Cell block samples from endobronchial ultrasound transbronchial needle aspiration provide sufficient material for ancillary testing in lung cancer—a quaternary referral centre experience
Introduction
Advances in the field of molecular biology and chemotherapeutic agents now mean that it is no longer sufficient to describe lung cancers as small cell lung cancer (SCLC) or non-small cell lung cancer (NSCLC). Immunohistochemistry (IHC) and molecular markers can provide important information to the clinician planning treatment. IHC is now essential to subtype NSCLC and plan patient management. The most well published example of molecular marking testing in lung cancer is in patients with advanced lung adenocarcinoma and somatic mutations in the epidermal growth factor receptor (EGFR) domain who can be treated with tyrosine kinase inhibitors to achieve prolonged progression free survival whether offered as first-line, second-line or maintenance treatment (1).
As these same medical oncology advancements have been made, so too have advances in endobronchial ultrasound guided biopsy (EBUS). EBUS fine needle aspirate and cell block samples are associated with fewer complications such as bleeding or pneumothorax than transbronchial lung biopsy or CT guided fine needle aspirations (FNA) or core biopsies (2-4). One concern, however, is that there may be less tissue in an EBUS sample and that this could be insufficient to perform the required ancillary IHC and molecular testing. Current evidence from other studies suggests that EBUS-transbronchial needles aspiration (TBNA) samples are usually sufficient for molecular genetic testing with diagnostic rates between 72–95.5% (5-8). Santis et al. [2011] had a diagnostic rate of 95.5% with the aid of rapid on site examination (ROSE) (8).
ROSE of aspirates by a pathologist can aid bronchoscopists by confirming best sites for aspiration in order to obtain sufficient tissue for diagnosis and ancillary testing. Nakajima et al. [2013] found that ROSE had no false positive results but a 5.7% false negative rate due to results found at later histological evaluation (9). It is therefore very useful but further information can be obtained after cell block analysis.
The aims of this study were to:
- Assess the overall diagnostic performance of a new EBUS-TBNA service in a quaternary referral centre;
- Evaluate the diagnostic accuracy of cytology smears from EBUS-TBNA samples versus cell block;
- Determine if EBUS-TBNA samples provide sufficient material for ancillary testing, in particular IHC and EGFR mutation analysis.
Methods
A retrospective audit of all EBUS-TBNA procedures at a single quaternary referral centre from its first EBUS procedure in July 2009 until end of July 2012 was performed. July 2012 was chosen as the cut-off date as at this point, hospital policy dictated that all NSCLC at this site would be routinely tested for the EGFR molecular marker. Royal Adelaide Hospital Ethics Committee approval was obtained. The Olympus linear EBUS scope UC 180-F was used for all procedures with either 21 or 22 gauge needle. The type of needle used for the cases was not recorded.
Procedures were performed under either general anaesthetic or light sedation by respiratory physicians or registrars under supervision. Respiratory physicians were fellows of the Royal Australian College of Physicians and registrars were undertaking specialist training in respiratory medicine. All procedures were attended by the pathology consultant or senior registrar in training. All pathologists were fellows of the Royal College of Pathologists of Australasia. Pathology registrars were undertaking specialist pathology training but had not yet received their fellowship from the college. They were considered senior enough to attend ROSE without an accompanying consultant pathologist once they had attended 50 supervised ROSE procedures. There were two consultants and one registrar on call for ROSE across all hospital departments each day during this study. The decision regarding whether a consultant or registrar attended the procedure depended on their availability.
TBNA smears were prepared by two bronchoscopy suite nursing staff. Nurses followed departmental protocol for slide preparation as follows:
The pathologist or pathology registrar then stained the slide for ROSE using Diff-Quick Romanovsky type stain (Hemacolor® Merck, Germany) and ROSE was performed. If ROSE confirmed sufficient sampling but ancillary tests were required, the pathologist would advise the proceduralist to take extra TBNA and these passes would go directly into Hank’s or saline solutions for later review at the laboratory. Smears were made until a diagnosis was obtained or until the procedure was abandoned due to patient intolerance. Dedicated passes for cell block only was left to the discretion of the thoracic physician performing the procedure. The number of passes per patient was recorded.
Cell blocks were prepared by qualified medical scientists in a NATA accredited laboratory according to a standardised protocol. The pre-prepared agar (Sigma®, Sigma-Aldrich, USA) is microwaved to make it molten and added to the centrifuged cell block. This then solidifies and the agar block is processed as per routine histopathology. Pas-D staining, thyroid transcription factor (TTF)-1, cytokeratin (CK)-7, CK-20 IHC was ordered routinely for adenocarcinoma cases. In cases with morphological features that could not clearly distinguish between squamous cell carcinoma and adenocarcinoma, squamous cell markers CK5/6 and p63 were also ordered. Further IHC tests were ordered depending on clinical history such as a history of a malignancy where metastatic disease was in the differential diagnosis. Molecular testing was performed on site at the Department of Molecular Pathology at the Institute of Medical and Veterinary Science, the pathology department of the Royal Adelaide Hospital. EGFR was the only molecular test ordered during this study done by Sanger sequencing of cell block material. Microdissection of paraffin-embedded cell block material was performed by a pathologist using a laser capture microscope to ensure that the majority of cells collected were tumour cells. DNA was then extracted using standard techniques and exons 18–21 inclusive in the EGFR gene were amplified by PCR and the products sequenced. Sequence variants were detected by comparison to a reference sequence (GenBank NM_005228.3) using Mutation Surveyor software (Mutation Surveyor®, SoftGenetics, USA). Although there is evidence for molecular testing on smears, the experience at our institution is that cell block provides better preservation of cells for testing (10,11). Currently our centre has switched to OncoFOCUS version 3 kit designed by AGENA for MALDI-TOF mass spectrometry which tests each tissue sample for EGFR, KRAS, NRAS, BRAF and KIT mutations.
A reference pathologist reviewed all smears and cell blocks for diagnostic accuracy. In cases where EGFR was not performed for clinical reasons, the reference pathologist reviewed the sample to see whether it was sufficient and suitable for molecular testing. To be considered suitable for molecular testing, tumour cells should comprise at least 20% of all cells in the cell block and there should be more than 100 tumour cells in total. Fisher’s exact test (GraphPad Prism version 6.04 for Windows, GraphPad Software, La Jolla, California, USA) was used to compare smear versus cell block diagnostic rates.
Results
Two hundred and thirty four EBUS-TBNA procedures were performed during the study period. The mean age of patients was 60 years with 65% (152/234) males and 35% (82/234) females. The average number of passes was 4.5 per patient.
Malignant cases
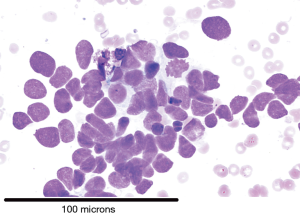
There were 101 cases of malignancy diagnosed with Table 1 showing the tissue types based on IHC. Diagnostic rate for malignancy was 96/101 (95%) for smears and 87/93 (93.5%) for cell block (P= not significant). Cases of definitive SCLC on smear (Figure 1) did not have a cell block made and hence the discrepancy in numbers. IHC was possible on 85/87 (97.7%) where cell block diagnosis was possible. Figure 2 is a picture of adenocarcinoma stained with Diff-Quick for ROSE. Figure 3 is adenocarcinoma staining positively for TTF1. Upon review by a reference pathologist, 69/87 (79.3%) of the cell blocks would have had sufficient tumour sample for molecular testing.
Full table
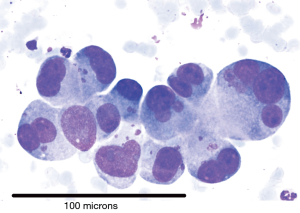
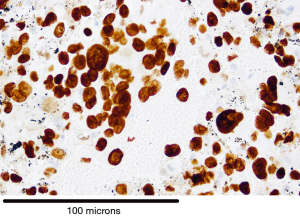
NSCLC adenocarcinoma cases
We assessed the clinical stage of the adenocarcinoma cases diagnosed as these would have had the most impact on management should they be EGFR mutation positive. Of the 30 adenocarcinoma cases, three were found to be EGFR positive. During this study time frame, only adenocarcinoma patients that were stage IIIB or IV with good performance status were routinely studied for EGFR as this was the patient group that would be considered for targeted therapy. There were nine cases where EGFR mutation status was not checked because radical intent treatment for stage IIIA disease was planned or the patient was too unwell for systemic therapy. In only one case was there insufficient material for EGFR mutation testing in patients eligible for potential treatment.
Benign cases
There were 107 cases with a benign diagnosis after adequate follow up and additional investigations where necessary. The diagnoses are outlined in Table 2.
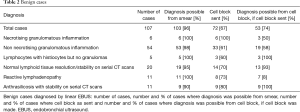
Full table
Non-diagnostic cases
There were 26/234 (11.1%) non diagnostic cases in this series. After further investigation, 11/234 (4.7%) were false negatives for malignancy with the remaining 15 cases being true negatives after adequate follow up and/or investigations. The 4.7% false negative rate for malignancy is similar to previous meta-analysis data published (2). Table 3 shows the conditions and the method by which the diagnosis was ultimately made. The three patients who had repeat EBUS-TBNA procedures had had their initial procedure performed under local sedation but did not tolerate it well. The repeat EBUS-TBNA procedures were performed under general anaesthetic and diagnosis was then possible in all cases.
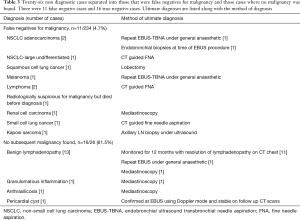
Full table
Discussion and conclusions
EBUS-TBNA is a well-established procedure for lung cancer mediastinal staging and diagnosis of central lesions outside of the airway lumen. It is now the recommended first technique for lymph node sampling in many lung cancer guidelines (12). Meta-analysis has shown a pooled sensitivity of 0.93 (95% CI, 0.91–0.94) and a pooled specificity of 1.00 (95% CI, 0.99–1.00) (2). Our overall diagnostic yield was comparable to published data, even considering the learning curve associated with the uptake of a new technique (13,14). In addition, all cases were used as training experience for senior thoracic registrars under direct supervision reducing the number of actual passes done by an experienced respiratory physician.
Rapid on site evaluation of samples by a pathologist assists the clinician in directing specimens for additional testing such as microbiological culture or flow cytometry, in ascertaining when sufficient biopsies have been taken for a diagnosis (9), and for further molecular marker and IHC testing (15). Onsite pathology advice reduces unnecessary TBNA when sufficient tissue has been obtained for diagnosis and all ancillary tests. More importantly this advice helps avoid repeat procedures for more tissue (16,17).
Usual practice at this site was to do one extra pass after diagnosis made to better allow for dedicated cell block ancillary testing. In this series, cell block yield was found to not be inferior to smears; this suggests that at sites where there are not sufficient resources for ROSE, cell block is a reasonable option. Cost-effectiveness of ROSE will vary between institutions but studies evaluating mathematical modelling for ROSE have found that it will be most cost-effective by avoiding repeat procedures if there are high fixed costs per procedure, there is a low per-pass adequacy rate and a short time per needle pass. If the repeat procedure is mediastinoscopy rather than EBUS, the savings are greater (18,19).
Although EBUS samples are smaller than transbronchial or CT FNA biopsies, they remain sufficient in most cases for ancillary testing. Our study looked at IHC and EGFR, one example of a molecular test that is changing management with the use of tyrosine kinase inhibitors in patients with adenocarcinoma and EGFR somatic mutation. Pathologist review found that IHC was possible in 97.7% of cell blocks tested and that 79.3% remained sufficient for EGFR testing. A larger pragmatic study by Navani et al. found very similar overall sensitivity [88% (95% CI, 66–77)], and diagnostic accuracy [91% (95% CI, 89–93)] (20). They reported a higher success rate for EGFR mutation testing of 90% but this was only in samples where the test was specifically requested so the overall success rate for that multi-centre analysis is unknown. Nevertheless, our pragmatic study results are reassuring, given molecular markers in cancer is a growing field and it is likely to offer future targets for chemotherapeutic agents. During this study, the only molecular test ordered was EGFR. Currently, we routinely perform EGFR molecular testing and ALK IHC on all primary lung adenocarcinoma and all non-small cell carcinoma NOS. If the ALK IHC is equivocal and there is no EGFR mutation, we proceed to ALK FISH.
In the year following this study, when molecular testing on lung adenocarcinoma or NSCLC NOS became routine, we performed a total of 170 linear EBUS cases, with an overall diagnostic rate of 84%. This is not statistically significantly different from our rate in this study. In total 78 (46%) of the cases were malignant with 92% of the cell blocks being suitable for IHC testing. Reassuringly, of the 34 cases where molecular testing for EGFR was ordered and one case where BRAF for melanoma was ordered, molecular testing was possible in 79% (27/34) of cases. This one year follow up audit supported the findings from our retrospective study. We plan to further evaluate the diagnostic yield of our procedures and cell block methodology in our centre to account for the multiple genetic markers now required not only in current clinical practice but also ongoing clinical trials.
Acknowledgements
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study had ethics approval from the Royal Adelaide Hospital Human Research Ethics Committee.
References
- Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 2013;105:595-605. [Crossref] [PubMed]
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96. [Crossref] [PubMed]
- Asano F, Aoe M, Ohsaki Y, et al. Deaths and complications associated with respiratory endoscopy: a survey by the Japan Society for Respiratory Endoscopy in 2010. Respirology 2012;17:478-85. [Crossref] [PubMed]
- Yeow KM, Su IH, Pan KT, et al. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 2004;126:748-54. [Crossref] [PubMed]
- Schuurbiers OC, Looijen-Salamon MG, Ligtenberg MJ, et al. A brief retrospective report on the feasibility of epidermal growth factor receptor and KRAS mutation analysis in transesophageal ultrasound- and endobronchial ultrasound-guided fine needle cytological aspirates. J Thorac Oncol 2010;5:1664-7. [Crossref] [PubMed]
- Garcia-Olivé I, Monsó E, Andreo F, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for identifying EGFR mutations. Eur Respir J 2010;35:391-5. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Suzuki M, et al. Assessment of epidermal growth factor receptor mutation by endobronchial ultrasound-guided transbronchial needle aspiration. Chest 2007;132:597-602. [Crossref] [PubMed]
- Santis G, Angell R, Nickless G, et al. Screening for EGFR and KRAS mutations in endobronchial ultrasound derived transbronchial needle aspirates in non-small cell lung cancer using COLD-PCR. PLoS One 2011;6:e25191. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Saegusa F, et al. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for nodal staging in patients with lung cancer. Ann Thorac Surg 2013;95:1695-9. [Crossref] [PubMed]
- Roy-Chowdhuri S, Chow CW, Kane MK, et al. Optimizing the DNA yield for molecular analysis from cytologic preparations. Cancer Cytopathol 2016;124:254-60. [Crossref] [PubMed]
- Knoepp SM, Roh MH. Ancillary techniques on direct-smear aspirate slides: a significant evolution for cytopathology techniques. Cancer Cytopathol 2013;121:120-8. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Kheir F, Alokla K, Myers L, et al. Endobronchial Ultrasound-Transbronchial Needle Aspiration of Mediastinal and Hilar Lymphadenopathy Learning Curve. Am J Ther 2016;23:e1016-9. [Crossref] [PubMed]
- Hu Y, Puri V, Crabtree TD, et al. Attaining proficiency with endobronchial ultrasound-guided transbronchial needle aspiration. J Thorac Cardiovasc Surg 2013;146:1387-1392.e1. [Crossref] [PubMed]
- Jurado J, Saqi A, Maxfield R, et al. The efficacy of EBUS-guided transbronchial needle aspiration for molecular testing in lung adenocarcinoma. Ann Thorac Surg 2013;96:1196-202. [Crossref] [PubMed]
- Oki M, Saka H, Kitagawa C, et al. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for diagnosing lung cancer: a randomized study. Respiration 2013;85:486-92. [Crossref] [PubMed]
- Trisolini R, Cancellieri A, Tinelli C, et al. Randomized Trial of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration With and Without Rapid On-site Evaluation for Lung Cancer Genotyping. Chest 2015;148:1430-7. [Crossref] [PubMed]
- Schmidt RL, Walker BS, Cohen MB. When Is Rapid On-Site Evaluation Cost-Effective for Fine-Needle Aspiration Biopsy? PLoS One 2015;10:e0135466. [Crossref] [PubMed]
- Bruno P, Ricci A, Esposito MC, et al. Efficacy and cost effectiveness of rapid on site examination (ROSE) in management of patients with mediastinal lymphadenopathies. Eur Rev Med Pharmacol Sci 2013;17:1517-22. [PubMed]
- Navani N, Brown JM, Nankivell M, et al. Suitability of endobronchial ultrasound-guided transbronchial needle aspiration specimens for subtyping and genotyping of non-small cell lung cancer: a multicenter study of 774 patients. Am J Respir Crit Care Med 2012;185:1316-22. [Crossref] [PubMed]