
Robotic-assisted thoracoscopic surgery first rib resection—surgical technique
Highlight box
Key findings
• Robotic-assisted thoracoscopic surgery (RATS) 1st rib resection is a safe and effective surgical technique for the treatment of thoracic outlet syndrome (TOS) providing an attractive alternative to video-assisted thoracoscopic surgery (VATS) with enhanced 3-dimensional (3D) visualization, manoeuvrability and avoidance of retraction of neurovascular structures with reduced morbidity. We present a detailed step-to-step 1st rib resection with clear visualization of the relevant anatomical landmarks.
What is conventional and what is novel/modified?
• The technique of RATS 1st rib resection has been established by different authors. It remains to be a hybrid surgical technique due to the lack of bone cutting robotic instruments.
• With the reduction from four to three minimally invasive incisions, we intended to minimize postoperative pain and requirement of morphine equivalents, length of stay and the aesthetic result for the predominantly young patients.
What is the implication, and what should change now?
• Robotic instruments allowing for a purely robotic-assisted cutting of bony structures are indispensable to move this hybrid technique to an independent robotic approach.
• Mainly owing to the unavailability of prospective studies, uniportal RATS (uRATS) has not yet been proven to further reduce postoperative morbidity as compared to multi-portal RATS approach. Randomized controlled studies will be necessary to reveal a potential benefit of uRATS over three-port RATS 1st rib resection.
• The introduction of standardized robotic training programs to help disseminate this superior surgical technique for certain indications including 1st rib resection are essential.
Introduction
Thoracic outlet syndrome (TOS) is a relatively rare but disabling disease primarily affecting young adults. The prevalence of TOS in the general population is not clearly defined because of the lack of epidemiologic data. It comprises a constellation of signs and symptoms originating from neurologic and/or vascular compression of the brachial plexus and subclavian vessels between the scalene muscles and the costoclavicular space (1). One needs to differentiate between three types of TOS: arterial (ATOS), venous (VTOS), and neurogenic (NTOS), of which NTOS is by far the most common type in about >95% (2). VTOS comprises 3–5% of all TOS cases (2,3). There are mixed cases of atrio-venous TOS (AVTOS) described, where both the arterial and venous structures are compressed in the thoracic outlet (TO).
VTOS is caused by subclavian vein (SV)-compression at the costoclavicular junction or less frequently the pectoralis minor space and mainly present as acute or chronic upper extremity swelling caused by deep venous thrombosis [Paget-Schroetter syndrome (PSS), “effort thrombosis”] or positional swelling (McCleery syndrome), first described by Paget in 1875 and Von Schrötter in 1884 (and edited by Nothnagel in 1899) (4,5). In VTOS, the SV-compression typically increases with overhead elevation of the upper limb.
Physical examination with the elevated arm stress test (EAST), the upper limb tension test (ULTT), the Halsted-, Wright-, and Adson-tests are usually performed to induce the symptomatology.
Pathophysiologically, the congenitally malformed abnormal medial aspect of the 1st rib often compresses the SV at the TO.
Conservative and interventional treatment comprise catheter-thrombolysis plus or minus venoplasty and oral anticoagulation as bridge to surgical decompression, however high thrombosis recurrence rates in especially young patients indicate oral anticoagulation followed by early surgical decompression in this patient group (2,6-8).
Surgical resection of the 1st rib has been shown to be a successful and curative treatment of symptomatic TOS (9-11).
The aim of the surgical management is relief from upper extremity swelling and neurologic symptoms, freedom from long-term anticoagulation, and a return to unrestricted arm activity. Published data showed that those results can be achieved in more than 90% of the patients (12).
While diverse open surgical approaches have been described (supraclavicular, infraclavicular, trans-axillary and hybrid approaches like the thoracoscopic assisted transaxillary approach (13,14), the advantages of minimally invasive techniques such as video-assisted (VATS) and robotic-assisted thoracoscopic surgery (RATS) nowadays predominate surgical 1st rib resection (15-17).
The aim of this surgical technique manual is to describe and highlight the precision of the RATS technique while presenting the key surgical steps. We present this article in accordance with the SUPER reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-702/rc).
Preoperative preparations and requirements
A detailed specific medical history along with a careful physical examination may already raise high suspicion of TOS. The symptoms are usually correlated to the components of the neurovascular bundle which are compressed. Upper extremity pain, heaviness or dysesthesia are common symptoms in NTOS; oedema and cyanosis of the upper limb may suggest a compression of the subclavian artery (SA); arm claudication with numbness and pain is typical in case of ATOS. Commonly, the symptoms are worsened by an abduction or flexion of the shoulder above the head.
The EAST, the ULTT, the Halsted-, Wright-, and Adson-tests are usually performed to induce the symptomatology (18,19). All these tests aim to narrow the TO by positioning of the upper limbs. During the EAST, the scalene triangle is narrowed by abducting the arms to 90° with flexed elbows and the shoulder rotated externally to tilt the forearms backwards (20,21). In the ULTT, both arms are abducted to 90° with the elbows straight. Both wrists are dorsiflexed followed by a neck tilting away from the tested limb. While abduction and extension of the wrists lead to symptoms on the ipsilateral side, the head tilt reveals symptoms on the contralateral side (1).
The Halsted-, Wright- and Adson manoeuvres work in a similar way, provoking a narrowing of the TO by upper limb positioning while the physician is palpating the radial artery to register a decrease or loss of pulse (1). In a positive Wright’s test, the radial pulse disappears when the arm is abducted and rotated externally (22). In the Adson test, the patient takes a long deep breath, elevates the chin, and turns it to the affected side (1). However, the above-mentioned provocation tests may raise suspicion of NTOS but have been found to test positive in healthy individuals too; hence they exhibit low diagnostic specificity (21,23). According to our institutional protocol, we perform a magnetic resonance imaging (MRI) of the chest for suspected compression of the brachial plexus along with nerve conduction studies (NCS) and needle electromyography (EMG) (24). Anterior scalene muscle block has not yet been implemented into our patient work-up when suspecting NTOS (25). In case of VTOS and ATOS, we prefer a combination of computed tomography (CT) of the chest with intravenous (iv) contrast and a duplex sonography of the SV and SA. All the diagnostic workups should be performed with upper limb in a neutral position and then during elevation of the upper extremity.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from all the patients for publication of this article, the accompanying images, and the video. A copy of the written consent is available for review by the editorial office of this journal.
Step-by-step description
- Under general anaesthesia with a left sided double lumen tube for single lung ventilation, the patient is placed in the lateral decubitus position using a soft roll underneath the patient to spread the rib spaces for better manoeuvrability of the robotic instruments The robot is brought towards the patient and the appropriate setting is selected on the robot touchscreen prior to deployment for docking.
- A three-port access with ports in the 3rd intercostal space (ICS) in the anterior axillary line (AAL), one port in the 5th ICS mid-axillary line (MAL) and a third incision in the posterior axillary line (PAL) in the 4th ICS, respectively, is chosen by the robotic-trained thoracic surgeon. Carbon dioxide (CO2) insufflation is started at 8 mmHg. Three robotic trocars of 8 mm with a 30° robotic camera, Cadiere Grasper® and a Maryland forceps® (Intuitive, Sunnyvale, CA, USA) are inserted (Figure 1).
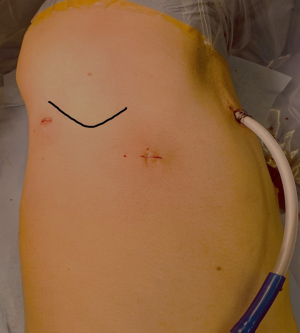 Figure 1 Three-port RATS access for 1st rib resection with incisions in the 3rd ICS in the AAL, in the 5th ICS MAL and in the PAL in the 4th ICS. Tip of scapula is highlighted as a landmark prior to incision. RATS, robotic-assisted thoracoscopic surgery; ICS, intercostal space; AAL, anterior axillary line; MAL, mid-axillary line; PAL, posterior axillary line.
Figure 1 Three-port RATS access for 1st rib resection with incisions in the 3rd ICS in the AAL, in the 5th ICS MAL and in the PAL in the 4th ICS. Tip of scapula is highlighted as a landmark prior to incision. RATS, robotic-assisted thoracoscopic surgery; ICS, intercostal space; AAL, anterior axillary line; MAL, mid-axillary line; PAL, posterior axillary line. - After entering the thoracic cavity through the camera port in the 5th ICS, multilevel intercostal nerve blocks to cover as many incisions as possible are applied, with a body weight adapted dosage of ropivacaine 0.75%, equally distributed to the different ICSs under direct vision. The first thoracic rib is identified below the most superior intercostal muscle fibres. With a 30° upwards view, the overlying parietal pleura on the upper border of the 1st rib is opened and the intercostal muscles are dissected off the 1st rib from the level of the internal thoracic vasculature anteriorly until the most posterior aspect of the 1st rib (Figure 2).
- In the next step, the parietal pleura at the inferior aspect of the 1st rib is opened under special precaution not to injure the underlying vasculature of the TO (Figure 3).
Afterwards, the ventral portion of the 1st rib is carefully divided with the 10-inch Kerrison Punch 90° degrees 1 cm lateral to the sternum. Due to the lack of robotic instruments for bone cutting, this important step has to be done with the assistance of a thoracoscopic device as described before, inserted through the most posterior port after undocking of the robot for this step (22). If necessary, the ports might have to be switched to limit the stress applied on intercostal structures through instrument angulation (Figure 4).
The 1st rib is then subsequently released and reflected towards the dorsal aspect and subluxated after carefully releasing the serratus anterior muscle, the scalene muscles and the costoclavicular ligament (Figure 5).

The rib is then stepwise cut in the paravertebral, dorsal aspect with the Kerrison Punch while taking care of the thoracic spinal nerve root one (T1) (Figure 6). To minimize recurrence or persistence in NTOS, a long posterior rib stump has to be avoided (26). Alternatively, a costovertebral exarticulation can be done in NTOS which has led to reduced recurrence (27).

The specimen is removed in an endo-catch bag through the assistant port in the 3rd ICS AAL without enlarging the incision. The medial and lateral stumps of the rib are then rounded with the Caspar Rongeur to prevent lesions of the visceral pleura after lung re-expansion (Figure 7).

Any fibrous membrane present over the neuro-vascular bundle should be removed. In our case, removal of fibrous tissue narrowing the SV, was done in Step 4 to create clear anatomical view on the important structures of the TO (Figure 8). A 24-French chest-tube is inserted, the lung is fully reventilated and skin closure is done intracutaneously (for all steps see Video 1).

Postoperative considerations and tasks
Major complications of 1st rib resection include Horner syndrome, pneumothorax, pleural effusion, chylothorax, hemothorax or extrapleural hematoma of which the last five can be diagnosed with a simple postoperative chest radiograph (3).
As recommended by the Society for Vascular Surgery for TOS in 2016, postoperative venous patency should be specifically reported at 3, 6, 12, and 24 months after surgical or interventional treatment. The recommended imaging modalities are duplex sonography, MRI, CT, or venography. The TOS disability score is strongly recommended for the duration of follow-up (28).
Postoperative anticoagulation should be continued as, e.g., Angle et al. have shown that 30–45% of the patients have residual stenosis due to post-thrombotic changes or fibrous strictures (29). Only after demonstration of SV patency on one of the above-mentioned imaging modalities at least 2–4 weeks postoperatively (of which duplex sonography is probably the most applied one), anticoagulation can be stopped.
Tips and pearls
- We strongly recommend to exclusively use bipolar energy during dissection around the first rib when operating near the neurovascular structures.
- It is important to properly release the relevant muscles (serratus anterior muscle, the scalene muscles and the costoclavicular ligament) in order to easily subluxate the rib with following exposure of the posterior face of the 1st rib.
- A gentle blunt dissection between the rib and the neurovascular bundle can safely be performed with the help of neurosurgical cotton stripes.
- To minimize the risk of injuring neurovascular structures, the camera angle may be changed from a 30° upwards view towards the upper border of the 1st rib to a more downwards view to achieve clear visualization of the vascular structures “behind” the rib.
- To avoid injuries to the parietal pleura, the robotic instruments should be used in a wristed-upwards position not to injure the parietal pleura with the backside.
- Exarticulation of the costo-vertebral joint rather than cutting the posterior aspect of the rib, which has led to reduced recurrence in NTOS (27).
- To reduce the risk of recurrent thrombosis in VTOS, potentially restricting fibrous tissue around the SV should be released meticulously.
- An angled Kerrison Punch to achieve a better angle to cut the posterior aspect of the 1st rib might be chosen instead of the standard straight Kerrison Punch.
- If brachial plexus neurolysis is necessary, the use of only sharp dissection and the avoidance of electrocoagulation is recommended.
- We recommend prompt and preferably seamless anticoagulation pre-/postoperatively when the chest radiograph doesn’t suggest any complications after chest drain removal in patients with thrombosis.
Results and single centre experience
In our tertiary care Division of Thoracic Surgery of the Cantonal Hospital Lucerne in Switzerland, we performed 20 RATS 1st rib resections between June 2020 and March 2024 in 18 patients with the mean age of 34 years [standard deviation (SD), ±12 years] at operation with equally distributed gender (50% male) (Table 1). All the procedures were done after diagnosis of TOS, of which 40% were VTOS, 30% ATOS, 15% AVTOS, 5% NTOS and 10% unspecified TOS. Sixty-five percent of the procedures were done on the right side, 35% on the left.
Table 1
| Variables | VTOS | ATOS | NTOS | TOS unspecified | AVTOS |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Age (years), mean ± SD [range] | 34±12 [17–57] | ||||
| Gender, n | |||||
| Female | 6 | 2 | 0 | 1 | 0 |
| Male | 2 | 4 | 1 | 1 | 3 |
| Laterality, n | |||||
| Left | 4 | 2 | 0 | 0 | 1 |
| Right | 4 | 4 | 1 | 2 | 2 |
| Paget-Schroetter, n | 6 | 0 | 0 | 0 | 1 |
| Cervical rib, n | 1 | 1 | 0 | 2 | 0 |
| Imaging/diagnostic, n | |||||
| MRI- and CT-angiography | 0 | 2 | 0 | 0 | 2 |
| MRI-angiography | 4 | 1 | 1 | 0 | 1 |
| CT-angiography | 4 | 2 | 0 | 1 | 0 |
| Duplex sonography | 2 | 4 | 0 | 1 | 2 |
| EMG/NCS | 0 | 0 | 1 | 0 | 0 |
| Outcomes | |||||
| Skin-to-skin (min), mean ± SD | 164±41 | ||||
| LOS (days), mean ± SD | 3±1 | ||||
| Follow-up (days), mean ± SD | 77±47 | ||||
| Anomalies on postoperative duplex | 1 patient with persistent post-thrombotic changes in duplex sonography | ||||
| Postoperative complications | |||||
| Pneumothorax | 2 patients, requiring chest drain | ||||
| Pleural effusion | 1 patient, requiring chest drain | ||||
| Revision | 1 patient (persistent airleak) | ||||
ATOS, arterial TOS; AVTOS, atrio-venous TOS; CT, computed tomography; EMG, electromyography; LOS, length of stay; MRI, magnetic resonance imaging; n, number; NCS, nerve conduction studies; NTOS, neurogenic TOS; RATS, robotic-assisted thoracoscopic surgery; SD, standard deviation; TOS, thoracic outlet syndrome; VTOS, venous TOS.
Preoperative imaging was done with CT-angiography in 39% (n=7), MRI-angiography in 39% (n=7) and the combination of CT- and MRI-angiography in n=4 (22%) of the patients.
Mean length of stay (LOS) was 3 days (SD, ±1 days). No intraoperative complications occurred, and the mean time of surgery was 164 minutes (SD, ±41 minutes). The skin-to-skin time did not significantly change throughout the years of practice.
Postoperative complications were recorded in three patients with two of them suffering from pneumothorax requiring reinsertion of a chest drain, one patient requiring pleural drainage for postoperative pleural effusion and one of the above-mentioned patients requiring re-operation for persistent air leak after chest drain re-insertion.
Median follow-up was 77 days (SD, ±47 days) with 89% of the patients (n=16 with n=18 operated sides) being clinically asymptomatic with unremarkable vascular flow signals in duplex sonography. One patient was found to have post-thrombotic changes after PSS and was kept on oral anticoagulation. Further follow up was done by the angiologist according to our in-house protocol.
Discussion
1st rib resection is the curative option of choice to treat TOS when conservative measures have failed. A structured multidisciplinary strategy involving different specialists like physiotherapists, neurologists, angiologist, vascular and thoracic surgeons as well as orthopaedic surgeons is necessary to choose the most appropriate treatment option after accurate diagnostic evaluation.
In 2016, Peek et al. systematically reviewed and reported, that surgical treatment of TOS was beneficial in most of the treated patients in the evaluated studies, with a convincing safety profile (30). Dua et al. added the fact of excellent long-term functional results after 1st rib resection in TOS through open approach in 2020 (31). Open approach had evolved over the last years providing different surgical accesses, especially chosen when vascular reconstruction was necessary (mainly supraclavicular or infraclavicular approach) (32).
Compared to open approach, minimally invasive surgery offers certain benefits. In both VATS and RATS, the 1st rib can be visualized immediately when entering the hemithorax. Under controlled conditions with a detailed overview of the surrounding anatomy, the 1st rib can be mobilised and removed with minimal dissection of involved muscles under direct vision (14,15,33). Burt et al. compared their supraclavicular and robotic approach for 1st rib resection in 116 patients and found RATS to be associated with significantly less brachial plexus injuries, lower postoperative visual analogue scale (VAS) scores and less analgesia-requirement (15).
VATS 1st rib resection resulted in short operative time (85 minutes; range, 65 to 90 minutes), LOS of 3 days, low postoperative morbidity and very good outcomes after 6 months in a study involving ten patients done by George et al. in 2017 (17). They highlight in addition, that VATS is an easy to teach procedure for trainees.
In comparison to VATS, RATS facilitates an improved manoeuvrability and 3D-visualization in anatomically confined spaces and removal of the 1st rib without retraction of sensible neurovascular structures while improving ergonomics for surgeons at the console as well (18,33). With a second robotic console, teaching is possible in a similar way to VATS.
RATS however, unless done uniportally, adds more ports than VATS (which has been done with two and three ports), hence results in greater short-term perioperative morbidity, longer hospitalization and operative times as well as greater cost than VATS. While some argue against RATS considering the perceived economic disadvantage, RATS has just recently shown to be of a similar cost to VATS, if not potentially cost-advantageous in comparison with VATS in studies for major lung resection (34,35).
Gharagozloo et al. 2021 published their excellent RATS 1st rib resection results with 100% patency of the SV in VTOS and >90% immediate relief in NTOS without any morbidity or mortality as did Zehnder et al. and our group in 2021 and 2023 respectively (9,10,22). Pupovac and colleagues described their experience with robotic 1st rib resection in 17 patients with short operative time of 113.2±55.3 minutes and mean LOS of 1.8±1.9 days with excellent postoperative outcomes in NTOS and VTOS (33).
As for VATS, where we are still trying to finally find the “most beneficial” number in incisions to achieve the lowest postoperative morbidity, clarifying prospective studies for RATS are lacking (36). Retrospective studies in VATS have shown a trend towards lower morphine-equivalent requirement and better postoperative VAS scores with less minimally invasive incisions; however, there is still no convincing evidence published. In comparison to the excellent work on RATS 1st rib resections as demonstrated by Burt et al., we managed to at least reduce the incisions to three rather than four in our series with the intention to reduce postoperative pain (37). The expected advantage of uniportal RATS (uRATS) 1st rib resection is less tissue damage through a single intercostal access resulting in lower postoperative morbidity. However, we are not certain, whether the increased forces due to leverage effects on the intercostal structures in uRATS will not have a detrimental effect on post-procedural pain. Clearly, more evidence is warranted on uRATS for 1st rib resection (38).
Conclusions
Robotic 1st rib resection is a safe and effective surgical technique for the treatment of TOS. RATS hereby provides an attractive alternative to VATS enhancing the 3D-visualization, manoeuvrability, and reduction of retraction of important neurovascular structures.
Acknowledgments
We thank Louise Croft for kindly reviewing our manuscript for English grammar and language.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-702/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-702/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-702/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from all the patients for publication of this article, the accompanying images, and the video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sanders RJ, Hammond SL, Rao NM. Diagnosis of thoracic outlet syndrome. J Vasc Surg 2007;46:601-4. [Crossref] [PubMed]
- Freischlag J, Orion K. Understanding thoracic outlet syndrome. Scientifica (Cairo) 2014;2014:248163. [Crossref] [PubMed]
- Hemp J, McGriff E, Kern J, et al. Thoracic Outlet Syndrome: Review of Surgical Approaches and Radiographic Complications. Appl Radiol 2022;51:18-22. [Crossref]
- Paget's Clinical Lectures and Essays. Br Foreign Med Chir Rev 1875;56:298-307. [PubMed]
- Von Schrötter L. Erkrankungen der Gefäße. In: Spezielle Pathologie und Therapie, Band XV, II. Theil, II. Hälfte: Erkrankungen der Venen, ed. by Nothnagel H. Vienna: Hölder; 1899:533-5.
- Lee JT, Karwowski JK, Harris EJ, et al. Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg 2006;43:1236-43. [Crossref] [PubMed]
- Elixène JB, Sadaghianloo N, Mousnier A, et al. Long-term functional outcomes and subclavian vein patency in patients undergoing thoracic outlet surgery for Paget-Schroetter Syndrome. J Cardiovasc Surg (Torino) 2017;58:451-7. [Crossref] [PubMed]
- Guzzo JL, Chang K, Demos J, et al. Preoperative thrombolysis and venoplasty affords no benefit in patency following first rib resection and scalenectomy for subacute and chronic subclavian vein thrombosis. J Vasc Surg 2010;52:658-62; discussion 662-3. [Crossref] [PubMed]
- Azenha LF, Kocher GJ, Kestenholz PB, et al. Thoracic outlet syndrome: a retrospective analysis of robotic assisted first rib resections. J Robot Surg 2023;17:891-6. [Crossref] [PubMed]
- Zehnder A, Lutz J, Dorn P, et al. Robotic-Assisted Thoracoscopic Resection of the First Rib for Vascular Thoracic Outlet Syndrome: The New Gold Standard of Treatment? J Clin Med 2021;10:3952. [Crossref] [PubMed]
- Gharagozloo F, Meyer M, Tempesta B, et al. Robotic transthoracic first-rib resection for Paget-Schroetter syndrome. Eur J Cardiothorac Surg 2019;55:434-9. [Crossref] [PubMed]
- Cook JR, Thompson RW. Evaluation and Management of Venous Thoracic Outlet Syndrome. Thorac Surg Clin 2021;31:27-44. [Crossref] [PubMed]
- Soukiasian HJ, Shouhed D, Serna-Gallgos D, et al. A video-assisted thoracoscopic approach to transaxillary first rib resection. Innovations (Phila) 2015;10:21-6. [Crossref] [PubMed]
- Madden N, Calligaro KD, Dougherty MJ, et al. Evolving strategies for the management of venous thoracic outlet syndrome. J Vasc Surg Venous Lymphat Disord 2019;7:839-44. [Crossref] [PubMed]
- Burt BM, Palivela N, Cekmecelioglu D, et al. Safety of robotic first rib resection for thoracic outlet syndrome. J Thorac Cardiovasc Surg 2021;162:1297-1305.e1. [Crossref] [PubMed]
- Samoila G, Twine CP, Williams IM. The infraclavicular approach for Paget-Schroetter syndrome. Ann R Coll Surg Engl 2018;100:83-91. [Crossref] [PubMed]
- George RS, Milton R, Chaudhuri N, et al. Totally Endoscopic (VATS) First Rib Resection for Thoracic Outlet Syndrome. Ann Thorac Surg 2017;103:241-5. [Crossref] [PubMed]
- Gkikas A, Lampridis S, Patrini D, et al. Thoracic Outlet Syndrome: Single Center Experience on Robotic Assisted First Rib Resection and Literature Review. Front Surg 2022;9:848972. [Crossref] [PubMed]
- Chang MC, Kim DH. Essentials of thoracic outlet syndrome: A narrative review. World J Clin Cases 2021;9:5804-11. [Crossref] [PubMed]
- Kuhn JE, Lebus V GF, Bible JE. Thoracic outlet syndrome. J Am Acad Orthop Surg 2015;23:222-32. [Crossref] [PubMed]
- Povlsen S, Povlsen B. Diagnosing Thoracic Outlet Syndrome: Current Approaches and Future Directions. Diagnostics (Basel) 2018;8:21. [Crossref] [PubMed]
- Gharagozloo F, Atiquzzaman N, Meyer M, et al. Robotic first rib resection for thoracic outlet syndrome. J Thorac Dis 2021;13:6141-54. [Crossref] [PubMed]
- Warrens AN, Heaton JM. Thoracic outlet compression syndrome: the lack of reliability of its clinical assessment. Ann R Coll Surg Engl 1987;69:203-4. [PubMed]
- Ferrante MA, Ferrante ND. The thoracic outlet syndromes: Part 1. Overview of the thoracic outlet syndromes and review of true neurogenic thoracic outlet syndrome. Muscle Nerve 2017;55:782-93. [Crossref] [PubMed]
- Burt BM. Thoracic outlet syndrome for thoracic surgeons. J Thorac Cardiovasc Surg 2018;156:1318-1323.e1. [Crossref] [PubMed]
- Mingoli A, Feldhaus RJ, Farina C, et al. Long-term outcome after transaxillary approach for thoracic outlet syndrome. Surgery 1995;118:840-4. [Crossref] [PubMed]
- Lassner F, Becker M, Prescher A. Relevance of Costovertebral Exarticulation of the First Rib in Neurogenic Thoracic Outlet Syndrome: A Retrospective Clinical Study. J Pers Med 2023;13:144. [Crossref] [PubMed]
- Illig KA, Donahue D, Duncan A, et al. Reporting standards of the Society for Vascular Surgery for thoracic outlet syndrome: Executive summary. J Vasc Surg 2016;64:797-802. [Crossref] [PubMed]
- Angle N, Gelabert HA, Farooq MM, et al. Safety and efficacy of early surgical decompression of the thoracic outlet for Paget-Schroetter syndrome. Ann Vasc Surg 2001;15:37-42. [Crossref] [PubMed]
- Peek J, Vos CG, Ünlü Ç, et al. Outcome of Surgical Treatment for Thoracic Outlet Syndrome: Systematic Review and Meta-Analysis. Ann Vasc Surg 2017;40:303-26. [Crossref] [PubMed]
- Dua A, Deslarzes-Dubuis C, Rothenberg KA, et al. Long-term Functional Outcomes Follow-up after 188 Rib Resections in Patients with TOS. Ann Vasc Surg 2020;68:28-33. [Crossref] [PubMed]
- Hempel GK, Shutze WP, Anderson JF, et al. 770 consecutive supraclavicular first rib resections for thoracic outlet syndrome. Ann Vasc Surg 1996;10:456-63. [Crossref] [PubMed]
- Pupovac SS, Lee PC, Zeltsman D, et al. Robotic-Assisted First Rib Resection: Our Experience and Review of the Literature. Semin Thorac Cardiovasc Surg 2020;32:1115-20. [Crossref] [PubMed]
- Coyan GN, Lu M, Ruppert KM, et al. Activity-Based Cost Analysis of Robotic Anatomic Lung Resection During Program Implementation. Ann Thorac Surg 2022;113:244-9. [Crossref] [PubMed]
- Kneuertz PJ, Singer E, D'Souza DM, et al. Hospital cost and clinical effectiveness of robotic-assisted versus video-assisted thoracoscopic and open lobectomy: A propensity score-weighted comparison. J Thorac Cardiovasc Surg 2019;157:2018-2026.e2. [Crossref] [PubMed]
- Perna V, Carvajal AF, Torrecilla JA, et al. Uniportal video-assisted thoracoscopic lobectomy versus other video-assisted thoracoscopic lobectomy techniques: a randomized study. Eur J Cardiothorac Surg 2016;50:411-5. [Crossref] [PubMed]
- Burt BM, Palivela N, Karimian A, et al. Transthoracic robotic first rib resection: Twelve steps. JTCVS Tech 2020;1:104-9. [Crossref] [PubMed]
- Ureña A, Déniz C, Muñoz A, et al. Uniportal robotic-assisted thoracoscopic surgery: resection of the first rib. Ann Cardiothorac Surg 2023;12:62-3. [Crossref] [PubMed]






