGRP)--a useful marker in small cell lung carcinomas. Anticancer Res 1999;19:2673-8. [PubMed]
- Molina R, Auge JM, Alicarte J, et al. Pro-gastrin-releasing peptide in patients with benign and malignant diseases. Tumour Biol 2004;25:56-61. [Crossref] [PubMed]
- Nakahama H, Tanaka Y, Fujita Y, et al. CYFRA 21-1 and ProGRP, tumor markers of lung cancer, are elevated in chronic renal failure patients. Respirology 1998;3:207-10. [Crossref] [PubMed]
- Inaji H, Komoike Y, Motomura K, et al. Demonstration and diagnostic significance of pro-gastrin-releasing peptide in medullary thyroid carcinoma. Oncology 2000;59:122-5. [Crossref] [PubMed]
- Niho S, Nishiwaki Y, Goto K, et al. Significance of serum pro-gastrin-releasing peptide as a predictor of relapse of small cell lung cancer: comparative evaluation with neuron-specific enolase and carcinoembryonic antigen. Lung Cancer 2000;27:159-67. [Crossref] [PubMed]
- Yamaguchi K, Aoyagi K, Urakami K, et al. Enzyme-linked immunosorbent assay of pro-gastrin-releasing peptide for small cell lung cancer patients in comparison with neuron-specific enolase measurement. Jpn J Cancer Res 1995;86:698-705. [Crossref] [PubMed]
- Sunaga N, Tsuchiya S, Minato K, et al. Serum pro-gastrin-releasing peptide is a useful marker for treatment monitoring and survival in small-cell lung cancer. Oncology 1999;57:143-8. [Crossref] [PubMed]
- Okusaka T, Eguchi K, Kasai T, et al. Serum levels of pro-gastrin-releasing peptide for follow-up of patients with small cell lung cancer. Clin Cancer Res 1997;3:123-7. [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Yang HJ, Gu Y, Chen C, et al. Diagnostic value of pro-gastrin-releasing peptide for small cell lung cancer: a meta-analysis. Clin Chem Lab Med 2011;49:1039-46. [Crossref] [PubMed]
- Dumesny C, Patel O, Lachal S, et al. Synthesis, expression and biological activity of the prohormone for gastrin releasing peptide (ProGRP). Endocrinology 2006;147:502-9. [Crossref] [PubMed]
- Cuttitta F, Carney DN, Mulshine J, et al. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. Nature 1985;316:823-6. [Crossref] [PubMed]
- Kim HR, Oh IJ, Shin MG, et al. Plasma proGRP concentration is sensitive and specific for discriminating small cell lung cancer from nonmalignant conditions or non-small cell lung cancer. J Korean Med Sci 2011;26:625-30. [Crossref] [PubMed]
- Yoshimura T, Fujita K, Kawakami S, et al. Stability of pro-gastrin-releasing peptide in serum versus plasma. Tumour Biol 2008;29:224-30. [Crossref] [PubMed]
- Korse CM, Holdenrieder S, Zhi XY, et al. Multicenter evaluation of a new progastrin-releasing peptide (ProGRP) immunoassay across Europe and China. Clin Chim Acta 2015;438:388-95. [Crossref] [PubMed]
- Wójcik E, Kulpa JK, Sas-Korczyńska B, et al. ProGRP and NSE in therapy monitoring in patients with small cell lung cancer. Anticancer Res 2008;28:3027-33. [PubMed]
- Holdenrieder S, von Pawel J, Dankelmann E, et al. Nucleosomes, ProGRP, NSE, CYFRA 21-1, and CEA in monitoring first-line chemotherapy of small cell lung cancer. Clin Cancer Res 2008;14:7813-21. [Crossref] [PubMed]
- Nisman B, Nechushtan H, Biran H, et al. New ARCHITECT plasma pro-gastrin-releasing peptide assay for diagnosing and monitoring small-cell lung cancer. Br J Cancer 2016;114:469-76. [Crossref] [PubMed]


