Thymic neoplasm: a rare disease with a complex clinical presentation
Introduction
Thymic neoplasms, thymoma and thymic carcinoma, are a rare heterogenous category of lesions with a broad spectrum of pathologic characteristics and clinical presentations. Thymoma is the most common primary anterior mediastinal mass, with an incidence of 1.5 cases per million, and the overall incidence of thymic neoplasms is 0.13 per 100,000 person years (1). Thymic carcinoma is much more rare than thymoma, but much more likely to spread. In fact, the five year survival rate for all stage thymoma is 78%, whereas only 40% for thymic cancer (2-4). Because of the rarity of these lesions, it is important to have a high index of clinical suspicion and to thoroughly consider the differential diagnosis of the anterior mediastinal mass.
Evaluation of the patient with an anterior mediastinal mass
Evaluation of a patient with an anterior mass must include the consideration of both malignant and benign processes. Neoplasms include thymoma, lymphoma, thymic carcinoma, thymic carcinoid, thymolipoma, germ cell tumors and lung metastases (5-7). Non-neoplasms include intrathoracic goiter, thymic cysts, lymphangiomas and aortic aneurysms (5-7). Although the etiology and risk factors for thymic tumors are unknown, previous irradiation and Epstein-Barr virus infections have been thought to play a role (8,9). There have also been reports of Class I and II HLA proteins being highly expressed in thymic epithelial cells and there may be an increased risk among Asians and Pacific Islanders (10), as well as an association with malignant fibrous histiocytoma among the Japanese (1). However, more research is required to determine a genetic predisposition to thymic tumors. Many of these masses are benign, especially in asymptomatic cases. However, most patients with symptoms present with malignant disease. Although most mediastinal malignant tumors are lung cancer metastases, most primary neoplasms in the anterior mediastinum are actually thymomas (11).
Because of the difference in the management of these lesions, it is important to differentiate between thymic malignancies and the other listed possibilities prior to initiating treatment. Lymphomas commonly present with generalized lymphadenopathy, but they can also present as primary anterior mediastinal lesions (6,12). Examples include nodular sclerosing Hodgkin’s disease and non-Hodgkin’s lymphomas, such as diffuse large B-cell lymphoma and acute lymphoblastic lymphoma (6,12). Thymic carcinoids are rare, but they present in association with multiple endocrine neoplasia type 1 syndrome (13,14). Although cases of thymoma usually have an indolent presentation, the presentation for lymphoma and germ cell tumors, for example, is much more rapid in onset (15).
Thymoma patients often present at a younger age (5th-8th decade of life) and better physiologic condition than those with other thoracic malignancies, often thus enabling thymoma patients to undergo more aggressive surgical and medical therapy when indicated (11). Of patients with thymoma, 30% are asymptomatic (16) and 30-50% present with Myasthenia Gravis (MG), but only 10-15% of MG patients have thymoma (16,17). MG is usually diagnosed by history (drooping eyelids, double vision, drooling, difficulty climbing stairs, hoarseness, dyspnea) and/or serum anti-acetylcholine receptor antibody levels, and they will require neurological treatment prior to surgical resection of the tumor (16,17). Even if they do not have clinical signs of MG, because of the perioperative risks associated with untreated MG, it is recommended to screen all these patients for MG prior to pursuing treatment for the thymoma (18). Pure red cell aplasia and hypo-gamma-globulinemia are the most common conditions associated with thymoma after MG, 2-6% of patients (16,19).
Other less commonly associated conditions include chronic ulcerative colitis, regional enteritis, systemic lupus erythematous, sarcoidosis, scleroderma, rheumatoid arthritis, polymyositis, dermatomyositis, pericarditis, Sjogren syndrome, Raynaud’s disease, thyroiditis, T-cell deficiency syndrome, pemphigus, alopecia, chronic candidiasis, Cushing’s syndrome, hypopituitarism, Addison’s disease, hypertrophic osteoarthropathy, macrogenitosomia praecox, nephrosis, minimal change nephropathy, red cell hypoplasia, pernicious anemia, erythrocytosis, agranulocytosis, multiple myeloma, hemolytic anemia, acute leukemia, and T-cell lymphocytosis (16,19). Although extremely rare with only one other case reported in the literature (20), we recently experienced a thymoma case who presented with ANA positive autoimmune hepatitis. Complete biochemical remission of his autoimmune hepatitis with a concomitant weaning of his steroids was achieved with thymectomy.
Local symptoms include pain, cough, hoarseness, and dyspnea (16,19,21). Superior Vena Cava syndrome and weight loss only occur in a small percentage of thymoma patients (16,19,21). It is uncommon for patients with thymoma to have metastatic disease at presentation (4,17), with the pleura being the most frequent site and extra-thoracic disease accounting for <10% of cases (22-24). Thymic carcinoma does present with distant metastasis more frequently than thymoma (6). Thymic tumors can rarely present as primary lesions outside the anterior mediastinum, such as the middle and posterior mediastinum, pleura, neck, and as intra-thyroidal lesions with histological characteristics of thymoma (SETTLE: spindle cell epithelial tumors of thymic-like epithelium) (10). It has been reported that 17-28% of patients with thymoma present with a second synchronous or metachronous malignancy, including lung, thyroid, gastrointestinal, prostate, lymphoma, brain, sarcoma, and leukemia, especially non-Hodgkin’s lymphoma, with an overall risk 1.5-7.1 times greater than population controls (25-27). Therefore, it is important to consider these factors in the evaluation of the patient with a possible thymic neoplasm.
To further evaluate the broad diagnostic spectrum of the anterior mediastinal mass, Chest computed tomography (CT) with intravenous contrast, serum beta-HCG, alpha fetoprotein (AFP), complete blood count, thyroid stimulating hormone (TSH), triiodothyronine (T3) and thyroxine (T4) levels should be obtained (5-7). Beta-HCG and AFP levels can be used to rule out germ cell tumors, and TSH, T3, and T4 levels to rule out mediastinal goiter (5-7). Because so many of these lesions are asymptomatic, they are often incidentally discovered on chest imaging for other diagnostic and screening purposes. For patients who cannot receive iodinated contrast, magnetic resonance imaging of the chest may play a role. Combined PET-CT may play a role for determining whether distant metastases are present, and it provides the benefit of correlation with anatomic structures as opposed to PET scan alone (28).
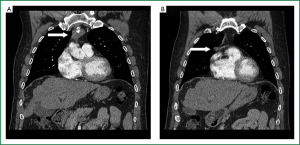
On CT scan, thymoma usually appears as a well-defined round or oval mass located anterior to the great vessels and heart, below the left innominate vein (15). Features suspicious for malignancy include vascular invasion, encasement, and pleural dissemination (15) (Figure 1). It has been reported that smooth contours and round shape suggest type A thymoma; irregular margins and enlarged lymph nodes suggest thymic carcinoma; calcifications suggest B1, B2 and B3 types; and the combination of homogenous enhancement and a high degree of enhancement suggest type A or AB thymoma; however, the false negative and false-positive rates are too high to apply these correlations broadly (29). There have been similar reports between the correlation of MRI characteristics and thymoma subtypes (29). On PET, there have been reports of higher maximum standard uptake value for B2 and B3 compared to A, AB, and B1, but still lower than C; that a value of 6.2 can differentiate thymoma from thymic carcinoma, and >7.1 completely differentiates the two (29,30). Therefore, combining these modalities may provide diagnostic value before considering biopsy (29).
In situations where clinical presentation and imaging cannot establish a diagnosis, where a patient requires induction chemotherapy, or where a metastatic lesion is suspected, tissue diagnosis is required (31-33). However, the biopsy should not violate the pleural space because of the propensity for pleural dissemination in thymic neoplasia (31-33). Although CT-guided needle biopsy is an option, the aspirated sample is often difficult to distinguish by cytology from other subtypes with a sensitivity of less than 60% (31-33). Core cutting biopsy that obtains multiple samples can improve the accuracy of the diagnosis (31-33). Larger samples can be obtained by incisional biopsy (e.g., anterior mediastinotomy or video-assisted thoracoscopy) with a diagnostic sensitivity >90% (31-33).
Staging and classification of thymic neoplasms
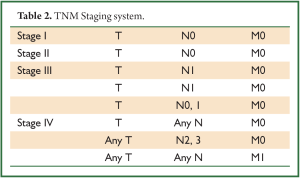
One of the challenges in managing patients with a thymic neoplasm is the controversy of the histology and staging classification systems of these lesions. Part of the reason is that there has been difficulty in correlating histological characteristics with clinical outcome (34). It is generally agreed that thymoma and thymic carcinoma originate from thymic epithelial cells and that the variable amount of lymphocytes present within the tumor is considered reactive (23). Historically, the Benatz classification defined 4 subtypes: lymphocytic, predominantly epithelial, mixed, and spindle cell; however, it provided no significant prognostic information on the course of the disease associated with these subtypes (35). There have also been multiple attempts to establish a TNM classification which correlated with outcome (23,36) (Tables 1,2); however, the system most widely accepted is the one proposed by Masaoka et al. in 1981 and modified by Koga in 1994 (4,37) (Table 3).

Full table
Full table

Full table
The Masaoka-Koga staging system takes into account direct tumor invasion into the capsule and surrounding structures, intra- versus extra-thoracic spread, including lymphatic dissemination, and this system does so while having been validated and correlated with the World Health Organization (WHO) histological classification system (16,38-40) (Tables 1-4). This combination relates thymoma epithelial cells to the differentiation process in the medullary and cortical areas of the normal gland (16,38-40). The WHO system categorizes the morphology of the epithelial cells and the lymphocytic/epithelial ratio into 3 types: A, B, and C (41) (Table 4). In fact, as lesions progress from A to C, there is also a progressive deterioration of the prognosis. However, the problem with this system is that there is a low intra- and inter- observer correlation of actually implementing the WHO classification system, resulting in poor reproducibility, especially among pathologists with limited experience in staging thymic neoplasms (23). Because a meta-analysis has demonstrated significant survival differences between four groupings of these categories (i.e. A+AB+B1 versus B2 versus B3 versus C) (23), Suster and Moran proposed to simplify the WHO system to define lesions as well-differentiated tumors for the traditional thymoma, poorly differentiated tumors for the traditional thymic carcinoma, and atypical thymoma for lesions with intermediate features (42,43).

Full table
Aside from staging and histology, there are other important prognostic factors to consider. First, completeness of resection is extremely important for prognosis, even for Stage III and IV lesions (44). Second, although MG once was associated with worse prognosis, advances in managing MG are such that most patients are diagnosed as stage I and II, and have a better outcome (19,24,45), whereas hypogammaglobulinemia and red blood cell aplasia are associated with a worse prognosis (46). Third, although the presence of lymph node metastasis worsens prognosis, it only occurs in <2% of cases and therefore its applications for staging all patients has thus been limited (46). Fourth, great vessel involvement worsens prognosis and increases the risk of recurrence with some even arguing that stage III be further subdivided based upon this criterion (45,47,48). Fifth, early recurrence has also been reported to be a poor prognostic indicator of overall survival (16).
Multimodality approach to thymic neoplasms: surgery, radiation, and systemic therapy
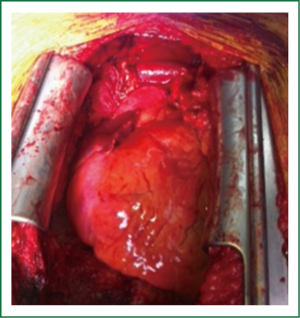
The gold standard for the management of all thymic neoplasms regardless of stage remains complete surgical resection (16). The operation is usually performed through a median sternotomy (Figure 2) and the entire thymus gland with all the surrounding mediastinal fat bordered laterally by the phrenic nerves should be removed (49) (Figure 3). Complete thymectomy is favored even in cases of only partial thymic gland involvement because of some reports of improved survival and multifocal thymoma (39). The oncologic equivalency of thoracoscopic and robotic assisted approaches has been reported, so long as capsule integrity has been maintained and tumor seeding has been prevented (49-52). However, it should be noted that 40% invade surrounding structures which may limit the ability to achieve R0 margins (16). Operative morbidity and mortality are generally reported as 20% and 2% respectively, with the 10-year overall survival and disease free survival rates of 90% and 94% for stage I; 70% and 88% for stage II; 55% and 56% for stage III; and 35% and 33% for stage IV, respectively (16).
Stage I lesions are managed by resection alone, with annual surveillance for recurrence by Chest CT for at least 10 years; however, for Stages II-IV, adjuvant therapy is an important consideration (47,53,54). Stage II lesions with capsular and mediastinal fat involvement have been reported to have recurrence and metastatic dissemination rates as high as 11%, even despite radiotherapy (55). Stage II B2, B3 and C lesions demonstrate a high extra-mediastinal recurrence rate and thus may require systemic therapy (56).
The oncologic principles of managing stage III lesions rest upon en bloc resection as a cornerstone, but only 50% achieve R0 margins due to the structures involved, ranging from 0-89% as determined by surgeon philosophy, judgment, experience and skill (57,58). Even with R0 resection, 10-year survival ranges from 35-53% with a 50% recurrence rate within 5 years, even with adjuvant radiotherapy, and chemotherapy is often also considered (16,59).
Stage IV lesions are also approached surgically in the setting of a multimodality approach. Because initial 5 yr and 10 yr survival rates were 50% and 0%, respectively, with the pleura being the favored host site, some have advocated a more aggressive approach including pleurectomy and extra-pleural pleuropneumonectomy (EPP), systemic or intra-pleural chemotherapy, photodynamic therapy, and irradiation, with some improved results (4,60-62). In fact, because 75% of recurrences are in the form of multiple pleural implants, some advocate not opening the mediastinal pleura and that minimally invasive transpleural approaches be used when possible (19,21). When recurrence occurs, complete resection, such as with EPP, has been reported to produce 72% survival at 5-years (63,64). Patients with a second recurrence are also offered surgery, but in the setting of multimodality therapy (63).
Radiotherapy is an important component of the multimodality approach to thymic neoplasms. For patients with unresectable disease, a dose of 60-70 Gy is recommended, whereas adjuvant radiotherapy doses range from 45-50 Gy for clear or close margins, 54 Gy for R1 margins, and 60 Gy or greater for R2 margins, 1.8 to 2.0 Gy per daily fraction (65-68). To prevent extra-mediastinal recurrence within the thorax, hemi- or entire thorax irradiation in addition to mediastinal irradiation, as opposed to mediastinal irradiation alone, has been advocated after Uematsu et al. reported 5 year relapse-free and survival rates of 100% and 96% versus 74% and 66%, respectively (69). However, this more aggressive approach did produce a 13% rate of symptomatic irradiation pneumonitis (69). It should be noted that care must be taken to adjust the dose when considering adjacent structures such as the heart (limit ≤30 Gy) as these patients as a population are younger and thus will potentially endure more of the long term effects of radiation than, for example, patients with non-small cell lung cancer from whom those dose limits for surrounding normal tissue are derived (65-68). In either case, the clinical target volume for adjuvant radiotherapy should be carefully reviewed with the thoracic surgeon to target the field and any potential sites of residual disease. Neoadjuvant radiation has not been advocated because reports have not demonstrated a survival advantage and because of the concerns of postoperative sternal and respiratory complications (21,58).
Although the role of radiotherapy has not been evaluated in prospective randomized trials, thymic neoplasms are radiosensitive and there are many retrospective reports which guide treatment. Stage I tumors completely resected do not require adjuvant radiotherapy. Because stage IIB tumors have a higher recurrence rate, especially WHO B2, B3 and C, there are reports of decreasing recurrence from 29-36.4% to 0-8% with radiotherapy, but 92% of the recurrences in the irradiated group were pleural dissemination (57,70,71). However, the role of radiation in stage II disease remains controversial and increasing evidence may be suggesting a lack of a benefit (47,53,54,72). For stages III and IVA, there are reports of adjuvant radiation reducing recurrence after complete resection from 50-53% down to 0-20% (73-75). After incomplete resection, studies have reported 5-year recurrence rates of 79% as opposed to 0% mediastinal failure rates at 5 years with adjuvant radiotherapy (21,73).
Patients with unresectable or recurrent thymic neoplasms are considered candidates for systemic chemotherapy; however, because of the rarity of these lesions, prospective trials comparing the different agents in the literature are rare (16,21,76-81). Patients who present with locally advanced disease can be restaged after treatment with chemotherapy to either undergo surgery if they are resectable, or to consider further radiotherapy and chemotherapy if they did not respond adequately (16,21,76-81). Patients with solitary metastasis should be evaluated for metastasectomy if complete resection can be achieved in the context of complete radical thymectomy with multimodality therapy.
First line therapy regimens include cisplatin, doxorubicin and cyclophosphamide (CAP); CAP with prednisone; cisplatin, doxorubicin, vincristine, and cyclophosphamide (ADOC); cisplatin and etoposide (PE); etoposide, ifosfamide, and cisplatin (VIP), or carboplatin and paclitaxel (16,21, 76-81). The last regimen is preferred for thymic cancer because it has demonstrated the highest response rate (82,83). Despite having 6 options for thymoma, the best outcomes have been reported using cisplatin/doxorubicin-based regimens, and many recommend CAP (53,84). However, PE, VIP, and carboplatin/paclitaxel may provide better options for those who cannot tolerate the more aggressive regimens (83). Second line therapy usually includes etoposide, ifosfamide, pemetrexed, octreotide, prednisone, 5-fluorouracil and leucovorin, gemcitabine, and paclitaxel (16,21,76-81); however, it should be noted that strong data are lacking regarding these regimens for thymic cancer (76).
Because of the importance of complete resection for prognosis, these agents have been employed in the setting of induction chemotherapy to convert unresectable tumors to resectable, but the exact selection criteria for induction chemotherapy have yet to be determined. Response rates have been reported as high as a 20% for complete pathologic response in the final specimen and 43% for radiological response and it is well tolerated in MG, with reports of producing MG remission in same cases (85). However, it should be noted that these studies are limited by small sample size. Thymic cancer is less responsive to systemic chemotherapy and its inclusion in some of the studies may have contributed to decreased survival (86-88). There is some evidence that steroids and octreotide may have a role in treatment as well; however, these agents have not been fully evaluated and are still under investigation (70,89).
In the era of targeted therapy, there have been investigations into specific targets in thymic neoplasms which may improve response to therapy. The presence of epidermal growth factor receptor (EGFR) mutations in thymic tumors is rare and is thought to explain why there have been poor responses reported to EGFR inhibitors (90). However, with the identification of HRAS/KRAS mutations that predict anti-EGFR drug resistance, there is a potential to determine which patients may respond to such agents (96-126). There is also a small subset of kit mutation containing thymic neoplasms which may respond to tyrosine kinase inhibitors (91,127-139). Similarly, insulin-like growth factor-1R expression in thymic tumors has led to ongoing Phase II trials to determine the efficacy of antibodies to this receptor (140).
There is also promise in the utilization of multi-kinase inhibitors, including those that target the vascular endothelial growth factor receptors (141). Because of the third and fourth pharyngeal pouch embryonic endodermal derivation of the thymus, it has been hypothesized that there may be oncogenic and therapeutic relationships to other pharyngeal neoplasia, and therefore, experimental studies targeting the COX-2 system which demonstrated promise on hypopharyngeal tumor cell lines may show future promise in thymic neoplasms (138,142). However, other markers which are over-expressed in thymic neoplasms that predict sensitivity to 5-fluoruracil-based agents, namely thymidine synthase and dihydropyrimidine dehydrogenase, failed to produce encouraging results in clinical studies (143). Therefore, it is important for a multidisciplinary approach to the study and treatment of this complex disease which includes basic scientists, oncologists, radiotherapists, pathologists and thoracic surgeons.
Conclusions
In summary, thymic neoplasms are a rare group of heterogenous lesions of the anterior mediastinum with a broad range of presentations and clinical courses, which require a high index of suspicion to appropriately diagnose and treat. Although diagnosis can often be made by clinical presentation and imaging alone, biopsy may be required to make the diagnosis or prior to the initiation of multimodality therapy. Surgical complete radical resection remains the gold standard of therapy, but it is important to consider it in the context of multimodality therapy, especially for stage II-IV disease. While thymic neoplasms are radiosensitive, these lesions do not respond as well to chemotherapy, and yet systemic therapy still does play an important role. Although there is no magic bullet in the treatment of systemic disease, as research continues there are some interesting possibilities for the future management of thymic neoplasms, especially in the era of targeted therapy.
Acknowledgements
Kazuaki Takabe is supported by United States National Institute of Health (R01CA160688) and Susan G. Komen for the Cure (Investigator Initiated Research Grant (12222224).
We would like to acknowledge the support of Dr. Junqi Qian and Dr. John Dalton of our Department of Pathology, and Dr. Claire Rezba of our Department of Anesthesiology.
Disclosure: The authors declare no conflict of interest.
References
- Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010;5:S260-5
- Proceedings of the First International Conference on Thymic Malignancies. August 20-21, 2009. Bethesda, Maryland, USA. J Thorac Oncol 2010;5:S259-370.
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84.
- Masaoka A, Yamakawa Y, Niwa H, et al. Thymectomy and malignancy. Eur J Cardiothorac Surg 1994;8:251-3.
- Marchevsky A, Marx A, Strobel P, et al. Policies and reporting guidelines for small biopsy specimens of mediastinal masses. J Thorac Oncol 2011;6:S1724-9.
- Strollo DC, Rosado de Christenson ML, Jett JR. Primary mediastinal tumors. Part 1: tumors of the anterior mediastinum. Chest 1997;112:511-22.
- Strollo DC, Rosado-de-Christenson ML, Jett JR. Primary mediastinal tumors: part II. Tumors of the middle and posterior mediastinum. Chest 1997;112:1344-57.
- Patton DF, Ribeiro RC, Jenkins JJ, et al. Thymic carcinoma with a defective Epstein-Barr virus encoding the BZLF1 trans-activator. J Infect Dis 1994;170:7-12.
- Patton DF, Shirley P, Raab-Traub N, et al. Defective viral DNA in Epstein-Barr virus-associated oral hairy leukoplakia. J Virol 1990;64:397-400.
- Verley JM, Hollmann KH. Thymoma. A comparative study of clinical stages, histologic features, and survival in 200 cases. Cancer 1985;55:1074-86.
- Detterbeck FC, Parsons AM. Management of stage I and II thymoma. Thorac Surg Clin 2011;21:59-67.
- Barth TF, Leithauser F, Joos S, et al. Mediastinal (thymic) large B-cell lymphoma: where do we stand? Lancet Oncol 2002;3:229-34.
- Ferolla P, Falchetti A, Filosso P, et al. Thymic neuroendocrine carcinoma (carcinoid) in multiple endocrine neoplasia type 1 syndrome: the Italian series. J Clin Endocrinol Metab 2005;90:2603-9.
- Teh BT. Thymic carcinoids in multiple endocrine neoplasia type 1. J Intern Med 1998;243:501-4.
- Marom EM. Imaging thymoma. J Thorac Oncol 2010;5:S296-303.
- Venuta F, Rendina EA, Longo F, et al. Long-term outcome after multimodality treatment for stage III thymic tumors. Ann Thorac Surg 2003;76:1866-72.
- Lewis JE, Wick MR, Scheithauer BW, et al. Thymoma. A clinicopathologic review. Cancer 1987;60:2727-43.
- Detterbeck FC, Moran C, Huang J, et al. Which way is up? Policies and procedures for surgeons and pathologists regarding resection specimens of thymic malignancy. J Thorac Oncol 2011;6:S1730-8.
- Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg 1996;112:376-84.
- Aigner E, Strassburg CP, Strasser M, et al. Transient autoimmune hepatitis induced by a thymoma. Am J Gastroenterol 2009;104:1332-4.
- Maggi G, Casadio C, Ardissone F, et al. Thymoma: results of 241 operated cases. Acta Otorhinolaryngol Ital 1990; 10:109-17.
- Chan JK, Rosai J. Tumors of the neck showing thymic or related branchial pouch differentiation: a unifying concept. Hum Pathol 1991;22:349-67.
- Pescarmona E, Rendina EA, Venuta F, et al. The prognostic implication of thymoma histologic subtyping. A study of 80 consecutive cases. Am J Clin Pathol 1990;93:190-5.
- Salyer WR, Eggleston JC. Thymoma: a clinical and pathological study of 65 cases. Cancer 1976;37:229-49.
- Pan CC, Chen PC, Wang LS, et al. Thymoma is associated with an increased risk of second malignancy. Cancer 2001;92:2406-11.
- Patella M, Anile M, Vitolo D, et al. Synchronous B3 thymoma and lung bronchoalveolar carcinoma. Interact Cardiovasc Thorac Surg 2011;12:75-6.
- Welsh JS. Thymoma and subsequent cancers. Int J Cancer 2004;108:327.
- Sung YM, Lee KS, Kim BT, et al. 18F-FDG PET/CT of thymic epithelial tumors: usefulness for distinguishing and staging tumor subgroups. J Nucl Med 2006;47:1628-34.
- Yanagawa M, Tomiyama N. Prediction of thymoma histology and stage by radiographic criteria. Thorac Surg Clin 2011;21:1-12.
- Demura Y, Tsuchida T, Ishizaki T, et al. 18F-FDG accumulation with PET for differentiation between benign and malignant lesions in the thorax. J Nucl Med 2003;44:540-8.
- Mehran R, Ghosh R, Maziak D, et al. Surgical treatment of thymoma. Can J Surg 2002;45:25-30.
- Murakawa T, Nakajima J, Kohno T, et al. Results from surgical treatment for thymoma. 43 years of experience. Jpn J Thorac Cardiovasc Surg 2000;48:89-95.
- Rendina EA, Venuta F, Martelli M, et al. Successful resection of a thymoma in an elderly patient. Ital J Surg Sci 1988;18:79-82.
- Yamakawa Y, Masaoka A, Hashimoto T, et al. A tentative tumor-node-metastasis classification of thymoma. Cancer 1991;68:1984-7.
- Bernatz PE, Harrison EG, Clagett OT. Thymoma: a clinicopathologic study. J Thorac Cardiovasc Surg 1961;42:424-44.
- Ricci C, Rendina EA, Pescarmona EO, et al. Correlations between histological type, clinical behaviour, and prognosis in thymoma. Thorax 1989;44:455-60.
- Tsuchiya R, Koga K, Matsuno Y, et al. Thymic carcinoma: proposal for pathological TNM and staging. Pathol Int 1994;44:505-12.
- Nakagawa K, Asamura H, Matsuno Y, et al. Thymoma: a clinicopathologic study based on the new World Health Organization classification. J Thorac Cardiovasc Surg 2003;126:1134-40.
- Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer 2002;94:624-32.
- Okumura M, Shiono H, Inoue M, et al. Outcome of surgical treatment for recurrent thymic epithelial tumors with reference to world health organization histologic classification system. J Surg Oncol 2007;95:40-4.
- Rosai J, Sobin L. Histological typing of tumors of the thymus. In: Rosai J, Sobin L. eds. Word Health Organization, international classifcation of tumors. Berlin: Springer, 1999:9-14.
- Moran CA, Suster S. Thymic carcinoma: current concepts and histologic features. Hematol Oncol Clin North Am 2008;22:393-407.
- Moran CA, Suster S. The World Health Organization (WHO) histologic classification of thymomas: a reanalysis. Curr Treat Options Oncol 2008;9:288-99.
- Jackson MA, Ball DL. Post-operative radiotherapy in invasive thymoma. Radiother Oncol 1991;21:77-82.
- Okumura M, Miyoshi S, Takeuchi Y, et al. Results of surgical treatment of thymomas with special reference to the involved organs. J Thorac Cardiovasc Surg 1999;117:605-13.
- Venuta F, Anile M, Diso D, et al. Thymoma and thymic carcinoma. Eur J Cardiothorac Surg 2010;37:13-25.
- Utsumi T, Shiono H, Kadota Y, et al. Postoperative radiation therapy after complete resection of thymoma has little impact on survival. Cancer 2009;115:5413-20.
- Wilkins KB, Sheikh E, Green R, et al. Clinical and pathologic predictors of survival in patients with thymoma. Ann Surg 1999;230:562-72; discussion 572-4.
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701.
- Komanapalli CB, Cohen JI, Sukumar MS. Extended transcervical video-assisted thymectomy. Thorac Surg Clin 2010;20:235-43.
- Limmer KK, Kernstine KH. Minimally invasive and robotic-assisted thymus resection. Thorac Surg Clin 2011;21:69-83.
- Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-42.
- Kondo K. Optimal therapy for thymoma. J Med Invest 2008;55:17-28.
- Korst RJ, Kansler AL, Christos PJ, et al. Adjuvant radiotherapy for thymic epithelial tumors: a systematic review and meta-analysis. Ann Thorac Surg 2009;87:1641-7.
- Chen G, Marx A, Chen WH, et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors: a clinicopathologic study of 200 thymoma cases from China. Cancer 2002;95:420-9.
- Venuta F, Anile M, Rendina EA, et al. The value of transcapsular invasion in patients with thymoma. Arch Pathol Lab Med 2009;133:1364-5.
- Haniuda M, Miyazawa M, Yoshida K, et al. Is postoperative radiotherapy for thymoma effective? Ann Surg 1996;224:219-24.
- Urgesi A, Monetti U, Rossi G, et al. Aggressive treatment of intrathoracic recurrences of thymoma. Radiother Oncol 1992;24:221-5.
- Venuta F, Rendina EA, Pescarmona EO, et al, et al. Multimodality treatment of thymoma: a prospective study. Ann Thorac Surg 1997;64:1585-91.
- Huang J, Rizk NP, Travis WD, et al. Feasibility of multimodality therapy including extended resections in stage IVA thymoma. J Thorac Cardiovasc Surg 2007;134:1477-83.
- Lucchi M, Davini F, Ricciardi R, et al. Management of pleural recurrence after curative resection of thymoma. J Thorac Cardiovasc Surg 2009;137:1185-9.
- Wright CD. Pleuropneumonectomy for the treatment of Masaoka stage IVA thymoma. Ann Thorac Surg 2006;82:1234-9.
- Regnard JF, Zinzindohoue F, Magdeleinat P, et al. Results of re-resection for recurrent thymomas. Ann Thorac Surg 1997;64:1593-8.
- Ruffini E, Mancuso M, Oliaro A, et al. Recurrence of thymoma: analysis of clinicopathologic features, treatment, and outcome. J Thorac Cardiovasc Surg 1997;113:55-63.
- Gomez D, Komaki R. Technical advances of radiation therapy for thymic malignancies. J Thorac Oncol 2010;5:S336-43.
- Gomez D, Komaki R, Yu J, et al. Radiation therapy definitions and reporting guidelines for thymic malignancies. J Thorac Oncol 2011;6:S1743-8.
- Mornex F, Resbeut M, Richaud P, et al. Radiotherapy and chemotherapy for invasive thymomas: a multicentric retrospective review of 90 cases. The FNCLCC trialists. Federation Nationale des Centres de Lutte Contre le Cancer. Int J Radiat Oncol Biol Phys 1995;32:651-9.
- Myojin M, Choi NC, Wright CD, et al. Stage III thymoma: pattern of failure after surgery and postoperative radiotherapy and its implication for future study. Int J Radiat Oncol Biol Phys 2000;46:927-33.
- Uematsu M, Yoshida H, Kondo M, et al. Entire hemithorax irradiation following complete resection in patients with stage II-III invasive thymoma. Int J Radiat Oncol Biol Phys 1996;35:357-60.
- Haniuda M, Kondo R, Numanami H, et al. Recurrence of thymoma: clinicopathological features, re-operation, and outcome. J Surg Oncol 2001;78:183-8.
- Haniuda M, Morimoto M, Nishimura H, et al. Adjuvant radiotherapy after complete resection of thymoma. Ann Thorac Surg 1992;54:311-5.
- Rena O, Papalia E, Oliaro A, et al. Does adjuvant radiation therapy improve disease-free survival in completely resected Masaoka stage II thymoma? Eur J Cardiothorac Surg 2007;31:109-13.
- Curran WJ Jr, Kornstein MJ, Brooks JJ, et al. Invasive thymoma: the role of mediastinal irradiation following complete or incomplete surgical resection. J Clin Oncol 1988;6:1722-7.
- Monden Y, Nakahara K, Iioka S, et al. Recurrence of thymoma: clinicopathological features, therapy, and prognosis. Ann Thorac Surg 1985;39:165-9.
- Urgesi A, Monetti U, Rossi G, et al. Role of radiation therapy in locally advanced thymoma. Radiother Oncol 1990;19:273-80.
- Girard N. Chemotherapy and targeted agents for thymic malignancies. Expert Rev Anticancer Ther 2012;12:685-95.
- Girard N, Mornex F. The role of radiotherapy in the management of thymic tumors. Thorac Surg Clin 2011;21:99-105.
- Kim ES, Putnam JB, Komaki R, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer 2004;44:369-79.
- Loehrer PJ Sr, Wick MR. Thymic malignancies. Cancer Treat Res 2001;105:277-302.
- Lucchi M, Mussi A, Basolo F, et al. The multimodality treatment of thymic carcinoma. Eur J Cardiothorac Surg 2001;19:566-9.
- Yokoi K, Matsuguma H, Nakahara R, et al. Multidisciplinary treatment for advanced invasive thymoma with cisplatin, doxorubicin, and methylprednisolone. J Thorac Oncol 2007;2:73-8.
- Furugen M, Sekine I, Tsuta K, et al. Combination chemotherapy with carboplatin and paclitaxel for advanced thymic cancer. Jpn J Clin Oncol 2011;41:1013-6.
- Lemma GL, Lee JW, Aisner SC, et al. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol 2011;29:2060-5.
- Rajan A, Giaccone G. Treatment of advanced thymoma and thymic carcinoma. Curr Treat Options Oncol 2008;9:277-87.
- Kosmidis PA, Iliopoulos E, Pentea S. Combination chemotherapy with cyclophosphamide, adriamycin, and vincristine in malignant thymoma and myasthenia gravis. Cancer 1988;61:1736-40.
- Yano M, Sasaki H, Moriyama S, et al. Number of recurrent lesions is a prognostic factor in recurrent thymoma. Interact Cardiovasc Thorac Surg 2011;13:21-4.
- Yano M, Sasaki H, Yokoyama T, et al. Thymic carcinoma with dissemination: a retrospective analysis of ten patients. Gen Thorac Cardiovasc Surg 2008;56:335-9.
- Yano M, Sasaki H, Yukiue H, et al. Thymoma with dissemination: efficacy of macroscopic total resection of disseminated nodules. World J Surg 2009;33:1425-31.
- Ferone D, van Hagen MP, Kwekkeboom DJ, et al. Somatostatin receptor subtypes in human thymoma and inhibition of cell proliferation by octreotide in vitro. J Clin Endocrinol Metab 2000;85:1719-26.
- Kurup A, Loehrer PJ Sr. Thymoma and thymic carcinoma: therapeutic approaches. Clin Lung Cancer 2004;6:28-32.
- Girard N, Shen R, Guo T, et al. Comprehensive genomic analysis reveals clinically relevant molecular distinctions between thymic carcinomas and thymomas. Clin Cancer Res 2009;15:6790-9.
- Azzoli CG, Park BJ, Pao W, et al. Molecularly tailored adjuvant chemotherapy for resected non-small cell lung cancer: a time for excitement and equipoise. J Thorac Oncol 2008;3:84-93.
- Chitale D, Gong Y, Taylor BS, et al. An integrated genomic analysis of lung cancer reveals loss of DUSP4 in EGFR-mutant tumors. Oncogene 2009;28:2773-83.
- Gao B, Sun Y, Zhang J, et al. Spectrum of LKB1, EGFR, and KRAS mutations in chinese lung adenocarcinomas. J Thorac Oncol 2010;5:1130-5.
- Girard N, Deshpande C, Azzoli CG, et al. Use of epidermal growth factor receptor/Kirsten rat sarcoma 2 viral oncogene homolog mutation testing to define clonal relationships among multiple lung adenocarcinomas: comparison with clinical guidelines. Chest 2010;137:46-52.
- Johnson ML, Sima CS, Chaft J, et al. Association of KRAS and EGFR mutations with survival in patients with advanced lung adenocarcinomas. Cancer 2013;119:356-62.
- Kawauchi K, Lazarus AH, Rapoport MJ, et al. Tyrosine kinase and CD45 tyrosine phosphatase activity mediate p21ras activation in B cells stimulated through the antigen receptor. J Immunol 1994;152:3306-16.
- Li C, Fang R, Sun Y, et al. Spectrum of oncogenic driver mutations in lung adenocarcinomas from East Asian never smokers. PLoS One 2011;6:e28204.
- Li C, Sun Y, Fang R, et al. Lung adenocarcinomas with HER2-activating mutations are associated with distinct clinical features and HER2/EGFR copy number gains. J Thorac Oncol 2012;7:85-9.
- Lovly CM, Dahlman KB, Fohn LE, et al. Routine multiplex mutational profiling of melanomas enables enrollment in genotype-driven therapeutic trials. PLoS One 2012;7:e35309.
- Lyustikman Y, Momota H, Pao W, et al. Constitutive activation of Raf-1 induces glioma formation in mice. Neoplasia 2008;10:501-10.
- Ma BB, Lui VW, Poon FF, et al. Preclinical activity of gefitinib in non-keratinizing nasopharyngeal carcinoma cell lines and biomarkers of response. Invest New Drugs 2010;28:326-33.
- Marks JL, Broderick S, Zhou Q, et al. Prognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinoma. J Thorac Oncol 2008;3:111-6.
- Marks JL, Gong Y, Chitale D, et al. Novel MEK1 mutation identified by mutational analysis of epidermal growth factor receptor signaling pathway genes in lung adenocarcinoma. Cancer Res 2008;68:5524-8.
- Marks JL, McLellan MD, Zakowski MF, et al. Mutational analysis of EGFR and related signaling pathway genes in lung adenocarcinomas identifies a novel somatic kinase domain mutation in FGFR4. PLoS One 2007;2:e426.
- Medema RH, Burgering BM, Bos JL. Insulin-induced p21ras activation does not require protein kinase C, but a protein sensitive to phenylarsine oxide. J Biol Chem 1991;266:21186-9.
- Miller VA, Riely GJ, Zakowski MF, et al. Molecular characteristics of bronchioloalveolar carcinoma and adenocarcinoma, bronchioloalveolar carcinoma subtype, predict response to erlotinib. J Clin Oncol 2008;26:1472-8.
- Oeste CL, Diez-Dacal B, Bray F, et al. The C-terminus of H-Ras as a target for the covalent binding of reactive compounds modulating Ras-dependent pathways. PLoS One 2011;6:e15866.
- Ohashi K, Sequist LV, Arcila ME, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci U S A 2012;109:E2127-33.
- Pao W. Defining clinically relevant molecular subsets of lung cancer. Cancer Chemother Pharmacol 2006;58:S11-5.
- Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol 2011;12:175-80.
- Pao W, Kris MG, Iafrate AJ, et al. Integration of molecular profiling into the lung cancer clinic. Clin Cancer Res 2009;15:5317-22.
- Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73.
- Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med 2005;2:e17.
- Pratilas CA, Hanrahan AJ, Halilovic E, et al. Genetic predictors of MEK dependence in non-small cell lung cancer. Cancer Res 2008;68:9375-83.
- Price KA, Azzoli CG, Krug LM, et al. Phase II trial of gefitinib and everolimus in advanced non-small cell lung cancer. J Thorac Oncol 2010;5:1623-9.
- Redente EF, Dwyer-Nield LD, Merrick DT, et al. Tumor progression stage and anatomical site regulate tumor-associated macrophage and bone marrow-derived monocyte polarization. Am J Pathol 2010;176:2972-85.
- Riely GJ, Johnson ML, Medina C, et al. A phase II trial of Salirasib in patients with lung adenocarcinomas with KRAS mutations. J Thorac Oncol 2011;6:1435-7.
- Riely GJ, Kris MG, Rosenbaum D, et al. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin Cancer Res 2008;14:5731-4.
- Riely GJ, Marks J, Pao W. KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc 2009;6:201-5.
- Rizvi NA, Rusch V, Pao W, et al. Molecular characteristics predict clinical outcomes: prospective trial correlating response to the EGFR tyrosine kinase inhibitor gefitinib with the presence of sensitizing mutations in the tyrosine binding domain of the EGFR gene. Clin Cancer Res 2011;17:3500-6.
- Solomon SB, Zakowski MF, Pao W, et al. Core needle lung biopsy specimens: adequacy for EGFR and KRAS mutational analysis. AJR Am J Roentgenol 2010;194:266-9.
- Su Z, Dias-Santagata D, Duke M, et al. A platform for rapid detection of multiple oncogenic mutations with relevance to targeted therapy in non-small-cell lung cancer. J Mol Diagn 2011;13:74-84.
- Sun Y, Ren Y, Fang Z, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol 2010;28:4616-20.
- Wang R, Hu H, Pan Y, et al. RET Fusions Define a Unique Molecular and Clinicopathologic Subtype of Non-Small-Cell Lung Cancer. J Clin Oncol 2012;30:4352-9.
- Zhang Y, Sun Y, Pan Y, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res 2012;18:1947-53.
- Buti S, Donini M, Sergio P, et al. Impressive response with imatinib in a heavily pretreated patient with metastatic c-KIT mutated thymic carcinoma. J Clin Oncol 2011;29:e803-5.
- Dirnhofer S, Zimpfer A, Went P. The diagnostic and predictive role of kit (CD117). Ther Umsch 2006;63:273-8.
- Giaccone G, Rajan A, Ruijter R, et al. Imatinib mesylate in patients with WHO B3 thymomas and thymic carcinomas. J Thorac Oncol 2009;4:1270-3.
- Girard N. Thymic tumors: relevant molecular data in the clinic. J Thorac Oncol 2010;5:S291-5.
- Hamada S, Masago K, Mio T, et al. Good clinical response to imatinib mesylate in atypical thymic carcinoid With KIT overexpression. J Clin Oncol 2011;29:e9-10.
- Iyoda M, Hudkins KL, Becker-Herman S, et al. Imatinib suppresses cryoglobulinemia and secondary membranoproliferative glomerulonephritis. J Am Soc Nephrol 2009;20:68-77.
- Massa S, Balciunaite G, Ceredig R, et al. Critical role for c-kit (CD117) in T cell lineage commitment and early thymocyte development in vitro. Eur J Immunol 2006;36:526-32.
- Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol 2005;13:205-20.
- Palmieri G, Marino M, Buonerba C, et al. Imatinib mesylate in thymic epithelial malignancies. Cancer Chemother Pharmacol 2012;69:309-15.
- Petrini I, Zucali PA, Lee HS, et al. Expression and mutational status of c-kit in thymic epithelial tumors. J Thorac Oncol 2010;5:1447-53.
- Schirosi L, Nannini N, Nicoli D, et al. Activating c-KIT mutations in a subset of thymic carcinoma and response to different c-KIT inhibitors. Ann Oncol 2012;23:2409-14.
- Ströbel P, Hartmann M, Jakob A, et al. Thymic carcinoma with overexpression of mutated KIT and the response to imatinib. N Engl J Med 2004;350:2625-6.
- Tsuchida M, Umezu H, Hashimoto T, et al. Absence of gene mutations in KIT-positive thymic epithelial tumors. Lung Cancer 2008;62:321-5.
- Haluska P, Shaw HM, Batzel GN, et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res 2007;13:5834-40.
- Azad A, Herbertson RA, Pook D, et al. Motesanib diphosphate (AMG 706), an oral angiogenesis inhibitor, demonstrates clinical efficacy in advanced thymoma. Acta Oncol 2009;48:619-21.
- Rieker RJ, Joos S, Mechtersheimer G, et al. COX-2 upregulation in thymomas and thymic carcinomas. Int J Cancer 2006;119:2063-70.
- Sasaki H, Fukai I, Kiriyama M, et al. Thymidylate synthase and dihydropyrimidine dehydrogenase mRNA levels in thymoma. Surg Today 2003;33:83-8.