
Nomogram model for predicting long-term survival in esophageal cancer patients with metastasis after treatment: a SEER-based study
Highlight box
Key findings
• A nomogram was established for predicting survival of metastatic esophageal cancer (EC) patients.
What is known and what is new?
• Nomogram is a reliable statistical predictive model with a simple intuitive graph that can accurately elucidate the risk of clinical events by incorporating important factors for oncology prognostics.
• This study established a nomogram model based on the Surveillance, Epidemiology and End Results (SEER) database for predicting 3-, 5-, and 8-year overall survival (OS) of EC patients after treatment.
What is the implication, and what should change now?
• The nomograms showed good efficiency in predicting 3-, 5-, and 8-year OS among EC patients with metastasis after surgery, radiotherapy and chemotherapy.
Introduction
Esophageal cancer (EC) is the seventh most prevalent malignancy and the sixth leading cause of cancer-related mortality worldwide (1). To date, preoperative and/or postoperative adjuvant therapy combined with surgical resection of the primary tumor and potential metastatic lymph nodes has been considered as the most effective treatment option for locally advanced EC (2). Nevertheless, the overall survival (OS) for EC remains dismal due to the metastasis and recurrence (3,4).
Tumor node metastasis (TNM) classification system, established by the American Joint Committee on Cancer (AJCC), has been widely used to evaluate tumor progression and risk stratification (5). However, this system is inadequate to predict the OS of EC patients because patients of the same TNM stage may have markedly different survival profiles even after the same treatment (6). Besides, other clinicopathological factors such as age, race, and lymph node ratio (LNR) may also affect the prognosis of EC (7,8). Therefore, a more effective tool is imperative to distinguish the prognosis of EC patients with different risk levels to guide treatment.
Nomogram is a reliable statistical predictive model with a simple intuitive graph that can accurately elucidate the risk of clinical events by incorporating important factors for oncology prognostics (9). It is more effective than the traditional staging systems in predicting the prognosis of the majority of cancers such as gastric (10), breast (11), colorectal (12), and lung (13) cancers. These lead us to investigate the feasibility of establishing a nomogram for predicting the prognosis of metastatic EC after treatment.
The Surveillance, Epidemiology and End Results (SEER) program provides the data of cancer diagnoses, treatment and survival from population-based cancer registries covering approximately 30% of the U.S. population. This makes it a reliable resource for studying the oncology (14). Based on the SEER database [2010–2014], Liu et al. developed a nomogram to predict 1- and 2-year OS and cancer-specific survival (CSS) for M1 stage EC (15). Besides, the nomogram constructed by Cao et al. with the SEER database between 1988 and 2007 predicted the probabilities of 3- and 5-year survival in EC patients after esophagectomy (16). However, there are limited studies focusing on predicting the long-term survival of metastatic EC patients after surgery, radiotherapy and chemotherapy. The purpose of this study was to establish a nomogram model based on SEER database for predicting 3-, 5-, and 8-year OS of metastatic EC patients after treatment. We present this article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-742/rc).
Methods
Data source
This retrospective study was based on the latest version of SEER database. Data of metastatic EC patients who underwent surgery, radiotherapy and chemotherapy from 2004 to 2015 was retrieved from the SEER database using the SEER*Stat software (version 8.4.2).
Patient population
We selected patients following International Classification Disease for Oncology, 3rd Edition (ICD-O3) topography codes for anatomic location in the esophagus: cervical esophagus (15.0), thoracic esophagus (15.1), abdominal esophagus (15.2), upper third of esophagus (15.3), middle third of esophagus (15.4), lower third of esophagus (15.5), overlapping lesion of esophagus (15.8) and esophageal lesions, not otherwise specified (15.9). We only considered patients over the age of 18 at diagnosis. To minimize bias caused by missing diagnostic and follow-up data, we referred to the following inclusion and exclusion criteria. Inclusion criteria were: (I) those with metastasis after surgery, chemotherapy and radiotherapy for the treatment; (II) data originating from hospitals or treatment centers; (III) complete data; and (IV) clearly classified data. Exclusion criteria were: (I) patients whose information was collected from autopsy and death certificates; and (II) patients with unknown or incomplete information of significant variables such as race, marital status, survival, and TNM stage. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Variables
Demographic and clinicopathological variables collected for each patient included age at diagnosis, gender, race, marital status, histological type, number of tumors, primary site (inferior esophagus and/or other sites), size of tumor, positive lymph nodes, poorly differentiated or undifferentiated (grade III or IV), T stage, N stage, radiation, and chemotherapy. The histological types included esophageal adenocarcinoma (EAC), esophageal squamous cell carcinoma (ESCC), and others according to ICD-O3. TNM stage classification was based on the AJCC 8th edition. The main outcome of this study was OS, which was defined as the time from diagnosis to death from any cause.
Statistical analysis
Patients included in this study were randomized into the training and the validation cohorts in a ratio of 7:3. Chi-squared test was used to determine the comparability of demographic and clinicopathological characteristics between the training and the validation cohorts. In the training cohort, univariate and complete subset regression analyses were performed to determine the independent predictors of OS. The hazard ratio (HR) and corresponding 95% confidence interval (CI) were used to show the effect of various factors on OS. A nomogram based on independent predictors was established for predicting 3-, 5-, and 8-year OS. The performance of the nomogram was assessed with respects to discrimination and calibration. The discriminative ability of the nomogram model was evaluated with area under the receiver operating characteristic (ROC) curve (AUC). Calibration was visualized by comparing nomogram-predicted versus observed survival probabilities. The survival curves were plotted by Kaplan-Meier method. Data were analyzed using R software (version 4.3.0). Survival analysis was performed using R packages survival, survminer and ggplot2. ROC curve was generated with R package timeROC. Nomogram and calibration curve were plotted using R package rms. The optimal cutoff values calculated by X-tile software (http://www.tissuearray.org/rimmlab) were utilized to stratify the patients into low- and high-risk groups for comparing the OS in different risk groups. A P value of less than 0.05 was considered statistically significant.
Results
Characteristics of the EC patients
Initially, 46,685 EC patients were screened, among which 14,491 showed metastasis (M1 stage). Among these cases, 860 patients received surgery, 587 underwent radiotherapy, and 561 underwent chemotherapy for the treatment, respectively. Finally, a total of 557 eligible EC patients underwent surgery, radiotherapy and chemotherapy were included in our analyses after excluding four cases with unknown N stage. Patients were randomly allocated into the training cohort (N=390) and the validation cohort (N=167) based on a ratio of 7:3. The demographic and clinicopathological characteristic of all patients are summarized in Table 1. The proportions of EAC, ESCC and other histological types were 71.63%, 15.62% and 12.75%, respectively. In terms of primary sites, lower portion of EC accounted for the highest proportion (83.84%), followed by others (13.29%), and overlapping lesion of esophagus (2.87%). A total of 61.58% of patients showed lymph node involvement. The vast majority (82.59%) of patients showed single tumor lesion.
Table 1
| Name | Whole cohort (n=557) | Training cohort (n=390) | Validation cohort (n=167) | P value |
|---|---|---|---|---|
| Age (years) | 60.08±9.56 | 59.61±9.73 | 61.20±9.07 | 0.07 |
| Sex, female | 62 (11.13) | 40 (10.26) | 22 (13.17) | 0.39 |
| Marital | 0.21 | |||
| Married | 405 (72.71) | 277 (71.03) | 128 (76.65) | |
| Single | 59 (10.59) | 47 (12.05) | 12 (7.19) | |
| Others | 93 (16.70) | 66 (16.92) | 27 (16.17) | |
| Number of tumors, 2 or more | 97 (17.41) | 65 (16.67) | 32 (19.16) | 0.56 |
| Primary site | 0.77 | |||
| EC, lower portion of esophagus | 467 (83.84) | 329 (84.36) | 138 (82.63) | |
| Other sites | 74 (13.29) | 51 (13.08) | 23 (13.77) | |
| Overlapping lesions | 16 (2.87) | 10 (2.56) | 6 (3.59) | |
| Size (mm) | 50.71±22.77 | 50.74±23.43 | 50.65±21.21 | 0.97 |
| Lymph node involvement | 343 (61.58) | 231 (59.23) | 112 (67.07) | 0.10 |
| III and IV grade | 335 (60.14) | 234 (60.00) | 101 (60.48) | 0.99 |
| T stage | 0.85 | |||
| T1 | 51 (9.16) | 37 (9.49) | 14 (8.38) | |
| T2 | 57 (10.23) | 42 (10.77) | 15 (8.98) | |
| T3 | 356 (63.91) | 247 (63.33) | 109 (65.27) | |
| T4 | 65 (11.67) | 43 (11.03) | 22 (13.17) | |
| Tx | 28 (5.03) | 21 (5.38) | 7 (4.19) | |
| N stage, N1 | 347 (62.30) | 243 (62.31) | 104 (62.28) | >0.99 |
| Histology | 0.36 | |||
| ESCC | 87 (15.62) | 64 (16.41) | 23 (13.77) | |
| EAC | 399 (71.63) | 281 (72.05) | 118 (70.66) | |
| Others | 71 (12.75) | 45 (11.54) | 26 (15.57) |
Data are presented as mean ± standard deviation or n (%) unless otherwise stated. EC, esophageal cancer; ESCC, esophageal squamous cell carcinoma; EAC, esophageal adenocarcinoma.
Identification of predictors in training cohort
Univariate and complete subset regression analyses were performed to investigate the correlation between variables and OS in the training cohort (Table 2). Univariate Cox analysis showed that age at diagnosis (P=0.04), female (HR: 0.62; 95% CI: 0.41, 0.94; P=0.02), primary malignant site (P=0.004), lymph node involvement (P=0.02), stage III or IV (P=0.002) was the potential predictors for OS. In complete subset regression analysis, age at diagnosis (HR: 1.01; 95% CI: 1.00, 1.02; P=0.04), EC of other sites (HR: 1.78; 95% CI: 1.29, 2.45; P<0.001), lymph node involvement (HR: 1.37; 95% CI: 1.08, 1.37; P=0.009), and stage III or IV (HR: 1.39; 95% CI: 1.20, 1.76; P=0.006) were the independent risk factors for poor OS. Female (HR: 0.58; 95% CI: 0.38, 0.88; P=0.01) showed reduced risk of poor OS compared with male population.
Table 2
| Name | Dead (n=308) | Alive/censored (n=82) | Univariate Cox regression | Complete subset regression | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Age (years) | 59.98±9.36 | 58.18±10.95 | 1.01 (1.00, 1.02) | 0.04 | 1.01 (1.00, 1.02) | 0.04 | |
| Sex, female | 25 (8.12) | 15 (18.29) | 0.62 (0.41, 0.94) | 0.02 | 0.58 (0.38, 0.88) | 0.01 | |
| Marital | |||||||
| Married | 222 (72.08) | 55 (67.07) | – | – | – | – | |
| Single | 35 (11.36) | 12 (14.63) | 0.83 (0.58, 1.18) | 0.29 | – | – | |
| Others | 51 (16.56) | 15 (18.29) | 0.99 (0.73, 1.35) | 0.96 | – | – | |
| Number of tumors, 2 or more | 53 (17.21) | 12 (14.63) | 0.84 (0.63, 1.13) | 0.26 | – | – | |
| Primary site | |||||||
| EC, lower portion of esophagus | 256 (83.12) | 73 (89.02) | – | – | – | – | |
| Other sites | 45 (14.61) | 6 (7.32) | 1.61 (1.17, 2.21) | 0.004 | 1.78 (1.29, 2.45) | <0.001 | |
| Overlapping lesions | 7 (2.27) | 3 (3.66) | 1.18 (0.56, 2.50) | 0.67 | 1.04 (0.48, 2.24) | 0.93 | |
| Size (mm) | 51.54±24.46 | 47.73±18.89 | 1.00 (1.00, 1.01) | 0.24 | – | – | |
| Lymph node involvement | 192 (62.34) | 39 (47.56) | 1.31 (1.04, 1.66) | 0.02 | 1.37 (1.08, 1.73) | 0.009 | |
| III and IV grade | 192 (62.34) | 42 (51.22) | 1.45 (1.15, 1.83) | 0.002 | 1.39 (1.20, 1.76) | 0.006 | |
| T stage | |||||||
| T1 | 29 (9.42) | 8 (9.76) | – | – | – | – | |
| T2 | 32 (10.39) | 10 (12.20) | 0.76 (0.46, 1.26) | 0.29 | – | – | |
| T3 | 197 (63.96) | 50 (60.98) | 0.93 (0.63, 1.38) | 0.72 | – | – | |
| T4 | 34 (11.04) | 9 (10.98) | 0.85 (0.52, 1.39) | 0.51 | – | – | |
| Tx | 16 (5.19) | 5 (6.10) | 1.06 (0.57, 1.95) | 0.86 | – | – | |
| N stage: N1 | 199 (64.61) | 44 (53.66) | 1.14 (0.90, 1.44) | 0.26 | – | – | |
| Histology | |||||||
| ESCC | 48 (15.58) | 16 (19.51) | – | – | – | – | |
| EAC | 228 (74.03) | 53 (64.63) | 1.06 (0.78, 1.45) | 0.70 | – | – | |
| Others | 32 (10.39) | 13 (15.85) | 0.98 (0.63, 1.54) | 0.95 | – | – | |
Data are presented as mean ± standard deviation or n (%) unless otherwise stated. EC, esophageal cancer; ESCC, esophageal squamous cell carcinoma; EAC, esophageal adenocarcinoma; HR, hazard ratio; CI, confidence interval.
Construction and evaluation of the nomogram for OS
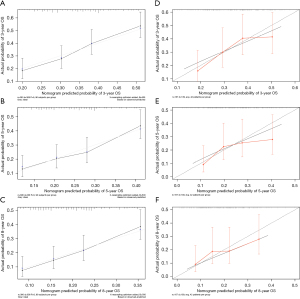
A nomogram was constructed based on the independent predictors identified by the univariate and complete subset regression analyses, including age at diagnosis, male, EC of other sites, lymph node involvement, and stage III or IV. The 3-, 5-, and 8-year OS were predicted using the constructed nomogram (Figure 1). The time-dependent ROC was used to evaluate the discriminative ability of the nomogram. In the training cohort, the AUC values of the nomogram in predicting the 3-, 5-, and 8-year OS were 0.676, 0.687 and 0.717, respectively (Figure 2A). In the validation cohort, the AUC values of the nomogram in predicting 3-, 5-, and 8-year survival were 0.653, 0.635, and 0.629, respectively (Figure 2B). These together indicated the well discriminative ability of the nomogram. The 3-, 5-, and 8-year calibration curves of the nomogram for the prediction of OS demonstrated a good consistency in training cohort (Figure 3A-3C) and validation cohort (Figure 3D-3F).

Performance of the nomogram in risk stratification
The risk scores of all EC patients were calculated. The cutoff point calculated by X-tile software was 0.5 for OS. Based on the cutoff point of OS in the training cohort, 321 cases were stratified into the low-risk group and 69 patients were stratified into high-risk group (Figure 4A). Kaplan-Meier curves for OS predicted by the nomogram were significantly distinct among low- and high-risk EC patients (P<0.001). In the validation cohort, the number of patients stratified into low- and high-risk groups was 126 and 41, respectively. Similarly, patients in the high-risk group were more likely to show a significantly reduced survival time compared with the low-risk group (P=0.002, Figure 4B).

Discussion
In the present study, we constructed a nomogram to predict 3-, 5-, and 8-year OS of EC patients with metastasis after surgery, radiotherapy and chemotherapy based on large-population SEER databases. Through univariate and complete subset regression analyses, we identified age at diagnosis, male, primary site, lymph node involvement and poorly differentiated or undifferentiated (grade III or IV) as independent prognostic factors. The nomogram established using these predictors were eventually proved to show good efficacy in predicting the 3-, 5-, and 8-year OS of these patients after surgery, radiotherapy and chemotherapy.
A handful of studies have shown that the survival of EC patients worsens with increasing age (16-18). Consistently, we found that the patients over 65 years old had a higher risk score than younger patients, which may be attributed to the poor nutritional status, decreased body resistance, and difficulty in recovering from surgery (19). In a previous study, based on univariate and multivariate analyses, Chen et al. reported that individuals aged 60 years or more and male sex were negative prognostic factors for OS in squamous cell carcinoma esophagus (SCCE) (20). Besides, in a study focused on the prognosis of EC patients with stage I–III, male sex and aged over 60 years mostly showed a poor prognosis (21). In this study, increasing age and male sex were confirmed to be independent risk factors for poor prognosis among EC patients with distal metastasis after surgery, radiation and chemotherapy.
The primary sites for EC are localized in the esophagus, while those with metastasis would show in the other sites demonstrating distal metastasis (22). Therefore, these patients would show a poor prognosis even after treatment. In this study, multiple site involvement (multiple distal metastasis) was identified as an independent risk factor for poor prognosis, which would induce an increased risk of poor prognosis (1.78-fold).
Selection of therapeutic strategies for patients is still a major challenge for clinicians as prognosis of EC patients remains poor, especially in advanced settings (23). In EC patients of stage III or IV, the cancer cells spread to distant lymph nodes or to other distant organs. Indeed, the worst outcome was noticed in cancer patients of stage III (HR: 1.35, 95% CI: 1.23–1.49, P<0.01) and stage IV (HR: 2.12, 95% CI: 1.94–2.32, P<0.01) (24). In this study, EC patients of stage III or IV were validated to be associated with increased risk of poor prognosis after treatment. Therefore, it is adopted as a predictor in the nomogram.
Lymph node status has been considered as the most important single prognostic factor for EC, and thereby detection of involved lymph nodes is crucial. Many studies have attempted to investigate the lymph node involvement in EC patients, with most of them performed in squamous cell carcinoma merely. For instance, stratification of lymph node metastasis could improve the diagnostic efficacy of EC, thereby improving the prognosis after treatment (25). In addition, extracapsular lymph node involvement was reported as a negative prognostic factor for locally advanced EC after neoadjuvant chemotherapy (26). Consequently, these patients showed poor prognosis even after treatment. In this study, lymph node involvement was identified as an independent risk factor for poor prognosis, and thereby was adopted into the nomogram.
Discrimination and calibration of the nomogram are assessed to avoid overfitting and to ensure the general application of the nomogram (9,27). Discrimination has usually been evaluated using ROC, and calibration is assessed by comparing the predicted survival probabilities from the nomogram with the actual survival probabilities. In our study, the AUC values of the nomograms to predict 3-, 5-, and 8-year OS revealed a well discrimination of the nomogram. The calibration plots showed acceptable agreement between the prediction probabilities and actual observations, which ensured the reliability and repeatability of the constructed nomogram. Furthermore, EC patients were divided into low-and high-risk groups based on the nomogram risk score. The Kaplan-Meier survival analysis suggested that the nomogram could help us accurately stratify the risk of EC patients.
Our study was hindered by several limitations. First, the retrospective nature of the SEER database may introduce the possibility of selection bias. Second, we could not collect the data from SEER since 2015 in this study even though there have been significant changes since that year. This was mainly associated with the limitations of data collection years and coding, and the coding was not uniform in the data after 2015. Third, the SEER database lacks some important clinical information that would confound our findings, such as specific regimens and timing of chemotherapy and radiotherapy, which are critical to the prognosis of EC patients. Fourth, the nomogram models were only internally validated, and external validation in other independent cohorts is needed to improve the accuracy and generalizability of the models. Finally, in this study, we could not compare the prediction efficacy of the nomogram with the real-world data (RWD) and the artificial intelligence (AI). Indeed, there are a few studies utilizing the application of AI and RWD to predict the risk of prognosis in cancer patients (28), or the integration of nomogram with the AI techniques for predicting the OS in tongue cancer (29). In the future, we will focus on their combination or their comparison in predicting the prognosis.
Conclusions
The nomograms based on these predictors showed satisfactory discrimination and calibration for predicting 3-, 5-, and 8-year OS. The nomograms could accurately stratify the risk of EC patients. Our findings may guide survival prediction and personalized treatment for EC patients after lymphadenectomy with different risk levels.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-742/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-742/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-742/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Wang Z, Mao Y, Gao S, et al. Lymph node dissection and recurrent laryngeal nerve protection in minimally invasive esophagectomy. Ann N Y Acad Sci 2020;1481:20-9. [Crossref] [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [Crossref] [PubMed]
- Robb WB, Messager M, Dahan L, et al. Patterns of recurrence in early-stage oesophageal cancer after chemoradiotherapy and surgery compared with surgery alone. Br J Surg 2016;103:117-25. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg 2017;6:119-30.
- Altorki NK, Zhou XK, Stiles B, et al. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg 2008;248:221-6. [Crossref] [PubMed]
- Kitamura Y, Oshikiri T, Takiguchi G, et al. Impact of Lymph Node Ratio on Survival Outcome in Esophageal Squamous Cell Carcinoma After Minimally Invasive Esophagectomy. Ann Surg Oncol 2021;28:4519-28. [Crossref] [PubMed]
- Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015;33:861-9. [Crossref] [PubMed]
- Dong D, Tang L, Li ZY, et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann Oncol 2019;30:431-8. [Crossref] [PubMed]
- Li S, Zhao J, Zhu L, et al. Development and validation of a nomogram predicting the overall survival of stage IV breast cancer patients. Cancer Med 2017;6:2586-94. [Crossref] [PubMed]
- Yu C, Zhang Y. Establishment of prognostic nomogram for elderly colorectal cancer patients: a SEER database analysis. BMC Gastroenterol 2020;20:347. [Crossref] [PubMed]
- Ouyang J, Hu Z, Tong J, et al. Construction and evaluation of a nomogram for predicting survival in patients with lung cancer. Aging (Albany NY) 2022;14:2775-92. [Crossref] [PubMed]
- Park HS, Lloyd S, Decker RH, et al. Overview of the Surveillance, Epidemiology, and End Results database: evolution, data variables, and quality assurance. Curr Probl Cancer 2012;36:183-90. [Crossref] [PubMed]
- Liu M, Wang C, Gao L, et al. A nomogram to predict long-time survival for patients with M1 diseases of esophageal cancer. J Cancer 2018;9:3986-90. [Crossref] [PubMed]
- Cao J, Yuan P, Wang L, et al. Clinical Nomogram for Predicting Survival of Esophageal Cancer Patients after Esophagectomy. Sci Rep 2016;6:26684. [Crossref] [PubMed]
- Du F, Sun Z, Jia J, et al. Development and Validation of an Individualized Nomogram for Predicting Survival in Patients with Esophageal Carcinoma after Resection. J Cancer 2020;11:4023-9. [Crossref] [PubMed]
- Farrow NE, Raman V, Jawitz OK, et al. Impact of Age on Surgical Outcomes for Locally Advanced Esophageal Cancer. Ann Thorac Surg 2021;111:996-1003. [Crossref] [PubMed]
- Kang M, Wang Y, Yang M, et al. Prognostic nomogram and risk factors for predicting survival in patients with pT2N0M0 esophageal squamous carcinoma. Sci Rep 2023;13:4931. [Crossref] [PubMed]
- Chen J, Zhu J, Pan J, et al. Postoperative radiotherapy improved survival of poor prognostic squamous cell carcinoma esophagus. Ann Thorac Surg 2010;90:435-42. [Crossref] [PubMed]
- Qiu MJ, Yang SL, Wang MM, et al. Prognostic evaluation of esophageal cancer patients with stages I-III. Aging (Albany NY) 2020;12:14736-53. [Crossref] [PubMed]
- Shaheen O, Ghibour A, Alsaid B. Esophageal Cancer Metastases to Unexpected Sites: A Systematic Review. Gastroenterol Res Pract 2017;2017:1657310. [Crossref] [PubMed]
- Puhr HC, Prager GW, Ilhan-Mutlu A. How we treat esophageal squamous cell carcinoma. ESMO Open 2023;8:100789. [Crossref] [PubMed]
- Then EO, Lopez M, Saleem S, et al. Esophageal Cancer: An Updated Surveillance Epidemiology and End Results Database Analysis. World J Oncol 2020;11:55-64. [Crossref] [PubMed]
- Li Z, Li Y, Liu X, et al. Stratification of lymph node metastasis improves diagnostic efficiency in thoracic esophageal squamous cell carcinoma. Dis Esophagus 2023;36:doad017. [Crossref] [PubMed]
- D'Journo XB, Avaro JP, Michelet P, et al. Extracapsular lymph node involvement is a negative prognostic factor after neoadjuvant chemoradiotherapy in locally advanced esophageal cancer. J Thorac Oncol 2009;4:534-9. [Crossref] [PubMed]
- Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188-95. [Crossref] [PubMed]
- Han T, Zhu J, Chen X, et al. Application of artificial intelligence in a real-world research for predicting the risk of liver metastasis in T1 colorectal cancer. Cancer Cell Int 2022;22:28. [Crossref] [PubMed]
- Alabi RO, Mäkitie AA, Pirinen M, et al. Comparison of nomogram with machine learning techniques for prediction of overall survival in patients with tongue cancer. Int J Med Inform 2021;145:104313. [Crossref] [PubMed]



