
Longitudinal assessment of quality of life indicators and prognosis in esophageal cancer patients with curative resection
Highlight box
Key findings
• Specific quality of life indicators were identified as independent prognostic factors at different time points after discharge for esophageal cancer patients. Worsening symptoms or better functional status in esophageal cancer patients may play a significant role in prognostic analysis for overall survival (OS).
What is known and what is new?
• A wide range of clinicopathological and molecular factors are identified as prognostic factors of esophageal cancer, such as tumor, node and metastasis stage, neoadjuvant treatment, surgical margin, CYFRA21-1and P53.
• The study found that certain quality of life indicators at different time points were independent prognostic factors for OS for esophageal cancer.
What is the implication, and what should change now?
• Healthcare professionals should incorporate these prognostic indicators into clinical practice to enhance patient care and potentially improve outcomes. The study also emphasizes the need for closer attention to independent prognostic factors, which can help identify patients with worsening symptoms and facilitate early clinical intervention to improve survival.
Introduction
Esophageal cancer remains an overwhelming global health challenge, which underscores an urgent need for improved prognostic analysis measures and treatment strategies. Despite advancements in medical science, the 5-year overall survival (OS) rate for patients diagnosed with esophageal cancer remains around 20% across all stages (1). In this context, the identification of reliable prognostic factors is crucial for refining therapeutic approaches as well as educating patients and their families on a realistic understanding of the disease trajectory, thereby facilitating patient care and lifestyle adjustments.
Traditionally, research on esophageal cancer has focused on clinicopathological and molecular predictors of outcome (2-5). However, there is an expanding body of evidence that emphasizes the significance of patient-reported outcomes, particularly health-related quality of life, as concerning indicators in managing chronic illnesses, including various cancer types. Quality of life assessments offer a unique perspective on how the disease and its treatments impact patients’ everyday experiences, providing a more holistic view of patient well-being. Despite the recognized importance of quality of life, studies specifically examining the link between post-esophagectomy quality of life and survival are limited. The surgical intervention itself has been identified as a determinant of quality of life outcomes (6-9), with emerging data suggesting that the baseline quality of life metrics could forecast complications and early disease recurrence in localized esophageal cancer cases (10). Pioneering work by Hiratsuka and colleagues has highlighted that deteriorations in patient-reported dyspnea, sleep disturbance, and depression within three months post-surgery correlate significantly with survival prospects (11). Similarly, Djärv et al.’s findings indicate that shifts in health-related quality of life at six months postoperatively can serve as a prognostic tool in esophagogastric cancers (12). These insights suggest that the trajectory of symptoms and functional status in cancer patients correlate with the underlying disease burden—whether it is remission, stability, or progression (13).
Given this background, our study hypothesized that changes in quality of life assessments post-esophagectomy offer valuable prognostic information. We aim to investigate the course of health-related quality of life in esophageal cancer patients over 2 years following surgery and analyze its relationship with long-term survival. By doing so, we hope to contribute to improving the understanding of esophageal cancer prognosis from the perspective of quality of life assessment and underscore the necessity for incorporating patient-centered metrics into clinical practice. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-311/rc).
Methods
Participants
The study population consisted of individuals diagnosed with cancer of the esophagus, esophagogastric junction, and cardia at a tertiary center, Sun Yat-sen University Cancer Center, Guangzhou, China, between January 21, 2012, and April 1, 2015. The inclusion criteria were established as follows: first, patients had to be capable of comprehending and completing the questionnaire in its entirety; secondly, only those who received a curative surgical procedure were considered; thirdly, the age was more than 18 years old. Exclusions were made for patients who did not achieve complete resection as confirmed by the postoperative pathological report, those who were lost to follow-up within 2 years after surgery, or those who died within 3 months after surgery. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) (14). The study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center (No. YB2016-070) and informed consent was obtained from all individual participants.
Data collection
Demographic, socioeconomic, and clinicopathological information was systematically documented in this study. The recorded variables included age, gender, occupation, marriage, education, financial status, tumor site, pathological subtype, anastomotic stoma site, as well as neoadjuvant and adjuvant therapy. To assess patient-reported symptoms and functioning status, we employed the Chinese versions of the European Organisation for Research and Treatment of Cancer (EORTC) 30-item core quality of life questionnaire (QLQ-C30) (15) and the disease-specific esophageal module (QLQ-OES18) (16). Both Chinese versions have been validated as valid and reliable with Cronbach’s α coefficients of all domains being >0.7 except for cognitive function (0.49) (17,18). The QLQ-C30 questionnaire consists of 30 items that were transformed into 15 scales, encompassing global health status and 5 functioning indicators (greater scores represent better health), and 9 symptom indicators (higher scores indicate worse status). On the other hand, the QLQ-OES18 questionnaire comprises 18 items that generate 10 scales, with higher scores indicating poorer status. It is worth noting that all items except the global health component in QLQ-C30, were rated on a four-point scale (ranging from “not at all” to “very much”), corresponding to scores of 1 to 4 In contrast, the items related to global health were rated on a seven-point scale (ranging from “very bad” to “very good”), denote as scores of 1 to 7.
Patients were followed up until death on May 22, 2022, or until the time of death. As part of routine practice, patients were requested to complete the QLQ-OES30 and QLQ-OES18 questionnaires based on a face-to-face interview to evaluate their quality of life at various time points. These time points included before surgery, at discharge, and when patients returned to the outpatient clinic for follow-up at 3 months, 6 months, 12 months, and 24 months after discharge. The OS time was defined by calculating the number of months between the assessment time and either the patient death or the last follow-up.
Statistical analysis
All statistical analyses were performed using R software, version 4.2.1 (June 23, 2022). Cases with missing data were removed. The distributions of the demographic, socioeconomic, and clinicopathological characteristics were summarized. The scores prior to the surgical intervention were regarded as the baseline scores. Based on our previous experience, surgical intervention in esophageal cancer patients can significantly impact their quality of life. Nevertheless, in situations where the quality of life is not compromised, patients typically report a general sense of well-being. Thus, to simplify the description, we defined the improved or stable status compared to the baseline as fine status. Descriptive statistics were performed for each time point on the QLQ-C30 and QLQ-OES18 scales. This involved calculating the mean, variance, and the number and percentage of patients who exhibited improved or stable status compared to baseline scores for each indicator at all postoperative time points. Because the data were collected by repeatedly measuring quality of life indicators at different time points in one population, we chose the Wilcox text to examine the difference in the quality of life indicators between adjacent time points, and the Kruskal-Wallis test to assess the statistical significance of the scale difference between 6, 12, and 24 months, which both are widely recognized as robust statistical methods for paired or related samples. To ensure interpretation accuracy and minimize bias, quality of life indicators with less than 10% or more than 90% of patients showing improved or stable status were excluded from the survival analysis. Univariate Cox analysis was conducted to examine the relationship between OS and demographic, socioeconomic, and clinicopathological characteristics as well as change status (i.e., whether the status was fine or worse compared to baseline) in selected quality of life indicators. Multivariate Cox analysis as a method to adjust confounding variables was then used to identify independent prognostic factors based on the results from univariate Cox analysis results. To demonstrate the effects of independent prognostic factors on OS, the hazard ratio with 95% confidence interval was calculated in the multivariate Cox analysis. Statistical significance was considered by using a P value threshold of <0.05 and a two-sided test.
Results
Study participants
A flowchart of patients’ inclusion and exclusion is illustrated in Figure 1. A total of 246 patients participated in preoperative assessment of quality of life. However, 11 patients were lost to follow-up, 1 patient died within 3 months after surgery, and 2 patients were R1 resected. Therefore, 232 patients were entered into the final analysis. The last follow-up date was May 22, 2022. The median follow-up time for the entire study population was 27 months, ranging from 5 to 118 months. Among the survivors until the last follow-up date, the median follow-up time was 98 months, ranging from 73 to 118 months. The 2-year survival rate was found to be 77.2% while the 5-year OS rate was 57.8%. 113 patients (48.7%) were older than 60 years old, and 62 patients (26.7%) were female. Demographic, socioeconomic, and clinicopathological characteristics are summarized in Table 1. Regarding to tumor site, 139 patients (59.9%) had tumors in the middle part of the chest while 49 patients (21.1%) had tumors in the lower part of the chest. The majority of patients (86.6%) had squamous cell carcinoma as the pathological type. For the anastomotic stoma site, 164 patients (70.7%) were anastomosed in the thoracic cavity. In terms of disease stage, 104 patients (44.8%) were classified as stage 3 or 4. Additionally, a subset of patients received different treatment modalities: 5 patients (2.2%) received neoadjuvant radiotherapy, 34 patients (14.7%) received neoadjuvant chemotherapy, 30 patients (12.9%) received adjuvant radiotherapy, and 88 patients (37.9%) received adjuvant chemotherapy.
Table 1
| Characteristics | Count (%) |
|---|---|
| Total number | 232 (100.0) |
| Age ≥60 years | 113 (48.7) |
| Gender (female) | 62 (26.7) |
| Occupation | |
| Worker | 23 (9.9) |
| Farmer | 58 (25.0) |
| Teacher | 5 (2.2) |
| Civil servant | 11 (4.7) |
| Individual business | 30 (12.9) |
| Others | 105 (45.3) |
| Marriage | |
| Unmarried | 1 (0.4) |
| Married | 223 (96.1) |
| Widowed | 8 (3.4) |
| Education | |
| Primary school | 87 (37.5) |
| Junior high school | 74 (31.9) |
| Senior high school | 50 (21.6) |
| Junior college | 12 (5.2) |
| Bachelor and above | 9 (3.9) |
| Financial status | |
| Bad | 60 (25.9) |
| Middle | 112 (48.3) |
| Good | 60 (25.9) |
| Tumor site | |
| UPT | 21 (9.1) |
| MPT | 139 (59.9) |
| LPT | 49 (21.1) |
| Junction | 23 (9.9) |
| Pathological subtype | |
| Squamous carcinoma | 201 (86.6) |
| Small cell carcinoma | 9 (3.9) |
| Adenocarcinoma | 22 (9.5) |
| Stage 3/4 | 104 (44.8) |
| Anastomotic stoma site | |
| Thoracic cavity | 164 (70.7) |
| Neck | 68 (29.3) |
| NeoRad (yes) | 5 (2.2) |
| NeoChemo (yes) | 34 (14.7) |
| Rad (yes) | 30 (12.9) |
| Chemo (yes) | 88 (37.9) |
UPT, the upper part of the chest; MPT, the middle part of the chest; LPT, the lower part of the chest; NeoRad, neoadjuvant radiotherapy; NeoChemo, neoadjuvant chemotherapy; Rad, adjuvant radiotherapy; Chemo, adjuvant chemotherapy.
Scores and changes in functioning and symptom
The scores of quality of life indicators from QLQ-C30 and QLQ-OES18 questionnaires at various time points, ranging from preoperative to 24 months after discharge are presented in Table 2. The distributions and detailed comparison of all quality of life indicators among different time points are demonstrated in Figure 2 for QLQ-C30 indicators and Figure 3 for QLQ-OES18 indicators. At the time of discharge, insomnia, constipation, cognitive functioning, choked when swallowing and problems with eating were similar to the preoperative time scores, while appetite loss, nausea and vomiting and trouble swallowing saliva showed improvement, and other indicators were significantly worse compared to the baseline. After 3 months of discharge, nausea and vomiting, social function, dysphagia, pain, and financial difficulties were either similar to or better than the scores at discharge. Social function, which worsened after surgery, returned to baseline levels at 3 months after discharge and further improved at 6 months. However, there was no significant difference in social function between 6, 12, and 24 months after discharge. It is worth noting that insomnia was comparable to the baseline score at discharge, and was even better than the baseline score at 3 months after discharge. At 6 months after discharge, pain and fatigue were either similar to or better than the baseline score for the first time. At 24 months after discharge, dry mouth and trouble with taste were either similar to or better than baseline for the first time. However, caution should be aroused as certain indicators (physical function, dyspnoea, fatigue, appetite loss, diarrhea, global health status, trouble with coughing, trouble talking, and reflux) remained worse even at 24 months after discharge. There was no significant difference in physical function, constipation, diarrhea, social function, global health status, and pain related to esophageal cancer between 6, 12, and 24 months after discharge.
Table 2
| Characteristics | Baseline | At discharge | 3 months after discharge | 6 months after discharge | 12 months after discharge | 24 months after discharge |
|---|---|---|---|---|---|---|
| Physical function | 97.39 (8.50) | 57.16 (14.65) | 86.00 (10.50) | 92.75 (13.62) | 91.45 (13.83)† | 90.90 (17.55)†§ |
| Role function | 56.54 (21.36) | 20.26 (25.37) | 61.11 (19.20) | 83.48 (22.84) | 85.84 (24.16)† | 86.10 (26.79)† |
| Dyspnoea | 1.15 (6.84) | 41.38 (21.32) | 13.42 (18.33) | 12.03 (21.41)† | 11.26 (20.07)† | 8.29 (19.21) |
| Pain | 9.20 (12.28) | 52.16 (22.85) | 22.29 (18.31) | 9.86 (18.94)‡ | 6.24 (14.34) | 6.91 (11.91)† |
| Fatigue | 20.31 (16.40) | 53.02 (24.89) | 30.83 (21.21) | 17.39 (23.87) | 18.67 (22.24)† | 13.69 (23.75) |
| Insomnia | 42.53 (21.30) | 45.40 (29.23)†‡ | 19.19 (23.51) | 16.37 (18.73)† | 12.48 (22.27) | 12.15 (26.29)† |
| Appetite loss | 38.65 (23.53) | 27.59 (28.18) | 18.04 (26.32) | 14.14 (19.16)† | 9.74 (21.33) | 8.29 (19.21)† |
| Nausea and vomiting | 9.20 (15.64) | 6.03 (12.96) | 9.52 (19.74)†‡ | 4.86 (13.01) | 4.87 (13.23)† | 3.04 (8.69)†§ |
| Constipation | 8.91 (15.42) | 9.91 (18.15)†‡ | 4.18 (14.47) | 4.04 (16.24)† | 1.83 (8.84)† | 3.13 (11.49)†§ |
| Diarrhea | 1.29 (7.16) | 28.74 (26.82) | 29.44 (26.36)† | 23.95 (26.07) | 25.88 (25.94)† | 22.10 (21.15)†§ |
| Cognitive function | 78.23 (17.59) | 77.16 (20.87)†‡ | 93.22 (12.42) | 92.17 (17.46)† | 92.47 (14.99)† | 91.07 (17.61)†§ |
| Emotional function | 60.90 (16.27) | 71.95 (18.53) | 84.96 (18.42) | 87.93 (20.92) | 86.72 (20.06)† | 92.27 (15.52) |
| Social function | 60.70 (23.91) | 22.99 (25.16) | 61.62 (18.08)‡ | 87.32 (23.92) | 90.26 (20.97)† | 90.42 (21.60)†§ |
| Financial difficulties | 22.41 (29.84) | 41.24 (27.04) | 14.29 (22.26) | 11.40 (25.25) | 7.15 (19.28) | 4.60 (15.22)† |
| Global health status | 139.94 (26.89) | 56.07 (11.32) | 69.08 (12.33) | 80.22 (18.03) | 79.79 (19.32)† | 81.49 (21.35)†§ |
| Dysphagia | 16.00 (12.35) | 50.77 (16.43) | 19.14 (16.87)‡ | 12.99 (20.60) | 19.63 (21.11)‡ | 20.56 (19.01)†‡ |
| Trouble swallowing saliva | 28.45 (39.29) | 9.20 (20.38) | 4.33 (11.65) | 3.50 (8.99)† | 2.89 (13.42) | 5.34 (21.71)† |
| Choked when swallowing | 30.75 (27.79) | 32.18 (28.74)†‡ | 24.24 (29.80) | 19.10 (22.29) | 13.55 (23.55) | 9.21 (17.25)† |
| Problems with eating | 26.15 (10.45) | 28.96 (17.49)†‡ | 32.11 (12.61)† | 27.78 (13.00) | 25.27 (12.06)‡ | 24.17 (9.47)†‡ |
| Dry mouth | 0.86 (5.30) | 20.69 (28.31) | 10.10 (19.26) | 5.19 (14.31) | 5.02 (12.78)† | 0.55 (4.27)‡ |
| Trouble with taste | 0.57 (5.34) | 13.36 (20.31) | 5.48 (14.54) | 2.60 (11.34) | 3.35 (13.12)† | 1.29 (6.45)†‡§ |
| Trouble with coughing | 0.29 (4.38) | 37.93 (28.39) | 16.16 (23.23) | 7.07 (16.24) | 3.81 (13.94) | 1.84 (7.64)† |
| Trouble talking | 0.57 (5.34) | 22.70 (31.51) | 11.83 (26.27) | 6.20 (17.72) | 2.59 (10.51) | 2.76 (10.47)† |
| Reflux | 0.86 (4.83) | 31.03 (22.41) | 29.58 (22.10)† | 19.48 (21.26) | 25.27 (19.70) | 28.82 (18.33) |
| Pain related to esophageal cancer | 11.06 (10.05) | 21.79 (11.85) | 9.24 (10.32) | 3.94 (9.12) | 2.84 (6.63)† | 3.62 (8.35)†§ |
Data are presented as mean (standard deviation). †, indicates no significant difference compared with the previous time point; ‡, indicates no significant difference compared with baseline; §, indicates no significant difference among 6, 12, 24 months after discharge. Quality of life indicators similar to or better than baseline levels at discharge: insomnia/constipation/cognitive function/choked when swallowing problems with eating/trouble swallowing saliva/physical function/role function/appetite loss/nausea and vomiting. Quality of life indicators similar to or better than baseline levels for the first time in 3 months after discharge: nausea and vomiting/social function/dysphagia pain related to esophageal cancer/financial difficulties. Quality of life indicators similar to or better than baseline levels for the first time in 6 months after discharge: pain/fatigue. Quality of life indicators similar to or better than baseline levels for the first time in 24 months after discharge: dry mouth/trouble with taste. Quality of life indicators that are always worse than baseline levels within 24 months after discharge are: physical function/dyspnoea/fatigue/appetite loss/diarrhea/global health status/trouble with coughing trouble talking/reflux/dry mouth/trouble with taste. Quality of life indicators that are always similar to or better than baseline levels within 24 months after discharge are role function/emotional function/financial difficulties/trouble swallowing saliva pain related to esophageal cancer/pain/insomnia/appetite loss/nausea and vomiting.


The number and percentage of patients with better or stable status in quality of life indicators compared to baseline at each postoperative time point are listed in Table 3. At discharge, the proportions of patients with better or stable status in physical function, pain, global health status, and dysphagia were less than 10. Notably, all patients experienced a decline in global health status, and even 24 months after discharge, the proportion remained below 10%, indicating a significant impact of surgery on global health. In contrast, there were more than 90% of patients reported better or stable insomnia and trouble swallowing saliva problems. In total, at 24 months after discharge, all indicators except for physical function, pain, global health status, and dysphagia had more than 50% of patients with better or stable status. At that time, among these indicators, more than 90% of patients had a fine status in 14 indicators. While global health status (8.8%), reflux (18.8%), and diarrhea (43.1%) had the lowest proportions of patients with a fine status.
Table 3
| Characteristics | At discharge | 3 months after discharge | 6 months after discharge | 12 months after discharge | 24 months after discharge |
|---|---|---|---|---|---|
| Number | 232 (100.0) | 231 (100.0) | 230 (100.0) | 219 (100.0) | 181 (100.0) |
| Physical function | 4 (1.7)‡ | 45 (19.5) | 131 (57.0) | 124 (56.6)† | 122 (67.4)† |
| Role function | 51 (22.0) | 156 (67.5) | 205 (89.1)†§ | 202 (92.2)‡ | 164 (90.6)‡ |
| Dyspnoea | 28 (12.1) | 146 (63.2) | 105 (47.9) | 161 (73.5)† | 150 (82.9)†§ |
| Pain | 8 (3.4)‡ | 84 (36.4)† | 178 (77.4) | 190 (86.8)† | 143 (79.0)† |
| Fatigue | 53 (22.8) | 99 (42.9) | 154 (67.0)† | 149 (68.0)†§ | 141 (77.9)†§ |
| Insomnia | 139 (59.9) | 208 (90.0) | 192 (83.8) | 204 (93.2)‡ | 165 (91.2)‡ |
| Appetite loss | 185 (79.7) | 204 (88.3) | 196 (85.6) | 205 (93.6)‡ | 169 (93.4)‡ |
| Nausea and vomiting | 186 (80.2) | 191 (82.7) | 210 (91.3)‡ | 191 (87.2) | 164 (90.6)‡ |
| Constipation | 185 (79.7) | 213 (92.2)‡ | 216 (93.5)‡ | 211 (96.3)‡ | 170 (93.9)‡ |
| Diarrhea | 91 (39.2) | 87 (37.7) | 109 (47.2) | 96 (43.8) | 78 (43.1)† |
| Cognitive function | 147 (63.4) | 207 (89.6) | 204 (88.7)† | 191 (87.2)† | 160 (88.4)†§ |
| Emotional function | 175 (75.4) | 204 (88.3) | 204 (88.7)†§ | 188 (85.8)†§ | 171 (94.5)‡ |
| Social function | 33 (14.2) | 150 (64.9) | 200 (87.0)† | 205 (93.6)‡ | 168 (92.8)‡ |
| Financial difficulties | 101 (43.5) | 181 (78.4) | 194 (84.0) | 199 (90.9)‡ | 172 (95.0)‡ |
| Global health status | 0 (0.0)‡ | 2 (0.9)‡ | 16 (7.0)‡ | 8 (3.7)‡ | 16 (8.8)‡ |
| Dysphagia | 6 (2.6)‡ | 136 (58.9) | 177 (76.6) | 133 (60.7) | 102 (56.4)† |
| Trouble swallowing saliva | 203 (87.5) | 215 (93.1)‡ | 200 (87.3) | 208 (95.0)‡ | 179 (98.9)‡ |
| Choked when swallowing | 161 (69.4) | 180 (77.9) | 167 (72.9) | 190 (86.8) | 155 (85.6)† |
| Problems with eating | 102 (44.2) | 95 (41.1) | 128 (55.4) | 133 (60.7) | 116 (64.1) |
| Dry mouth | 139 (59.9) | 172 (74.5) | 198 (85.7) | 188 (85.8) | 178 (98.3)‡ |
| Trouble with taste | 155 (66.8) | 197 (85.3) | 217 (93.9)‡ | 203 (92.7)‡ | 174 (96.1)‡ |
| Trouble with coughing | 54 (23.3) | 141 (61.0) | 187 (81.0)†§ | 201 (91.8)‡ | 171 (94.5)‡ |
| Trouble talking | 136 (58.6) | 183 (79.2)† | 199 (86.1) | 205 (93.6)‡ | 168 (92.8)‡ |
| Reflux | 48 (20.7) | 54 (23.4) | 109 (47.2) | 53 (24.2) | 34 (18.8) |
| Pain related to esophageal cancer | 88 (37.9) | 177 (76.6) | 210 (90.9)‡ | 212 (96.8)‡ | 163 (90.1)‡ |
Data are presented as count (%). †, indicates significant variables in univariable analysis; ‡, indicates that this variable is not included in univariable analysis; §, indicates significantly independent variables in multivariable analysis.
Survival by changes in scales of QLQ-C30 and QLQ-OES18
The detailed results of the univariable Cox analysis results of the demographic, socioeconomic, and clinicopathological characteristics and changes in quality of life indicators at postoperative time points can be found in Table 4. The multivariable Cox analysis results based on quality of life indicators at various time points are available in Table 5 (at discharge), Table 6 (at 3 months after discharge), Table 7 (at 6 months after discharge), Table 8 (at 12 months after discharge) and Table 9 (at 24 months after discharge).
Table 4
| Characteristics | Not quality of life indicators | At discharge | 3 months after discharge | 6 months after discharge | 12 months after discharge | 24 months after discharge |
|---|---|---|---|---|---|---|
| Age group | 0.90 | |||||
| Gender | 0.02* | |||||
| Occupation | 0.19 | |||||
| Marriage | 0.07 | |||||
| Education | 0.41 | |||||
| Financial status | 0.86 | |||||
| Tumor site | 0.17 | |||||
| Pathology | 0.61 | |||||
| Stage | <0.001* | |||||
| Operation approach | 0.86 | |||||
| NeoRadiotherapy | 0.68 | |||||
| NeoChemotherapy | 0.74 | |||||
| Radiotherapy | 0.66 | |||||
| Chemotherapy | 0.04* | |||||
| Physical function | 0.78 | 0.83 | 0.05* | <0.001* | ||
| Role function | 0.97 | 0.36 | <0.001* | |||
| Dyspnoea | 0.10 | 0.81 | 0.19 | 0.04* | <0.001* | |
| Pain | 0.03* | 0.35 | 0.03* | <0.001* | ||
| Fatigue | 0.29 | 0.39 | 0.02* | <0.001* | <0.001* | |
| Insomnia | 0.84 | 0.27 | ||||
| Appetite loss | 0.73 | 0.97 | 0.17 | |||
| Nausea and vomiting | 0.29 | 0.68 | 0.20 | |||
| Constipation | 0.14 | |||||
| Diarrhea | 0.86 | 0.87 | 0.16 | 0.07 | 0.03* | |
| Cognitive function | 0.77 | 0.68 | 0.005* | 0.01* | <0.001* | |
| Emotional function | 0.31 | 0.16 | <0.001* | <0.001* | ||
| Social function | 0.79 | 0.96 | <0.001* | |||
| Financial difficulties | 0.94 | 0.76 | 0.23 | |||
| Global health status | ||||||
| Dysphagia | 0.58 | 0.45 | 0.98 | 0.002* | ||
| Trouble swallowing saliva | 0.49 | 0.83 | ||||
| Choked when swallowing | 0.64 | 0.08 | 0.24 | 0.16 | 0.01* | |
| Problems with eating | 0.62 | 0.09 | 0.60 | 0.97 | 0.13 | |
| Dry mouth | 0.10 | 0.51 | 0.39 | 0.21 | ||
| Trouble with taste | 0.58 | 0.49 | ||||
| Trouble with coughing | 0.80 | 0.42 | 0.003* | |||
| Trouble talking | 0.17 | 0.90 | 0.62 | |||
| Reflux | 0.49 | 0.07 | 0.17 | 0.13 | 0.20 | |
| Pain related to esophageal cancer | 0.21 | 0.63 |
*, indicates the corresponding factor was significantly associated with overall survival in the univariable Cox analysis.
Table 5
| Characteristics | Coef | Exp(coef) | se(coef) | z | Pr(>|z|) | Lower 95% CI | Upper 95% CI | Sig |
|---|---|---|---|---|---|---|---|---|
| Gender (F vs. M) | −0.45 | 0.64 | 0.23 | −1.92 | 0.06 | 0.4 | 1.01 | |
| Stage (3/4 vs. 1/2) | 0.71 | 2.04 | 0.19 | 3.81 | <0.001* | 1.41 | 2.94 | * |
| Chemotherapy (yes vs. no) | 0.29 | 1.34 | 0.19 | 1.58 | 0.12 | 0.93 | 1.93 |
*, indicates the corresponding factor is statistically significant in multivariable Cox analysis. Coef, coefficient; Exp(coef), hazard ratio; se, standard error; 95% CI, 95% confidence interval of hazard ratio; Sig, significance.
Table 6
| Characteristics | Coef | Exp(coef) | se(coef) | z | Pr(>|z|) | Lower 95% CI | Upper 95% CI | Sig |
|---|---|---|---|---|---|---|---|---|
| Pain | 0.31 | 1.36 | 0.19 | 1.65 | 0.10 | 0.94 | 1.98 | |
| Gender (F vs. M) | −0.47 | 0.63 | 0.23 | −1.98 | 0.05 | 0.4 | 0.99 | |
| Stage (3/4 vs. 1/2) | 0.66 | 1.93 | 0.19 | 3.49 | <0.001* | 1.33 | 2.8 | * |
| Chemotherapy (yes vs. no) | 0.27 | 1.3 | 0.19 | 1.42 | 0.16 | 0.9 | 1.88 |
*, indicates the corresponding factor is statistically significant in multivariable Cox analysis. Coef, coefficient; Exp(coef), hazard ratio; se, standard error; 95% CI, 95% confidence interval of hazard ratio; Sig, significance.
Table 7
| Characteristics | Coef | Exp(coef) | se(coef) | z | Pr(>|z|) | Lower 95% CI | Upper 95% CI | Sig |
|---|---|---|---|---|---|---|---|---|
| Role function | −0.96 | 0.38 | 0.32 | −2.95 | <0.001* | 0.2 | 0.73 | * |
| Fatigue | −0.01 | 0.99 | 0.23 | −0.03 | 0.97 | 0.63 | 1.56 | |
| Cognitive function | 0.16 | 1.18 | 0.41 | 0.41 | 0.69 | 0.53 | 2.61 | |
| Emotional function | −0.79 | 0.46 | 0.39 | −2.04 | 0.04 | 0.21 | 0.97 | * |
| Social function | −0.14 | 0.87 | 0.39 | −0.35 | 0.72 | 0.4 | 1.88 | |
| Trouble with coughing | −0.68 | 0.51 | 0.23 | −3.03 | <0.001* | 0.32 | 0.79 | * |
| Gender (F vs. M) | −0.7 | 0.5 | 0.24 | −2.87 | <0.001* | 0.31 | 0.8 | * |
| Stage (3/4 vs. 1/2) | 0.76 | 2.14 | 0.19 | 3.98 | <0.001* | 1.47 | 3.12 | * |
| Chemotherapy (yes vs. no) | 0.28 | 1.32 | 0.19 | 1.45 | 0.15 | 0.91 | 1.93 |
*, indicates the corresponding factor is statistically significant in multivariable Cox analysis. Coef, coefficient; Exp(coef), hazard ratio; se, standard error; 95% CI, 95% confidence interval of hazard ratio; Sig, significance.
Table 8
| Characteristics | Coef | Exp(coef) | se(coef) | z | Pr(>|z|) | Lower 95% CI | Upper 95% CI | Sig |
|---|---|---|---|---|---|---|---|---|
| Dyspnoea | −0.12 | 0.89 | 0.27 | −0.45 | 0.65 | 0.52 | 1.5 | |
| Fatigue | −0.53 | 0.59 | 0.25 | −2.12 | 0.03 | 0.36 | 0.96 | * |
| Cognitive function | −0.19 | 0.83 | 0.33 | −0.55 | 0.58 | 0.43 | 1.6 | |
| Emotional function | −0.66 | 0.52 | 0.33 | −2.03 | 0.04 | 0.27 | 0.98 | * |
| Physical function | −0.2 | 0.82 | 0.26 | −0.77 | 0.44 | 0.5 | 1.36 | |
| Pain | −0.07 | 0.93 | 0.29 | −0.23 | 0.82 | 0.52 | 1.67 | |
| Gender (F vs. M) | −0.92 | 0.4 | 0.28 | −3.34 | <0.001* | 0.23 | 0.68 | * |
| Stage (3/4 vs. 1/2) | 0.51 | 1.67 | 0.2 | 2.58 | 0.01 | 1.13 | 2.47 | * |
| Chemotherapy (yes vs. no) | 0.35 | 1.41 | 0.21 | 1.67 | 0.09 | 0.94 | 2.12 |
*, indicates the corresponding factor is statistically significant in multivariable Cox analysis. Coef, coefficient; Exp(coef), hazard ratio; se, standard error; 95% CI, 95% confidence interval of hazard ratio; Sig, significance.
Table 9
| Characteristics | Coef | Exp(coef) | se(coef) | z | Pr(>|z|) | Lower 95% CI | Upper 95% CI | Sig |
|---|---|---|---|---|---|---|---|---|
| Dyspnoea | −0.82 | 0.44 | 0.35 | −2.37 | 0.02 | 0.22 | 0.87 | * |
| Fatigue | −0.39 | 0.67 | 0.38 | −1.02 | 0.31 | 0.32 | 1.43 | |
| Diarrhea | −0.4 | 0.67 | 0.27 | −1.47 | 0.14 | 0.39 | 1.14 | |
| Cognitive function | −1.38 | 0.25 | 0.4 | −3.44 | <0.001* | 0.12 | 0.55 | * |
| Choked when swallowing | −0.06 | 0.94 | 0.35 | −0.18 | 0.86 | 0.47 | 1.88 | |
| Physical function | −0.06 | 0.94 | 0.36 | −0.18 | 0.86 | 0.46 | 1.89 | |
| Pain | −0.94 | 0.39 | 0.31 | −3.06 | <0.001* | 0.22 | 0.71 | * |
| Dysphagia | −0.41 | 0.66 | 0.29 | −1.39 | 0.17 | 0.37 | 1.18 | |
| Gender (F vs. M) | −1 | 0.37 | 0.34 | −2.93 | <0.001* | 0.19 | 0.72 | * |
| Stage (3/4 vs. 1/2) | 0.48 | 1.62 | 0.25 | 1.9 | 0.06 | 0.99 | 2.67 | |
| Chemotherapy (yes vs. no) | −0.11 | 0.89 | 0.28 | −0.41 | 0.68 | 0.51 | 1.55 |
*, indicates the corresponding factor is statistically significant in multivariable Cox analysis. Coef, coefficient; Exp(coef), hazard ratio; se, standard error; 95% CI, 95% confidence interval of hazard ratio; Sig, significance.
It was observed that at discharge, the changing status of all quality of life indicators showed no prognostic significance (P>0.05). However, in the subsequent postoperative time points, certain indicators were associated with OS in the univariable Cox analysis. These indicators included pain and trouble talking at 3 months after discharge, role function, fatigue, cognitive function, emotional function, social function, and trouble with coughing at 6 months after discharge, physical function, dyspnoea, pain, fatigue, cognitive function, and emotional function at 12 months after discharge and physical function, dyspnoea, pain, fatigue, diarrhea, cognitive function, dysphagia, and choked when swallowing at 24 months. The survival outcomes of the two groups based on the worse and similar or better status of independent prognostic factors are depicted in Figure 4.
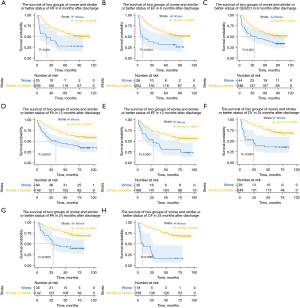
In multivariate analysis, role function, emotional function, and trouble with coughing at 6 months after discharge, fatigue and emotional function at 12 months after discharge, and dyspnoea, pain, and cognitive function at 24 months after discharge were identified as independent prognostic factors. For example, at 6 months after discharge, patients who reported better or stable role function had a 62% reduction in mortality compared with those who reported worse role function [P<0.001, hazard ratio (HR): 0.38, 95% confidence interval (CI): 0.20–0.73]. Similarly, mortality decreased by 54% for emotional function (P=0.04, HR: 0.46, 95% CI: 0.21–0.97) and by 49% for trouble with coughing (P=0.003, HR: 0.51, 95% CI: 0.32–0.79). At 12 months after discharge, patients who reported improved or stable fatigue had a 41% reduction in mortality compared with those who reported worse fatigue (P=0.03, HR: 0.59, 95% CI: 0.36–0.96). Likewise, mortality decreased by 48% for emotional function (P=0.04, HR: 0.52, 95% CI: 0.27–0.98). At 24 months after discharge, patients with an improved or stable status in dyspnoea had a 56% reduction in mortality compared with those who reported worse dyspnoea (P=0.02, HR: 0.44, 95% CI: 0.22–0.87). Mortality decreased by 75% for cognitive function (P<0.0001, HR: 0.25, 95% CI: 0.12–0.55) and by 61% for pain (P<0.0001, HR: 0.39, 95% CI: 0.22–0.71). These findings highlight the prognostic significance of certain quality of life indicators in relation to OS at different postoperative time points.
Discussion
To our knowledge, this is the first study to longitudinally assess patients with esophageal cancer for up to two years and to investigate the relationship between changes in quality of life indicators at five different time points and OS. Our findings revealed that certain quality of life scores at discharge were similar to those before surgery, while others remained stable over time. For example, scores for insomnia, constipation, cognitive function, choked when swallowing and problems with eating scores at discharge were comparable to those before surgery. Furthermore, there were no significant alterations in nausea and vomiting, diarrhea, problems with eating, and reflux from discharge to 3 months after discharge. Similarly, physical function, constipation, diarrhea, social function, global health status, and pain related to esophageal cancer at 6, 12, and 24 months after discharge did not change significantly. The study also found that scores for insomnia, constipation, cognitive function, choked when swallowing, and problems with eating at discharge remained similar to baseline scores. Additionally, no significant difference was observed in nausea and vomiting, diarrhea, problems with eating, and reflux at 3 months after discharge compared to those scores at discharge. Overall, scores for physical function, constipation, diarrhea, social function, global health status, and pain related to esophageal cancer remained stable at 6, 12, and 24 months after discharge. It is likely to be observed that symptoms or functions in esophagectomy patients tend to improve over time which represents a positive adaptation. Notably, a previous study has demonstrated that the long-term quality of life of esophagectomy patients who have survived for a minimum of 3 years exhibits improvement when compared with the time of diagnosis, and more importantly, reaches the level of the general population (19,20).
Our study revealed that specific quality of life indicators at defined post-discharge intervals are independent prognostic factors for OS in esophageal cancer patients after curative resection. Notably, stable or better experience with role function, emotional function, and coughing difficulty at 6 months, fatigue and emotional function at 12 months, and dyspnea, pain, and cognitive function at 24 months post-discharge were significantly associated with better prognosis, which was linked to a reduction in mortality, ranging from 41% to 75%. Conversely, deterioration from baseline in the identified quality of life indicators at specific post-discharge intervals predicted a poorer survival outcome. The order of prognostic impact was cognitive function at 24 months, role function at 6 months, pain and dyspnoea at 24 months post-discharge, followed by emotional function and trouble with coughing at 6 months, and emotional function and fatigue at 12 months. These insights enhance the precision of prognostic assessment for esophageal cancer patients after undergoing surgery.
Previous studies have explored the prognostic relevance of various quality of life indicators across various cancer types, though no single indicator is universally significant (21,22). Quinten and colleagues did a pooled analysis of whether quality of life assessments had prognostic value for different cancers and highlighted that no consistent prognostic indicator in all cancers was recognized (23). However, certain indicators, such as role function in prostate and testicular cancers and emotional function in breast and head and neck cancers, were found to be predictive for survival. Djärv’s research identified key quality of life indicators—such as global health-related quality of life, physical function, social function, fatigue, pain, dyspnoea, appetite loss, dysphagia, and esophageal pain which were associated with an elevated hazard ratio of mortality (24). Consistent with these findings, a study among gastroesophageal cancer patients reported a significant association between physical function, role function, cognitive function, social function, global quality of life, fatigue, nausea, vomiting, pain, dyspnoea, appetite loss, constipation, and cancer-specific survival (25). Additionally, another research group discovered that patients with impaired cognitive function experienced a 1.72-fold higher risk of recurrence, and a 1.90-fold higher mortality risk respectively (25).
This study offers a distinct perspective on the prognostic significance of quality of life indicators in esophageal cancer patients Nieuwenhuizen and colleagues’s study showed that deterioration in physical and emotional function at 6 and 12 months after treatment, as well as global quality of life and social function at 12 months, were linked to reduced survival (26). However, our findings did not corroborate this association, possibly due to the highly morbid nature of esophagectomy, which may have resulted in minimal improvement or stability in global health status among patients over the two-year post-discharge period. Wikman et al.’s study identified symptom clusters, including reflux, cough, and eating difficulties, in esophageal cancer patients at 6 months post-surgery, which were associated with a significantly increased risk of mortality (27), which aligns with Djärv’s research (28). The potential influence of surgery on symptoms might explain why our study did not observe the same correlations, as the surgical impact could overshadow other factors.
Our findings suggest that the prognostic value of quality of life indicators can vary over time. To mitigate bias, we excluded certain indicators from survival analysis when they showed a significant skew at some time points. For example, role function was an independent prognostic factor at 6 months after discharge but was not at 12 and 24 months as most patients (more than 90%) reported improved conditions at the later stages. This fluctuation in prognostic significance likely stems from the complex interplay of factors that influence quality of life. Quality of life is largely perceived through the alignment of personal aspirations with actual achievements. As these factors vary over time, they cause the distribution of certain quality of life indicators to differ, resulting in varying analytical outcomes.
We revealed a time-dependent emergence of quality of life indicators as prognostic factors. The number of prognostic indicators at discharge and in 3 months after discharge was much less than that in later stages, and even there was no independent prognostic factor found during these early stages. We assumed that initial similarity in quality of life among patients with varying outcomes may be due to the persistence of acute post-surgical side effects, which mask differences in the prognostic value of quality of life indicators. Most acute side effects often resolve within 6 months, a timeframe consistent with the recovery from head and neck tumors as reported previously (29,30). Worsening symptoms beyond this period may indicate undetected tumor recurrence or disease progression.
Oncologists always underestimate the incidence and severity of a patient’s symptoms (31,32). A decline in quality of life measures after surgery may suggest disease progression, warranting rigorous follow-up or examination. Compared with routine clinical practice, intensive symptom monitoring can improve cancer patient survival (31) by enabling early intervention. We proposed that physicians should closely monitor these prognostic factors to identify worsening symptoms, perform thorough examinations to diagnose disease progression and adjust treatment plans accordingly. By incorporating these indicators into clinical practice, healthcare professionals can enhance patient care and potentially improve outcomes.
We held several advantages in this study, including its prospective design that mitigates recall bias through time-based data collection. The low loss to follow-up ensures a robust dataset, and the extended follow-up period allows for a comprehensive assessment of long-term outcomes. We also employed multivariable analysis to adjust for confounding factors, enhancing the reliability of our findings. The use of validated patient-reported quality of life questionnaires further solidifies the credibility of our assessment tools. However, there’s no doubt to acknowledge certain limitations. Firstly, it was conducted at a single center, which may limit the generalizability of our findings to other settings or populations. Secondly, there was heterogeneity in cancer stages and variability in neoadjuvant and adjuvant therapy, as major confounding factors, potentially impacting the relationship between quality of life indicators and prognosis. Thirdly, the loss of patients to follow-up might introduce bias, as these individuals may have poorer symptomatology or functional outcomes.
Conclusions
In conclusion, our study highlights the fluctuating nature of quality of life indicators concerning survival outcomes across different post-discharge time points in esophageal cancer patients. Notably, the deterioration of role function, emotional function, and coughing difficulty at 6 months, fatigue and emotional function at 12 months, and dyspnea, fatigue, and cognitive function at 24 months post-discharge were significantly associated with a poorer prognosis. These findings suggest that vigilant monitoring of symptomatic and functional changes in esophageal cancer patients is crucial for enhancing prognostic assessments.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-311/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-311/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-311/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-311/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center (No. YB2016-070) and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Zhang SW, Wu LL, Yang H, et al. Effect of the Active Cycle of Breathing Technique on Perioperative Outcome in Individuals With Esophagectomy: A Quasi-Experimental Study. Front Surg 2021;8:735947. [Crossref] [PubMed]
- Wu LL, Zhong JD, Zhu JL, et al. Postoperative survival effect of the number of examined lymph nodes on esophageal squamous cell carcinoma with pathological stage T1-3N0M0. BMC Cancer 2022;22:118. [Crossref] [PubMed]
- Wu LL, Wang JL, Huang W, et al. Prognostic Modeling of Patients Undergoing Surgery Alone for Esophageal Squamous Cell Carcinoma: A Histopathological and Computed Tomography Based Quantitative Analysis. Front Oncol 2021;11:565755. [Crossref] [PubMed]
- Liu X, Wu L, Zhang D, et al. Prognostic impact of lymph node metastasis along the left gastric artery in esophageal squamous cell carcinoma. J Cardiothorac Surg 2021;16:124. [Crossref] [PubMed]
- Cheng N, Li X, Zhao C, et al. Microarray expression profile of long non-coding RNAs in EGFR-TKIs resistance of human non-small cell lung cancer. Oncol Rep 2015;33:833-9. [Crossref] [PubMed]
- Jezerskyte E, Saadeh LM, Hagens ERC, et al. Long-term health-related quality of life after McKeown and Ivor Lewis esophagectomy for esophageal carcinoma. Dis Esophagus 2020;33:doaa022. [Crossref] [PubMed]
- Eyck BM, Klevebro F, van der Wilk BJ, et al. Lasting symptoms and long-term health-related quality of life after totally minimally invasive, hybrid and open Ivor Lewis esophagectomy. Eur J Surg Oncol 2022;48:582-8. [Crossref] [PubMed]
- Barbour AP, Cormack OMM, Baker PJ, et al. Long-term Health-related Quality of Life Following Esophagectomy: A Nonrandomized Comparison of Thoracoscopically Assisted and Open Surgery. Ann Surg 2017;265:1158-65. [Crossref] [PubMed]
- Healy LA, Ryan AM, Moore J, et al. Health-related quality of life assessment at presentation may predict complications and early relapse in patients with localized cancer of the esophagus. Dis Esophagus 2008;21:522-8. [Crossref] [PubMed]
- Hiratsuka Y, Kim YJ, Suh SY, et al. The association between changes in symptoms or quality of life and overall survival in outpatients with advanced cancer. Ann Palliat Med 2022;11:2338-48. [Crossref] [PubMed]
- Djärv T, Metcalfe C, Avery KN, et al. Prognostic value of changes in health-related quality of life scores during curative treatment for esophagogastric cancer. J Clin Oncol 2010;28:1666-70. [Crossref] [PubMed]
- Bouchard LC, Aaronson N, Gondek K, et al. Cancer symptom response as an oncology clinical trial end point. Expert Rev Qual Life Cancer Care 2018;3:35-46. [Crossref] [PubMed]
- World Medical Association Declaration of Helsinki. ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. [Crossref] [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [Crossref] [PubMed]
- Blazeby JM, Conroy T, Hammerlid E, et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer 2003;39:1384-94. [Crossref] [PubMed]
- Wan C, Meng Q, Yang Z, et al. Validation of the simplified Chinese version of EORTC QLQ-C30 from the measurements of five types of inpatients with cancer. Ann Oncol 2008;19:2053-60. [Crossref] [PubMed]
- Dai Z, Lang W, Yang H, et al. Validation of EORTC QLQ-OES18 for Chinese patients with esophageal cancer. Dis Esophagus 2017;30:1-7. [Crossref] [PubMed]
- Katz A, Nevo Y, Ramírez García Luna JL, et al. Long-Term Quality of Life After Esophagectomy for Esophageal Cancer. Ann Thorac Surg 2023;115:200-8. [Crossref] [PubMed]
- Darling GE. Quality of life in patients with esophageal cancer. Thorac Surg Clin 2013;23:569-75. [Crossref] [PubMed]
- Gotay CC, Kawamoto CT, Bottomley A, et al. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol 2008;26:1355-63. [Crossref] [PubMed]
- Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol 2009;10:865-71. [Crossref] [PubMed]
- Quinten C, Martinelli F, Coens C, et al. A global analysis of multitrial data investigating quality of life and symptoms as prognostic factors for survival in different tumor sites. Cancer 2014;120:302-11. [Crossref] [PubMed]
- Djärv T, Blazeby JM, Lagergren P. Predictors of postoperative quality of life after esophagectomy for cancer. J Clin Oncol 2009;27:1963-8. [Crossref] [PubMed]
- de Graeff A, de Leeuw JR, Ros WJ, et al. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. Eur J Cancer 2001;37:332-9. [Crossref] [PubMed]
- van Nieuwenhuizen AJ, Buffart LM, Langendijk JA, et al. Health-related quality of life and overall survival: a prospective study in patients with head and neck cancer treated with radiotherapy. Qual Life Res 2021;30:1145-53. [Crossref] [PubMed]
- Wikman A, Johar A, Lagergren P. Presence of symptom clusters in surgically treated patients with esophageal cancer: implications for survival. Cancer 2014;120:286-93. [Crossref] [PubMed]
- Djärv T, Lagergren P. Six-month postoperative quality of life predicts long-term survival after oesophageal cancer surgery. Eur J Cancer 2011;47:530-5. [Crossref] [PubMed]
- Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes 2009;7:102. [Crossref] [PubMed]
- Basch E, Deal AM, Dueck AC, et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 2017;318:197-8. [Crossref] [PubMed]
- Laugsand EA, Sprangers MA, Bjordal K, et al. Health care providers underestimate symptom intensities of cancer patients: a multicenter European study. Health Qual Life Outcomes 2010;8:104. [Crossref] [PubMed]
- Nekolaichuk CL, Bruera E, Spachynski K, et al. A comparison of patient and proxy symptom assessments in advanced cancer patients. Palliat Med 1999;13:311-23. [Crossref] [PubMed]



