
Association between reflux esophagitis and pulmonary function in patients with chronic obstructive pulmonary disease
Highlight box
Key findings
• This study investigated reflux esophagitis (RE) has an effect on pulmonary function in patients with chronic obstructive pulmonary disease (COPD).
What is known and what is new?
• Previous studies have found that gastroesophageal reflux disease has an effect on lung function in patients with COPD.
• In fact, there is a strong correlation between RE and pulmonary function test results.
What is the implication, and what should change now?
• Clinicians should pay attention to the management of RE in patients with COPD.
Introduction
Due to the notable prevalence of chronic obstructive pulmonary disease (COPD) and gastroesophageal reflux disease (GERD), scholars have acknowledged the potential interplay between these conditions. It is theorized that GERD may exacerbate the clinical manifestations of COPD, while mechanical alterations induced by COPD may heighten the severity of GERD symptoms (1). Reflux esophagitis (RE) is characterized by inflammation of the esophageal mucosa resultant from GERD, wherein gastric contents regurgitate into the esophagus, mouth, throat, or stomach, giving rise to distressing symptoms and potential complications (2). Irrespective of the manifestation of GERD symptoms, the occurrence of erosive esophagitis varies, ranging from 6.4% in China to 15.5% in Sweden. Furthermore, among individuals not facing GERD symptoms, the prevalence of erosive esophagitis spans from 6.1% in China to 9.5% in Sweden (3-6). COPD denotes a persistent and progressive respiratory ailment typified by obstructed bronchial passages that remain refractory to complete reversal via bronchodilator therapy (7). While primary COPD predominantly impacts pulmonary function, it is acknowledged as a multifaceted condition characterized by persistent systemic inflammation, often accompanied by additional comorbidities (8). The exacerbation of COPD correlates with a rapid decline in lung function, a decline in quality of life, a notable socioeconomic burden, and heightened mortality rates (9). RE is prevalent among COPD patients and represents a common comorbidity in this population (10). Nonetheless, limited research has delved into the interplay between RE and COPD. Thus, our investigation aims to elucidate the relationship between RE and COPD by evaluating the impact of RE on pulmonary function through pulmonary function tests (PFTs). We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-817/rc).
Methods
Study population
Information was collected from individuals who had received both endoscopic evaluations and PFTs at The First Affiliated Hospital of Dalian Medical University between April 2021 and October 2023. Inclusion criteria considered patients who had undergone endoscopic procedures within a one-year interval preceding and following their PFTs. Exclusion criteria involved patients diagnosed with asthma, idiopathic pulmonary fibrosis, bronchiectasis, lung tumors, pneumonia, as well as those who had undergone lobectomy. Patients who had previously undergone stomach surgery and patients received PPIs, antibiotics, H2RA receptor antagonist, and pectic bismuth one month before endoscopic examination were also excluded from participation. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and approved by the Institutional Ethics Committee of The First Affiliated Hospital of Dalian Medical University (No. PJ-KS-KY-2024-268). Written informed consent was obtained from patients prior to enrollment.
Data collection
Various parameters including sex, height (cm2), weight (kg), body mass index [BMI; calculated as weight (kg)/height2, m2], smoke, drink, PFTs, and endoscopic findings underwent meticulous analysis.
PFTs were conducted on patients within our designated pulmonary function testing facility using the Cosmed PFT4 Ergo equipment from Rome, Italy. The accompanying software, PFT suite version 8.0b, was utilized for data analysis. Adherence to standardized protocols was ensured by performing all measurements in accordance with the updated Pulmonary Function Test Guidelines, established jointly by the American Thoracic Society and the European Respiratory Society in 2005 (11). Recorded PFT parameters included forced expiratory volume in 1 second to forced vital capacity ratio (FEV1/FVC), FEV1, peak expiratory flow (PEF), maximum mid-expiratory flow (MMEF75/25), expiratory reserve volume (ERV), and maximal voluntary ventilation (MVV). Subsequently, all respiratory indices were expressed as percentages relative to predicted values.
Persistent airflow limitation, indicative of COPD, was confirmed in patients exhibiting post-bronchodilator results and FEV1/FVC of <0.70. These specific criteria have been employed consistently across various clinical trials (12). Severity of COPD was graded based on The Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) criteria for airflow restriction, which categorizes patients into four groups: GOLD1 (mild, FEV1 ≥80% predicted), GOLD2 (moderate, 50%≤ FEV1 <80% predicted), GOLD3 (severe, 30%≤ FEV1 <50% predicted), and GOLD4 (very severe, FEV1 <30% predicted) (12).
Esophagogastroduodenoscopy (EGDS; was utilized for endoscopic assessments, employing the Olympus Evis Exera II-2951 system manufactured by Olympus Medical System Corp. in Tokyo, Japan). A proficient endoscopist conducted the procedures, ensuring their successful completion and well-tolerated nature among all participants. Subsequently, patients exhibiting evident signs of RE based on the endoscopic findings were identified through careful screening.
The Los Angeles (LA) classification stands as the predominant scoring system employed to delineate the endoscopic characteristics of the esophageal mucosa and to categorize the severity of RE (13). Esophagitis severity is assessed and classified according to the LA criteria (14). Under this classification, grade A (LA-A) denotes a mucosal break of no more than 5 mm, whereas grade B (LA-B) signifies a mucosal break exceeding 5 mm. Grade C (LA-C) denotes continuous mucosal involvement covering less than 75% of the esophageal circumference, while grade D (LA-D) indicates mucosal involvement extending to at least 75% of the esophageal perimeter.
Outcomes
The primary outcome was the effect of RE on lung function measures including FEV1 (%), FEV1/FVC, MMEF 75/25, ERV, and PEF in patients with COPD, the relationship between the severity of RE and COPD, and the risk factors for COPD with RE. The secondary outcome is a comparison of lung function between patients with RE and normal controls.
Statistical analysis
Statistical computations were executed utilizing SPSS Statistics software (version 29.0; IBM Co., Armonk, NY, USA). Descriptive statistics were presented as mean values with corresponding ± standard deviation (SD). Group comparisons were conducted through the application of the Kruskal-Wallis test, t-test, chi-squared test as appropriate. Spearman correlation coefficients (based on ranks) were calculated for correlations. Univariate and multivariate regression analysis were conducted to analyze risk factors for patients with COPD to the development of RE. A significance threshold of P<0.05 was adopted to ascertain statistical significance.
Results
Baseline
Patients initially diagnosed with RE and then being found to have COPD and patients with known COPD and then being diagnosed with RE were enrolled as subjects in the R-C group. Among the 388 participants enrolled in the investigation, 51 individuals presented with both COPD and RE (R-C =51), 82 exhibited RE alone (R + NC =82), 81 were diagnosed solely with COPD (NR + C =81), and 174 individuals who underwent routine check-up and being without RE and COPD were enrolled as normal controls. Various parameters including sex, height (cm2), weight (kg), BMI [calculated as weight (kg)/height2, m2], smoke, drink, PFTs, and endoscopic findings underwent meticulous analysis. Interestingly, there were no statistically significant disparities in age, height, weight, and BMI across the four delineated groups (Table 1).
Table 1
| Characteristic | NC (n=174) | NR + C (n=81) | R + NC (n=82) | R-C (n=51) | P value |
|---|---|---|---|---|---|
| Age (years) | 62.8±11.4 | 64.9±10.7 | 62.1±11.0 | 62.6±10.2 | 0.36 |
| Sex | <0.001 | ||||
| Male | 44 (25.3) | 44 (54.3) | 43 (52.4) | 33 (64.7) | |
| Female | 130 (74.7) | 37 (45.7) | 39 (47.6) | 18 (35.3) | |
| Height (cm) | 164.5±8.2 | 165.2±8.2 | 166.4±9.2 | 164.9±8.8 | 0.30 |
| Weight (kg) | 67.4±13.0 | 67.1±10.9 | 69.8±14.1 | 68.6±14.6 | 0.44 |
| BMI (kg/m2) | 24.8±3.7 | 24.7±3.8 | 25.1±3.9 | 25.0±4.2 | 0.89 |
| History of smoking | <0.001 | ||||
| Never | 139 (79.9) | 27 (33.3) | 31 (37.8) | 16 (31.4) | |
| Frequent | 35 (20.1) | 54 (66.7) | 51 (62.2) | 35 (68.6) |
Values are reported as mean ± SD or as numerical values accompanied by percentages. BMI, body mass index; NC, normal control; NR + C, non-RE + COPD; R + NC, RE + non-COPD; R-C, RE + COPD; RE, reflux esophagitis; COPD, chronic obstructive pulmonary disease; SD, standard deviation.
Effect of RE on pulmonary function
The PFT outcomes displayed notable abnormalities in individuals diagnosed with RE as opposed to their normal counterparts (Table 2). Among patients with RE, various pulmonary function indicators including (FEV1/FVC, P<0.001; MMEF75/25, P<0.001; and PEF, P=0.048) were all markedly diminished.
Table 2
| Variable | RE (n=82) | NC (n=174) | P value |
|---|---|---|---|
| FEV1 (%) | 101.0±15.6 | 104.7±13.0 | 0.051 |
| FEV1/FVC | 83.1±5.5 | 86.5±4.9 | <0.001 |
| MMEF75/25 | 84.1±25.8 | 96.3±19.8 | <0.001 |
| ERV | 98.3±50.0 | 99.4±45.8 | 0.85 |
| MVV | 85.3±20.5 | 90.5±19.1 | 0.050 |
| PEF | 93.0±22.7 | 98.3±18.5 | 0.048 |
Values are reported as mean ± SD. PFT, pulmonary function test; RE, reflux esophagitis; NC, normal control; FEV1, forced expiratory volume in 1 s; FEV1/FVC, the ratio of forced expiratory volume in 1s/forced vital capacity; MMEF75/25, maximum mid-expiratory flow; ERV, expiratory reserve volume; MVV, maximal voluntary ventilation; PEF, peak expiratory flow; SD, standard deviation.
Effect of RE on pulmonary function in patients with COPD
Among the cohort of 132 patients diagnosed with COPD, 51 were additionally diagnosed with RE (Table 3). Our analysis revealed differences in FEV1 (%) (P=0.04), FEV1/FVC (P<0.001), MMEF75/25 (P=0.03), ERV (P=0.003), and PEF (P=0.002) between patients with both RE and COPD and patients with COPD alone.
Table 3
| Variable | RE (+) (n=51) | RE (−) (n=81) | P value |
|---|---|---|---|
| FEV1 (%) | 62.3±16.9 | 69.2±19.5 | 0.04 |
| FEV1/FVC | 52.9±6.9 | 60.7±9.7 | <0.001 |
| MMEF75/25 | 25.0±10.0 | 30.4±15.2 | 0.03 |
| ERV | 71.1±24.9 | 94.6±51.1 | 0.003 |
| PEF | 51.9±14.3 | 61.9±20.2 | 0.002 |
Values are delineated as mean ± SD. PFT, pulmonary function test; COPD, chronic obstructive pulmonary disease; RE, reflux esophagitis; FEV1, forced expiratory volume in 1 s; FEV1/FVC, the ratio of forced expiratory volume in 1 s/forced vital capacity; MMEF75/25, maximum mid-expiratory flow; ERV, expiratory reserve volume; PEF, peak expiratory flow; SD, standard deviation.
Association between severity of RE and pulmonary function
All COPD patients were evaluated, among several measures that differed between the RE+ and RE− groups, four indicators showed negative correlation with the severity of RE as defined by the LA classification: FEV1 (r=−0.19, P=0.03), FEV1/FVC (r=−0.50, P<0.001), ERV (r=−0.23, P=0.01), PEF (r=−0.23, P=0.01).
Risk factors for COPD and RE
We conducted an analysis to investigate potential influencing variables among patients diagnosed with COPD, with or without RE. Parameters including age, sex, BMI, FEV1, FEV1/FVC, and, MMEF75/25, smoke and drink were examined. The findings revealed statistically significant discrepancies in FEV1 [odds ratio (OR) 1.10; 95% confidence interval (CI): 1.04–1.17; P=0.001] and FEV1/FVC ratios (OR 0.79; 95% CI: 0.71–0.88; P<0.001) across the respective groups (Table 4). Furthermore, multiple logistic regression analysis demonstrated that the FEV1/FVC ratio served as a protective factor (Table 5) with OR being 0.87 (95% CI: 0.82–0.93, P<0.001).
Table 4
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Sex | 0.42 | 0.16 to 1.16 | 0.09 |
| Age (years) | 0.96 | 0.92 to 1.01 | 0.14 |
| BMI | 1.06 | 0.94 to 1.20 | 0.35 |
| FEV1 | 1.10 | 1.04 to 1.17 | 0.001 |
| FEV1/FVC | 0.79 | 0.71 to 0.88 | <0.001 |
| MMEF75/25 | 0.95 | 0.89 to 1.01 | 0.13 |
| Smoke | 0.92 | 0.40 to 2.11 | 0.84 |
| Drink | 1.55 | 0.65 to 3.70 | 0.32 |
COPD, chronic obstructive pulmonary disease; RE, reflux esophagitis; BMI, body mass index; FEV1, forced expiratory volume in 1 s; FEV1/FVC, the ratio of forced expiratory volume in 1 s/forced vital capacity; MMEF75/25, maximum mid-expiratory flow; CI, confidence interval.
Table 5
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| FEV1 | 1.03 | 0.99 to 1.07 | 0.10 |
| FEV1/FVC | 0.87 | 0.82 to 0.93 | <0.001 |
COPD, chronic obstructive pulmonary disease; RE, reflux esophagitis; FEV1, forced expiratory volume in 1 s; FEV1/FVC, the ratio of forced expiratory volume in 1 s/forced vital capacity; CI, confidence interval.
Association between RE and COPD severity
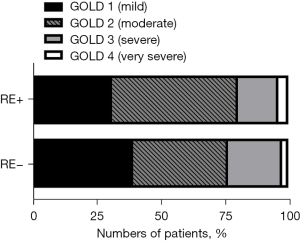
Patients diagnosed with COPD were split into two groups based on the presence or absence of RE, namely RE+ (n=51) and RE− (n=81) groups. COPD severity was categorized according to the GOLD classification system, revealing that within the RE+ group, 16 patients (31.4%) were classified as GOLD1, 25 (49.0%) as GOLD2, 8 (15.7%) as GOLD3, and 2 (3.9%) as GOLD4. In contrast, among RE− patients, 32 individuals (39.5%) were categorized as GOLD1, 30 (37.0%) as GOLD2, 17 (21.0%) as GOLD3, and 2 (2.5%) as GOLD4. The collective analysis indicated no discernible pattern associating RE with the severity of COPD (P=0.55, Figure 1).
Effect of COPD on the severity of RE
Among the 133 patients diagnosed with RE, 51 were also diagnosed with COPD, while 82 did not exhibit COPD. The severity of esophagitis was assessed using the LA classification system. In patients with COPD, the distribution of LA grades was as follows: 44 (86.3%) were classified as LA-A, 1 (2.0%) as LA-B, 1 (2.0%) as LA-C, and 5 (9.8%) as LA-D. Conversely, among patients with RE but without COPD, the distribution of LA grades was as follows: 62 individuals (75.6%) were LA-A, 10 (12.2%) were LA-B, 1 (1.2%) was LA-C, and 9 (11.0%) were LA-D. Overall, the analysis revealed that the presence of COPD did not exert a significant impact on the severity of RE (P=0.21, Figure 2).

Discussion
The correlation between RE and COPD remains a topic of contention within scholarly discourse. While RE has been observed to occur frequently among individuals diagnosed with COPD, existing evidence does not suggest a direct association between RE and acute exacerbations of COPD or airflow limitation (15). Scholarly literature has documented a causal association between COPD and GERD (16). Individuals diagnosed with COPD exhibit an elevated susceptibility to developing esophageal disorders, with the COPD diagnosis itself increasing the likelihood of experiencing symptoms related to GERD (17). Notably, within the initial year following the COPD diagnosis, patients concurrently diagnosed with COPD and GERD demonstrate a notably heightened incidence and risk of hospitalization compared to those without GERD (18). Two primary mechanisms implicated in GERD’s impact on COPD severity include vagus-mediated reflex bronchoconstriction and pulmonary microaspiration (16). The presence of GERD exerts a comprehensive influence on nearly all outcomes associated with COPD. In their study, Bonacin et al. observed statistically significant distinctions in FVC (P=0.03), FEV1 (P=0.002), FEV1/FVC (P=0.001), and PEF (P=0.001) between patients with and without GERD (7,19). The swift deterioration in FEV1 serves as a robust prognostic indicator for both mortality risk and COPD-related hospital admissions (20). A subset of individuals diagnosed with COPD experiencing recurrent exacerbations is classified as exhibiting a “frequent exacerbations” phenotype. The pathophysiological mechanisms underlying this phenotype are intricate, with affected individuals typically demonstrating an accelerated decline in FEV1, rendering them more susceptible to COPD exacerbations (21). In a study by Baldomero et al. GERD was identified as an independent predictor of the rapid decline in FEV1 (22). Wang et al. observed notable decrements in PFT outcomes, specifically FEV1 (%) and FEV1/FVC, among patients experiencing acute exacerbations who tested positive for the Ryan antigen compared to those who tested negative. Furthermore, individuals who tested positive for the Ryan antigen demonstrated significant enhancements in FEV1 (%) and FEV1/FVC ratios relative to baseline following treatment with proton pump inhibitors and primary therapy (23). Our investigation unveiled a potential adverse impact of RE on pulmonary function indicators among COPD patients. Specifically, compared to COPD patients without RE, those with RE exhibited deteriorated FEV1/FVC, FEV1, PEF, MMEF75/25 and ERV. The severity of RE is associated with the deterioration of several of these lung function measures. The precise mechanisms underlying the influence of RE on PFTs results remain inadequately explored and warrant further comprehensive inquiry. Our investigation aimed to ascertain the potential reciprocal influence between RE and COPD severity. Findings indicated a lack of significant impact of RE on COPD severity, and conversely, COPD did not exhibit a discernible effect on the severity of RE. Prior research endeavors have similarly failed to establish a substantial correlation between RE severity and COPD (24).
Age, sex, and BMI are independent risk factors for GERD in patients with COPD (16). However, these factors may not be independent risk factors for RE in patients with COPD. In COPD, smoking increases the risk of GERD (21). However, smoking in COPD patients was not found to be a risk factor for RE in our study, which is consistent with the findings of a previous study (24). Use of theophylline may increase the risk of developing RE in patients with COPD while inhaled anticholinergic drugs may reduce the risk of RE (24,25). Our analysis revealed that the FEV1/FVC ratio served as a protective factor for patients concurrently diagnosed with COPD and RE, indicating a close association between this index and RE. A decline in the FEV1/FVC ratio may independently pose a heightened risk for RE development.
In individuals exhibiting normal lung function, the presence of erosive RE do not demonstrate any influence on the annual decrease in FEV1 or FVC, nor dose it impact the decline in overall lung function (15). However, our investigation revealed that there was a difference in PFT outcomes in patients with RE compared to those with normal lung function.The differences between the results concerned in the two study may be due to the confounding factor-smoking. The precise mechanisms through which RE influences the results of lung function tests remain an area requiring further elucidation, highlighting the need for additional comprehensive studies in this domain.
This study encountered several limitations. Firstly, the data were sourced from a solitary institution, and the patient cohort was relatively modest in size, gathered over a restricted timeframe. Additionally, the enrollment of participants was limited, with a notably smaller proportion presenting with esophagitis grades B, C, and D in comparison to grade A. Secondly, despite the performance of endoscopic examinations within a year of PFTs, a temporal gap persisted between the two procedures, necessitating further investigation into the precise relationship between PFT outcomes and RE. Thirdly, it is imperative to explore additional potential independent risk factors associated with the development of both COPD and RE, such as prolonged utilization of pharmaceutical agents linked to respiratory ailments. Further extensive investigations are warranted to elucidate the potential impact of these pharmaceutical agents on the prevalence of RE among individuals diagnosed with COPD.
Additionally, our study did not delve into the potential relationship between RE and acute exacerbations of COPD, nor did it explore whether the prolonged presence of RE may precipitate acute exacerbations of COPD. Furthermore, our analysis solely encompassed selected PFT outcomes, thereby precluding a comprehensive examination of the potential association between RE and other PFT parameters. Moreover, our study employed objective test results to evaluate the association between RE and COPD, underscoring the significance of early identification and intervention for RE in mitigating the risk of impaired pulmonary function. Clinicians should remain vigilant regarding the presence of RE and institute appropriate therapeutic interventions along with the management of respiratory disorders.
Conclusions
Our investigation revealed a detrimental impact of RE on PFT outcomes. While RE may exacerbate declines in lung function among individuals with COPD, it does not appear to influence the severity of COPD itself. Likewise, COPD did not exhibit a discernible effect on RE severity. Nonetheless, our findings suggest that a diminished ratio of FEV1/FVC could independently predispose patients with COPD to the development of RE.
Acknowledgments
The authors would like to acknowledge pulmonary function tests room of The First Affiliated Hospital of Dalian Medical University for the contributions to the manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-817/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-817/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-817/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-817/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and approved by the Institutional Ethics Committee of The First Affiliated Hospital of Dalian Medical University (No. PJ-KS-KY-2024-268). Written informed consent was obtained from patients prior to enrollment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zou M, Zhang W, Xu Y, et al. Relationship Between COPD and GERD: A Bibliometrics Analysis. Int J Chron Obstruct Pulmon Dis 2022;17:3045-59. [Crossref] [PubMed]
- Azer SA, Hashmi MF, Reddivari AKR. Gastroesophageal Reflux Disease (GERD). In: StatPearls. Treasure Island (FL): StatPearls Publishing; May 1, 2024.
- Dent J, Becher A, Sung J, et al. Systematic review: patterns of reflux-induced symptoms and esophageal endoscopic findings in large-scale surveys. Clin Gastroenterol Hepatol 2012;10:863-873.e3. [Crossref] [PubMed]
- Ronkainen J, Aro P, Storskrubb T, et al. High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: a Kalixanda study report. Scand J Gastroenterol 2005;40:275-85. [Crossref] [PubMed]
- Richter JE, Rubenstein JH. Presentation and Epidemiology of Gastroesophageal Reflux Disease. Gastroenterology 2018;154:267-76. [Crossref] [PubMed]
- Zou D, He J, Ma X, et al. Epidemiology of symptom-defined gastroesophageal reflux disease and reflux esophagitis: the systematic investigation of gastrointestinal diseases in China (SILC). Scand J Gastroenterol 2011;46:133-41. [Crossref] [PubMed]
- Kahnert K, Jörres RA, Behr J, et al. The Diagnosis and Treatment of COPD and Its Comorbidities. Dtsch Arztebl Int 2023;120:434-44. [Crossref] [PubMed]
- Negewo NA, Gibson PG, McDonald VM. COPD and its comorbidities: Impact, measurement and mechanisms. Respirology 2015;20:1160-71. [Crossref] [PubMed]
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60:925-31. [Crossref] [PubMed]
- Jung KS. Reflux esophagitis is one of highly prevalent comorbidities among patients with chronic obstructive pulmonary disease. Korean J Intern Med 2014;29:428-9. [Crossref] [PubMed]
- Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019;200:e70-88. [Crossref] [PubMed]
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [Crossref] [PubMed]
- Ge H, Zhou X, Wang Y, et al. Development and Validation of Deep Learning Models for the Multiclassification of Reflux Esophagitis Based on the Los Angeles Classification. J Healthc Eng 2023;2023:7023731. [Crossref] [PubMed]
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999;45:172-80. [Crossref] [PubMed]
- Kang HR, Lee YJ, Lee HY, et al. The Impact of Erosive Reflux Esophagitis on the Decline of Lung Function in the General Population. J Korean Med Sci 2021;36:e29. [Crossref] [PubMed]
- Lee AL, Goldstein RS. Gastroesophageal reflux disease in COPD: links and risks. Int J Chron Obstruct Pulmon Dis 2015;10:1935-49. [Crossref] [PubMed]
- Martinez CH, Mannino DM, Divo MJ. Defining COPD-Related Comorbidities, 2004-2014. Chronic Obstr Pulm Dis 2014;1:51-63. [Crossref] [PubMed]
- Tsai CL, Lin YH, Wang MT, et al. Gastro-oesophageal reflux disease increases the risk of intensive care unit admittance and mechanical ventilation use among patients with chronic obstructive pulmonary disease: a nationwide population-based cohort study. Crit Care 2015;19:110. [Crossref] [PubMed]
- Bonacin D, Fabijanić D, Radić M, et al. Gastroesophageal reflux disease and pulmonary function: a potential role of the dead space extension. Med Sci Monit 2012;18:CR271-5. [Crossref] [PubMed]
- Mannino DM, Reichert MM, Davis KJ. Lung function decline and outcomes in an adult population. Am J Respir Crit Care Med 2006;173:985-90. [Crossref] [PubMed]
- Broers C, Tack J, Pauwels A. Review article: gastro-oesophageal reflux disease in asthma and chronic obstructive pulmonary disease. Aliment Pharmacol Ther 2018;47:176-91. [Crossref] [PubMed]
- Baldomero AK, Wendt CH, Petersen A, et al. Impact of gastroesophageal reflux on longitudinal lung function and quantitative computed tomography in the COPDGene cohort. Respir Res 2020;21:203. [Crossref] [PubMed]
- Wang H, Fu Z, Xu P, et al. Proton pump inhibitor treatment improves pulmonary function in acute exacerbations of COPD patients with 24-hour Dx-pH monitoring-diagnosed laryngopharyngeal reflux. Clin Respir J 2021;15:558-67. [Crossref] [PubMed]
- Kang HH, Seo M, Lee J, et al. Reflux esophagitis in patients with chronic obstructive pulmonary disease. Medicine (Baltimore) 2021;100:e27091. [Crossref] [PubMed]
- Kim SW, Lee JH, Sim YS, et al. Prevalence and risk factors for reflux esophagitis in patients with chronic obstructive pulmonary disease. Korean J Intern Med 2014;29:466-73. [Crossref] [PubMed]


