The feasibility of molecular testing on cell blocks created from brush tip washings in the assessment of peripheral lung lesions
Introduction
Lung cancer remains the single largest contributor to cancer-related deaths worldwide. Most patients are diagnosed with advanced disease and are not surgical candidates. Indeed, 57% of lung cancer is metastatic at the time of diagnosis in the United States, and for these patients systemic chemotherapy is the primary mode of management.
Radial endobronchial ultrasound (EBUS) is commonly performed for diagnosis of suspected peripheral lung cancer. Multiple sampling modalities may be utilized, such as forceps biopsy [transbronchial lung biopsy (TBLB)], transbronchial needle aspiration (TBNA), brushings, and washings, and studies indicate diagnostic yield is maximized where all specimen types are combined (1). Our unit also routinely creates brush tip washings (BTW) during EBUS, which utilizes the cells that remain on the bronchoscope cytology brush following smearing onto cytology slides. This material would otherwise be discarded, and limited reports suggest BTW may contribute to diagnostic utility of radial EBUS (2).
With the rapid development of genotype-guided therapies, molecular testing is increasingly important in the routine work up of lung cancer. Epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) testing is now considered part of the standard investigation of non-squamous non-small cell lung cancer (NSCLC), as both have therapeutic implications (3). Additional genes sometimes tested include Kirsten rat sarcoma viral oncogene homolog (KRAS) B-Raf proto-oncogene (BRAF), ROS proto-oncogene 1 (ROS1), ret proto-oncogene (RET), and HER2 (ERBB2) (4).
Here we show the utility of cell blocks derived from BTW specimens for performing routine molecular analysis.
Methods
Study design
We conducted a prospective observational study at a single tertiary institution in Melbourne, Australia. Patients who had had a bronchoscopy +/− endobronchial ultrasound performed at the Royal Melbourne Hospital were recruited over a 24-month period from January 2014 to January 2016. They were included if they had brushings taken during the procedure, a cell block created from the BTW, and molecular testing requested on that cell block.
Technique
Bronchoscopic investigation of peripheral pulmonary lesions was performed with intravenous sedation and topical lignocaine 2% as previously described (5). Experienced physicians performed the procedure using a standard video-bronchoscope (BF-P160, Olympus, Tokyo, Japan) using the guide sheath technique, with a 20-MHz radial EBUS probe (UM-BS20e26R; Olympus, Tokyo, Japan). After the lesion was located, sampling instruments were passed down the sheath and specimens collected under fluoroscopic vision. Routine brushings were taken and smeared onto two slides, one for rapid on site examination (ROSE) using rapid Romanowsky stain and the other fixed in 95% alcohol for Papanicolau staining, as previously described (6). Once the smears were collected from the brushings, the brush tip was rinsed with 0.9% sodium chloride solution. This process was repeated each time the peripheral lesion was sampled with the bronchoscope cytology brush. The fluid from the BTW was then processed into a formalin fixed paraffin embedded cell block. The BTW was concentrated into a cell sediment, this was then re-suspended in 10% formalin for at least half an hour. The suspension was then spun down into a cell pellet and a small amount of agar added. This was then processed using routine histological methods into a paraffin embedded cell block.
Molecular testing was performed in a National Association of Testing Authorities Australia (NATA) accredited laboratory. A single H&E slide from the cell block was examined by a pathologist to determine tumour cell content. If there were no identifiable tumour cells in the H&E slide prepared from the cell block, the sample was deemed inadequate and did not undergo molecular testing. Any cell block with identifiable tumour cells on a H&E stained section underwent molecular testing. A pathologist identified the area of maximum tumour cells in the H&E slide, and this area was macrodissected from four 10 μ unstained sections from the cell block, followed by DNA extraction using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). Targeted sequences of BRAF exon 15, KRAS exons 2, 3 and 4, and EGFR exons 18, 19, 20, and 21 were amplified by polymerase chain reaction, and sequenced on a MiSeq sequencer (Illumina, San Diego, USA).
Results
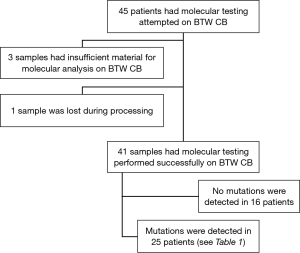
From January 2014 to January 2016 we identified 45 patients in whom molecular testing was requested on cell block specimens created from BTW (BTW-CB). The outcomes have been summarized in Figure 1. All except one case had a final diagnosis of adenocarcinoma or NSCLC not otherwise specified (NOS). Forty-nine percent of the population was female, with a mean age of 69 (range, 47 to 88) years.
Forty-one of 45 (91%) specimens were adequate for molecular analysis. For 3 patients (7%) there was inadequate tumour cells in the H&E slide prepared from the cell block for molecular testing, although interestingly all three demonstrated diagnostic malignant cells on routine pathologic evaluation of BTW smears. One sample was lost during cell-block processing, and therefore could not be tested.
The results of molecular testing from the 41 patients with tumour cells present in the BTW derived cell block are reported in Table 1. Of these, BTW-CB was the sole diagnostic specimen available for testing in 60%.

Full table
A total of 35 of the 41 patients that underwent molecular testing had adenocarcinoma, in which the frequency of detecting a mutation in any of the three assayed genes was 66%. The EGFR mutations and the BRAF mutation were detected in women, and 63% of the KRAS mutations were found in men.
Discussion
This study showed that molecular testing can be successfully performed on cell blocks created from BTW. Ninety-one percent of the cell blocks created in this cohort had tumour cells present and were considered suitable for molecular testing. Importantly, the frequency of mutations detected in the three assayed genes in adenocarcinomas (66%) was similar to that previously reported (48%) by standard detection methods (7), suggesting that false wild type results due to inadequate tumour cells were not common in our series. There were inadequate tumour cells in 7% (3) of the cell blocks, slightly lower than the 11.3% of “inadequate” samples reported in the 2014 study looking at molecular testing on cell blocks created from fine needle aspirate (8).
The ability to perform molecular analysis of BTW is an important advance in assessment of NSCLC patients. Multiple studies, including the multicenter randomized phase 3 EURTAC trial, have demonstrated that patients with NSCLC with certain activating EGFR mutations benefit from EGFR tyrosine kinase inhibitors as first line chemotherapy over standard chemotherapy (9). Adenocarcinomas with ALK rearrangements have been shown to shrink in size or remain stable in the majority of patients treated with ALK tyrosine kinase inhibitors (10).
Currently there are few bronchoscopic specimen types where molecular testing is established. This includes formalin-fixed transbronchial lung biopsy tissue, which can be limited by variable diagnostic sensitivity, which may be as low as 45% (11). Thus despite undergoing a diagnostic procedure, a significant proportion of patients may not have specimens amenable to molecular testing. Multiple studies have confirmed maximal diagnostic yield of radial EBUS is achieved by including bronchial brushings in sampling performed (1). As such, cytology brushings may be the sole diagnostic specimen, and use of BTW-CB can significantly improve the proportion of procedure amenable to molecular testing. In addition, we have previously used on-site cytologic examination to confirm diagnostic bronchial brushings specimens which we have shown may improve diagnostic yield, reduce procedure time and complication risk, and obviate the need for TBLB (6). In this study, it also allowed greater confidence that BTW specimens were more likely to contain diagnostic malignant tissue, and therefore be suitable for molecular analysis.
While many patients in whom biologic agents may be used will have metastatic disease, an increasingly common patient group is the early stage NSCLC patients who are inoperable (12). In such patients, bronchoscopic specimens may be the only source of diagnostic tissue. In addition, pneumothorax complicating TBLB is more frequent in such patients (13) and the consequences of pneumothorax may be clinically more severe. Hence the ability to obtain molecular diagnosis with low morbidity BTW could be of significant value. BTW are easily prepared using cells that would otherwise be discarded, and cost of this technique is minimal. The preparation of BTW sample is quick, under an hour including fixation time, easily prepared and cost effective, using minimal reagents.
Despite being first described in the literature in 1975 by Zavala, and despite the ease with which BTW are created, research into the role of BTW in molecular testing is limited. Only one previous study has attempted molecular analysis of BTW specimens. Tsai and colleagues used TRI reagent solution to rinse the brush tip before performing reverse PCR multi-gene analysis (14) with confirmed tumour cells on brushings in (68.9% of patients) and apparently successful molecular testing in 95.2% of those samples containing tumour cells (14).
KRAS was the most frequently mutated gene in our series (76%), similar to previous reports (15). The role of KRAS mutations is still under investigation, with the suggestion that they may provide value in predicting tumour sensitivity to MEK inhibitors (3). As the clinical role of molecular characterization of NSCLC becomes greater, it is likely that techniques such as BTW, which increase the likelihood of successful molecular diagnosis, will increase in importance.
Study limitations
This is a small single institution cohort study. Our study demonstrates the feasibility of performing molecular analysis of BTW specimens, though future studies are required to validate our findings and to examine the sensitivity of this technique. Factors that contribute to specimen adequacy remain undetermined and further studies to evaluate the optimal method of developing BTW for molecular analysis are also required.
Conclusions
Molecular testing of brush tip wash specimens obtained at diagnostic bronchoscopy is feasible. The use of less invasive specimen collection techniques that are suitable for molecular testing, such as BTW may obviate the need for TBLB and associated morbidity and mortality.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Melbourne Health Human Research Ethics Committee (NO. LNR/15/MH/289).
References
- Boonsarngsuk V, Kanoksil W, Laungdamerongchai S. Comparison of diagnostic performances among bronchoscopic sampling techniques in the diagnosis of peripheral pulmonary lesions. J Thorac Dis 2015;7:697-703. [PubMed]
- Kawaraya M, Gemba K, Ueoka H, et al. Evaluation of various cytological examinations by bronchoscopy in the diagnosis of peripheral lung cancer. Br J Cancer 2003;89:1885-8. [Crossref] [PubMed]
- Aisner DL, Marshall CB. Molecular pathology of non-small cell lung cancer: a practical guide. Am J Clin Pathol 2012;138:332-46. [Crossref] [PubMed]
- Morgensztern D, Campo MJ, Dahlberg SE, et al. Molecularly targeted therapies in non-small-cell lung cancer annual update 2014. J Thorac Oncol 2015;10:S1-63. [Crossref] [PubMed]
- Bonney A, Beaty A, See K, et al. Diagnostic Utility of Bronchial Brush-Tip Washings for the Immunohistochemical Assessment of Peripheral Lung Lesions. Acta Cytol 2016;60:74-8. [Crossref] [PubMed]
- Steinfort DP, Leong TL, Laska IF, et al. Diagnostic utility and accuracy of rapid on-site evaluation of bronchoscopic brushings. Eur Respir J 2015;45:1653-60. [Crossref] [PubMed]
- Dearden S, Stevens J, Wu YL, et al. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol 2013;24:2371-6. [Crossref] [PubMed]
- Rafael OC, Aziz M, Raftopoulos H, et al. Molecular testing in lung cancer: fine-needle aspiration specimen adequacy and test prioritization prior to the CAP/IASLC/AMP Molecular Testing Guideline publication. Cancer Cytopathol 2014;122:454-8. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Botana-Rial M, Núñez-Delgado M, Pallarés-Sanmartín A, et al. Multivariate study of predictive factors for clearly defined lung lesions without visible endobronchial lesions in transbronchial biopsy. Surg Endosc 2010;24:3031-6. [Crossref] [PubMed]
- Dudani S, Leighl NB, Ho C, et al. Approach to the non-operative management of patients with stage II non-small cell lung cancer (NSCLC): A survey of Canadian medical and radiation oncologists. Lung Cancer 2016;94:74-80. [Crossref] [PubMed]
- Tukey MH, Wiener RS. Population-based estimates of transbronchial lung biopsy utilization and complications. Respir Med 2012;106:1559-65. [Crossref] [PubMed]
- Tsai TH, Yang CY, Ho CC, et al. Multi-gene analyses from waste brushing specimens for patients with peripheral lung cancer receiving EBUS-assisted bronchoscopy. Lung Cancer 2013;82:420-5. [Crossref] [PubMed]
- Jurado J, Saqi A, Maxfield R, et al. The efficacy of EBUS-guided transbronchial needle aspiration for molecular testing in lung adenocarcinoma. Ann Thorac Surg 2013;96:1196-202. [Crossref] [PubMed]