
The diagnostic value of hydroxyproline combined with tuberculosis infection T lymphocyte spot assay in pulmonary tuberculosis
Highlight box
Key findings
• Our study found that the detection of hydroxyproline (HYP) expression alone or combined with tuberculosis infection T lymphocyte spot assay (TSPOT.TB) could aid in the auxiliary diagnosis of pulmonary tuberculosis (PTB).
What is known and what is new?
• The role of HYP expression in PTB has been reported to some extent, but systematic studies of this relationship are lacking.
• We systematically analyzed the expression changes of HYP in serum, urine, and bronchoalveolar lavage fluid and evaluated its value in diagnosing PTB when used alone or in combination with TSPOT.TB.
What is the implication, and what should change now?
• The evaluation of HYP as a molecular marker in the diagnosis of PTB indicated that it could aid in diagnosis when used alone or in combination with TSPOT.TB.
Introduction
Pulmonary tuberculosis (PTB) is a respiratory pulmonary disease infection caused by Mycobacterium tuberculosis (MTB) (1). Although there are well-developed treatment methods, only a minority of patients are successfully diagnosed with PTB due to individual differences and the subjective interpretation of specimens (2). With the deepening of research, a variety of auxiliary diagnostic indicators of PTB have been applied to clinical practice, but these each have certain limitations (3). At present, the common etiological diagnosis, such as the gold standard detection for tuberculosis (TB) diagnosis (MTB culture), has a low positive rate of etiological laboratory detection and a long culture time (4). Although the polymerase chain reaction (PCR) of MTB, including TB-DNA and TB-RNA, can shorten the detection time, it has high requirements for specimen quality and cannot solve the practical detection problem of a low positivity rate (5). The GeneXpert/RIF assay has obvious advantages such as easy operation and simultaneous detection of rifampicin resistance, but it can only detect body fluid specimens with a high degree of infection (6). The TB infection T lymphocyte spot assay (TSPOT.TB) is a method for directly screening effector T cells without interference from patient specimens or clinical symptoms (7). Currently, it is widely used in the clinical screening for TB infection. However, although it solves the problem of non-contamination of specimens, it cannot distinguish between active and latent TB infection (8). Meanwhile, although bronchial brush microscopy can overcome the challenges involving positivity rate and cost, it is invasive and potentially harmful to patients (9). Therefore, it is urgent to develop new diagnostic molecular markers or combined diagnostics in PTB.
Hydroxyproline (HYP) is a nonessential amino acid and a critical component of collagen (10). Studies have shown that HYP plays an important role in a variety of diseases, such as liver fibrosis, skin-associated diseases, graft-versus-host disease (GVHD), wound healing, and cardiovascular diseases (11-15). The anti-inflammatory, antioxidant, and tissue repair effects of HYP in diseases have also been widely recognized (16). During the occurrence and development of PTB, lung tissue damage and tissue repair are important evaluation indicators of the occurrence, development, and treatment outcomes in those with TB (17). After the MTB invades the lung tissue, it first leads to exudative lesions of the lung, which manifest as congestion, edema, and leukocyte infiltration. In the early exudative lesions, neutrophils are mainly present and then are gradually replaced by lymphocytes and macrophages (18). Exudative lesions are short-lived and are followed by proliferative lesions, with Langerhans giant cells potentially appearing in the center of the lesion. Epithelioid cells, Langerhans cells, and lymphocytes infiltrate to form a typical epithelioid granulomatous nodule, which is the characteristic lesion of TB (19). If progression occurs, the inflammatory cells release proteolytic enzymes after death, causing the tissue to dissolve and necrotize, forming coagulative necrosis (19). Study using animal experiments reported that changes in HYP concentration are significantly associated with the degree of pulmonary inflammation in TB and that HYP expression is a prognostic predictor after anti-TB treatment (20). This suggests that HYP may figure prominently in the occurrence, development, and prognosis of PTB. However, the expression level of HYP in different body fluid samples of TB and its application value in clinical diagnosis have not been systematically investigated.
Thus, in our study, the expression of HYP in blood, urine, and bronchoalveolar lavage fluid (BALF) of PTB was determined, and the correlation between HYP and several clinical indicators was analyzed. The aim of this study was to assess the diagnostic efficacy of HYP alone or in combination with TSPOT.TB in the preliminary screening of PTB. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1585/rc).
Methods
Study participants
Patients with PTB attending the Affiliated Nantong Hospital of Shanghai University (The Sixth People’s Hospital of Nantong) from August 2019 to December 2020 were selected as the study participants. Blood, urine, and BALF samples were collected from patients with PTB. Meanwhile, healthy individual and those with general pneumonia group were included as a healthy control (HC) group and a respiratory control (RC) group, respectively. The inclusion criteria for HCs were as follows: blood and urine samples collected during the same period as physical, no history of TB or history of exposure to TB, and normal computed tomography (CT) examination and a negative TSPOT.TB result. Meanwhile, the inclusion criteria for the RC groups were as follows: BALF collected from RC patients, TB ruled out by CT examination, and a negative TSPOT.TB result. The presence of TB (the PTB group) was determined mainly according to the Guidelines for the Diagnosis and Treatment of Tuberculosis and diagnosed by clinicians following the diagnostic criteria of the Tuberculosis Branch of the Chinese Medical Association. Chronic diseases such as human immunodeficiency virus (HIV) infection, other chronic illness, adrenocorticosteroid use, and use of other immunosuppressives were excluded from the study. All individuals were included according to age and sex, and all selected participants were of Han ethnicity with no blood relationships between them. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All data and sample collection in this study were subject to informed consent and were approved by the Ethics Committee of the Affiliated Nantong Hospital of Shanghai University (The Sixth People’s Hospital of Nantong) (No. NTLYLL2019017).
Acquisition of three types of body fluid specimens
The specimens of patients with TB included serum, urine, and BALF before and after treatment. Blood and urine specimens were collected from each group and stored in a biobank. BALF specimens are mainly obtained through bronchoscopy by professional TB clinicians.
TSPOT.TB procedure
TB infection was detected with a TSPOT.TB kit (Shanghai Fuxing Changzheng Biotechnology Co., Ltd., Shanghai, China). The steps of the assay procedure were as follows: 5 mL of peripheral blood was collected with a heparin anticoagulant tube and poured into a 15-mL centrifuge tube. The same volume of Ficoll lymphocyte separation solution was added proportionally. Gradient centrifugation was completed, and a thin layer of mononuclear cells in the middle was absorbed and counted. Cells (2×105/L) were then seeded in the plate and cultured in a 5% CO2 incubator at 37 ℃. According to requirements mentioned in the kit, a blank control well (without cells), a positive control well (phytohemagglutinin stimulation), TB-specific antigens ESAT6 stimulation group, and CFP-10 stimulation group were established. After enzyme-conjugate secondary antibody was added into all wells, a color-rendering solution was added to the washing plate for color rendering. A special detector supplied by Shanghai Fuxing Changzheng Biotechnology Co., Ltd. was used to read the number of spots and assess the experimental results. Results were analyzed according to the manufacturer’s instructions in TSPOT.TB kit.
HYP detection
HYP expression was determined according to a HYP detection kit (Sigma-Aldrich, Merck Group, Darmstadt, Germany). Serum, urine, or BALF samples (100 µL each) were transferred into a sealed polypropylene Eppendorf tube with a liner lid. After the addition of 12 M of 100 µL hydrochloric acid (Sigma-Aldrich) and reaction at 120 ℃ for 3 hours, 4 mg of activated carbon (Sigma-Aldrich) per tube was added and centrifuged at 10,000 g for 3 min. Subsequently, 10–50 µL of supernatant was directly transferred to a 96-well plate. The preparation method of the HYP standard was as follows: 10 µL of 1 mg/mL HYP standard solution was diluted with 90 µL of water into a 0.1-mg/mL standard storage solution. Following this, 0.1 mg/mL of HYP standard liquid in volumes of 0, 2, 4, 6, 8, and 10 µL was added to the 96-well plate to generate 0 (blank), 0.2, 0.4, 0.6, 0.8, and 1.0 µg/well standard liquids, respectively. Chloramine T/oxidation buffer (100 µL) was then added to the sample and standard well and incubated at room temperature for 5 min. After 100 µL of diluted dimethylaminobenzaldehyde reagent was added into each well and incubated at 60 ℃ for 90 min, the absorbance value (A value) was measured at 560 nm (A560).
Statistical analysis
All data were analyzed and processed using GraphPad Prism 8.0 (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS 17.0 (IBM Corp., Armonk, NY, USA). Unpaired and paired samples were statistically analyzed through variance analysis, the t-test, or the Chi-square test. Count data and measurement data of included cases were calculated according to percentile and mean ± standard deviation (SD). The sensitivity and specificity of HYP diagnosis, 95% confidence interval (CI), and area under the curve (AUC) were analyzed using the receiver operating characteristic (ROC). Spearman correlation coefficient was used to measure the correlation between two quantitative variables. All tests were two-tailed, and a P value <0.05 was generally considered to indicate statistical significance.
Results
General information of included participants
In order to examine the diagnostic role of HYP in PTB, 144 participants were included in the study: 43 in the HC group, 39 in the RC group, and 42 in the PTB group. The general information of patients is shown in Table 1. As indicated by the table, the lymphocyte and monocyte count of the PTB group were significantly increased compared to those of the HC group (lymphocyte: P=0.02; monocyte: P=0.02) as were the levels of immunoglobin A (IgA; P=0.02) and C-reactive protein (CRP; P<0.001); however, there was significant decrease in serum protein (TP; P<0.001; ALB; P<0.001). Among the patients with PTB, TSPOT.TB, sputum smear, and sputum culture were positive in 78.57%, 71.43%, and 76.19%, respectively; meanwhile, the positive rates of the TB-RNA and the GeneXpert assay were 64.29% and 90.48%, respectively. Compared with the RC group, the PTB group had a higher neutrophil count (P=0.04) and neutrophil percentage (P=0.02), but there was no significant difference in the other indicators between these groups (P>0.05).
Table 1
| Characteristics | HC (n=43) | RC (n=39) | PTB (n=42) | P value | |
|---|---|---|---|---|---|
| PTB vs. HC | PTB vs. RC | ||||
| Sex (male/female) | 16/27 | 21/18 | 22/20 | 0.20 | 0.37 |
| Age (years) | 41.33±12.89 | 49.35±14.12 | 45.14±13.52 | 0.25 | 0.69 |
| WBC (×109/L) | 6.78±3.26 | 7.37±2.29 | 6.21±2.39 | 0.33 | 0.09 |
| LYM (×109/L) | 18.15±0.83 | 1.48±0.71 | 1.44±0.69 | 0.02* | 0.62 |
| LMY (%) | 29.66±11.68 | 20.41±7.83 | 20.08±10.13 | 0.02* | 0.24 |
| MON (×109/L) | 0.38±0.18 | 0.52±0.19 | 0.50±0.27 | 0.02* | 0.90 |
| MON (%) | 5.89±2.20 | 10.30±16.33 | 8.18±2.84 | <0.001*** | 0.56 |
| NEU (×109/L) | 4.46±2.87 | 5.19±2.02 | 4.03±1.99 | 0.42 | 0.04* |
| NEU (%) | 62.00±13.43 | 69.49±9.33 | 63.77±10.17 | 0.44 | 0.02* |
| RBC (×1012/L) | 4.49±0.61 | 4.24±0.65 | 4.42±0.72 | 0.68 | 0.56 |
| HGB (g/L) | 133.63±18.30 | 127.42±21.25 | 130.02±21.26 | 0.41 | 0.96 |
| TP (g/L) | 73.46±13.84 | 66.60±4.85 | 66.02±5.37 | <0.001*** | 0.34 |
| ALB (g/L) | 44.48±2.78 | 40.56±3.73 | 39.90±3.86 | <0.001*** | 0.42 |
| IgA(g/L) | 2.20±1.13 | 2.41±0.90 | 2.93±1.31 | 0.02* | 0.14 |
| IgG (g/L) | 11.01±2.36 | 10.40±1.78 | 10.81±2.70 | 0.65 | 0.11 |
| IgM (g/L) | 1.46±0.62 | 1.39±0.70 | 1.26±0.50 | 0.09 | 0.22 |
| IgE (IU/mL) | 66.70±70.38 | 249.45±543.29 | 123.52±274.55 | 0.19 | 0.40 |
| ESR (mm/h) | 14.09±9.97 | 24.06±32.60 | 27.83±31.11 | 0.007 | 0.62 |
| CRP (mg/L) | 5.10±4.71 | 22.60±36.30 | 41.57±60.74 | <0.001*** | 0.08 |
| TSPOT.TB | 2 (4.65) | 0 (0.00) | 33 (78.57) | <0.001*** | <0.001*** |
| MTB-smear (+) | 0 (0.00) | 0 (0.00) | 30 (71.43) | <0.001*** | <0.001*** |
| MTB-culture (+) | 0 (0.00) | 0 (0.00) | 32 (76.19) | <0.001*** | <0.001*** |
| TB-RNA (+) | 0 (0.00) | 0 (0.00) | 27 (64.29) | <0.001*** | <0.001*** |
| GeneXpert/RIF (+) | 0 (0.00) | 0 (0.00) | 38 (90.48) | <0.001*** | <0.001*** |
Data are presented as n, mean ± standard deviation, or n (%). Significant value: *, P<0.05; ***, P<0.001. HC, healthy control; RC, respiratory control; PTB, pulmonary tuberculosis; WBC, white blood cell count; LYM, lymphocyte count; MON, monocyte count; NEU, neutrophils count; RBC, red blood cell count; HGB, hemoglobin; TP, total protein; ALB, albumin; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; IgE; immunoglobulin E; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; TSPOT.TB, tuberculosis infection T lymphocyte spot assay; MTB, Mycobacterium tuberculosis; RIF, rifampicin.
Expression of HYP in different specimen types
To investigate HYP expression in different body fluids, we analyzed HYP expression in serum, urine, and BALF. Compared with that in the HC group, the expression of HYP in serum of patients with PTB was significantly increased (P=0.03), while the expression of HYP in urine was not significantly different (P>0.05). Compared with that of the RC group, HYP expression in the BALF of patients with PTB was significantly increased (P<0.001), as shown in Figure 1. Statistical analysis showed that the expression of HYP with PTB in different body fluids had certain correlations (serum HYP vs. urine HYP: r=0.8556, P<0.001; serum HYP vs. BALF HYP: r=0.4274, P=0.005; urine HYP vs. BALF HYP: r=0.4980, P<0.001) (Figure 2). Moreover, the expression of HYP in the serum and BALF of patients with PTB was higher than that of the control group, indicating HYP may have certain diagnostic value.


Correlation analysis between HYP and relevant clinical indicators
After analyzing the clinical test indicators in the PTB group, we found that serum HYP expression was positively correlated with CRP level (r=0.4661, P=0.002), neutrophil count (r=0.3338, P=0.03), and monocyte count (r=0.3462, P=0.02) and negatively correlated with albumin level (r=−0.3575, P=0.02) (Figure 3). The expression of HYP in urine was positively correlated with neutrophil count (r=0.3508, P=0.02), neutrophil percentage (r=0.3804, P=0.047), and monocyte count (r=0.3263, P=0.04) and negatively correlated with albumin level (r=−0.4031, P=0.008) (Figure 4). HYP expression in BALF showed a positive correlation with CRP level (r=0.3652, P=0.02) (Figure 5).



Diagnostic analysis of HYP and TSPOT.TB detection in PTB
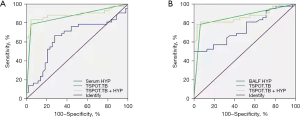
The ROC curve was used to determine the diagnostic performance of HYP in PTB. The results showed that the sensitivity, specificity, and AUC of serum HYP were 66.67%, 72.09%, and 0.6481, respectively, while the sensitivity, specificity, and AUC of HYP in BALF were 67.74%, 64.29%, and 0.7435, respectively. As TSPOT.TB is a commonly used blood test index, we analyzed the diagnostic efficacy of the combined detection of HYP and TSPOT.TB in PTB. We found that the AUC of the combined detection of serum HYP and TSPOT.TB was 0.8690, the sensitivity was 78.57%, and the specificity was 96.77%. The AUC area of the combined detection of HYP and TSPOT.TB in BALF was 0.8606, the sensitivity was 78.59%, and the specificity was 93.55%, as shown in Figure 6. These results indicate that HYP may be a valuable auxiliary indicator in the diagnosis of TB and that the combined diagnosis of HYP and TSPOT.TB can effectively improve the diagnostic efficacy.
Discussion
Although PTB has been studied in depth, and its associated vaccines and therapeutic drugs have been widely used, PTB remains a major disease across the globe (21). In clinics, the diagnosis of PTB is mainly dependent on etiological detection; however, traditional methods are reliant upon the quality of sputum and other respiratory specimens, resulting in a low detection rate (22). A number of studies have shown that MTB infection detection in the body fluid samples from patients, such as blood, can avoid the subjectivity in interpretation (23,24). Therefore, identifying novel blood molecular markers is a critical need for the early diagnosis of PTB.
HYP is a relatively traditional molecular marker and is mainly used in the diagnosis of several conditions, such as injury repair, skin diseases, liver fibrosis, and cardiovascular diseases (11-15). Although there have been some insightful reports regarding the role of HYP in PTB and its related diseases, there has been no systematic study or evaluation related to the diagnostic utility of HYP in PTB (25,26). Our study thus analyzed the role of HYP in PTB diagnosis by collecting serum, urine, and BALF from patients with PTB. Among these people included in this study, the lymphocyte and monocyte count of the PTB group were significantly increased compared to those of the HC group as were the levels of IgA and CRP. There was significant decrease in serum protein. Among the patients with PTB, TSPOT.TB, sputum smear, and sputum culture were positive in 78.57%, 71.43%, and 76.19%, respectively; meanwhile, the positive rates of the TB-RNA and the GeneXpert assay were 64.29% and 90.48%, respectively. Compared with the RC group, the PTB group had a higher neutrophil count and neutrophil percentage, but there was no significant difference in the other indicators between these groups. The results showed that compared with the HC group, the HYP expression in serum of patients with PTB was significantly increased, while that from urine was not. Compared with that in the RC group, the HYP expression in BALF of patients with PTB was significantly increased. In addition, statistical analysis suggested that in patients with PTB, HYP expression was positively correlated among serum, urine, and BALF specimens. These results indicate that the expression of HYP in serum and BALF of patients with PTB is higher than that of healthy individuals, which may have certain diagnostic significance. Subsequently, ROC curves were used to analyze the diagnostic efficacy of HYP in serum and BALF for PTB. The results showed that the sensitivity of HYP in serum was 66.67%, the specificity was 72.09%, and the AUC was 0.6481; meanwhile, the sensitivity, specificity, and AUC of HYP in BALF were 67.74%, 64.29%, and 0.7435, respectively. Finally, the sensitivity, specificity, and AUC of HYP in serum combined with TSPOT.TB were 78.57%, 96.77%, and 0.8690, respectively, while the sensitivity, specificity, and AUC of HYP in BALF combined with TSPOT were 78.59%, 93.55%, and 0.8606, respectively. These results suggest that HYP may serve as an auxiliary molecular marker in the diagnosis of PTB. The combined diagnosis of HYP and TSPOT demonstrated significantly improved the specificity and could effectively improve the diagnostic efficacy for PTB. Previous studies have shown that the urine excretion of HYP is increased in patients with TB (27,28), but we did not find this to be significantly different our in our study. This could be due to factors such as outdated literature on urine HYP detection, the low specificity of its detection methods, or different population characteristics.
Research also indicates that macrophages play a critical role in the host anti-TB infection as an important constituent of TB granuloma, which is also composed of dendritic cells, neutrophils, T cells, and B cells (29). The expression changes of neutrophils, mononuclear macrophages, and some inflammatory factors are accompanied by host anti-TB infection (30). Our study found that HYP was positively correlated with some clinical test indicators related to infection and inflammation, such as CRP level, neutrophil count, monocyte count, and neutrophil percentage. These results suggest that neutrophils, monocytes, and inflammatory cytokine CRP play an important role in the process of tissue injury and repair.
Studies have shown that around a quarter of TB cases globally are caused by malnutrition, which increases the risk of TB by 6 to 10 times (31,32). A lower albumin level is one of the main manifestations of malnutrition in patients with TB. An analysis of 1,100 patients with TB aged 65 years or older found that the average albumin level of patients with nutritional risk was significantly lower than that of patients without nutritional risk (34.15 vs. 38.18 g/L) (33). Another case-control study showed that the albumin level of patients with PTB was significantly lower than that of the control group (3.3 vs. 3.9 g/dL) (34). The main mechanism underlying this association is that patients with PTB are prone to gastrointestinal dysfunction and loss of appetite due to the influence of inflammation and disturbance of feeding factors, which increases the level of serotonin in hypothalamus (35). In addition, patients with TB are often unable to synthesize proteins due to the “anabolic disorder” of proteins, and the amino acids they ingestion are mainly used for oxidation and energy supply, resulting in the loss of protein libraries in the body (36). In addition, the downregulation of albumin in those with TB is not conducive to lesion absorption, cavity closure, or drug absorption, and drug-induced liver injury is more likely to occur during anti-TB treatment, which hinders the rehabilitation of patients with TB (37). Our study found that in the PTB group, the expression of HYP was negatively correlated with the albumin level. This indicates that the downregulation of nutritional protein in the body might lead to the slowing down of lung tissue repair during the onset of TB, resulting in the decline of the body’s ability to clear MTB, which is not conducive to the reconstruction and repair of lung tissue and leads to increased HYP expression.
Conclusions
Our findings suggest that the expression of HYP in patients with PTB is significantly increased, and thus HYP expression may serve as an auxiliary diagnostic molecular marker of TB. Furthermore, HYP expression is correlated with relevant clinical indicators and can reflect the disease status well. Due to the limitations of sample size and single-center study, although the study has achieved certain positive diagnostic results, we hope to conduct multi-center study and expand the sample size to further confirm the value of HYP in PTB in the future, and we are looking for researchers work together and join us.
Acknowledgments
Funding: This work was supported financially by grants from
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1585/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1585/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1585/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-1585/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of the Affiliated Nantong Hospital of Shanghai University (The Sixth People’s Hospital of Nantong) (No. NTLYLL2019017). Informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lai P, Cai W, Qu L, et al. Pulmonary Tuberculosis Notification Rate Within Shenzhen, China, 2010-2019: Spatial-Temporal Analysis. JMIR Public Health Surveill 2024;10:e57209. [Crossref] [PubMed]
- Vega V, Cabrera-Sanchez J, Rodríguez S, et al. Risk factors for pulmonary tuberculosis recurrence, relapse and reinfection: a systematic review and meta-analysis. BMJ Open Respir Res 2024;11:e002281. [Crossref] [PubMed]
- Li Z, Wang J, Xiu X, et al. Evaluation of different diagnostic methods for spinal tuberculosis infection. BMC Infect Dis 2023;23:695. [Crossref] [PubMed]
- Ismail N, Dippenaar A, Morgan G, et al. Microfluidic Capture of Mycobacterium tuberculosis from Clinical Samples for Culture-Free Whole-Genome Sequencing. Microbiol Spectr 2023;11:e0111423. [Crossref] [PubMed]
- Zou X, Zhu Y, Qin Y, et al. Value analysis of next-generation sequencing combined with Xpert in early precise diagnosis of pulmonary tuberculosis. Diagn Microbiol Infect Dis 2023;107:115921. [Crossref] [PubMed]
- Kim KH, Jeong N, Lim JU, et al. Clinical relevance of false-negative interferon-gamma release assays in patients with tuberculous pleurisy in an intermediate tuberculosis burden country. J Thorac Dis 2022;14:1009-19. [Crossref] [PubMed]
- Xu T, Lai Q, Qu N, et al. Diagnostic Values of Peripheral Blood T-Cell Spot Test for Tuberculosis (T-SPOT.TB) for Spinal Tuberculosis. Surg Infect (Larchmt) 2023;24:534-40. [Crossref] [PubMed]
- Herai Y, Yahaba M, Taniguchi T, et al. Factors Influencing the Indeterminate Results in a T-SPOT.TB test: A Matched Case-control Study. Intern Med 2023;62:3321-6. [Crossref] [PubMed]
- Li J, Jing Q, Hu Z, et al. Mycobacterium tuberculosis-specific memory T cells in bronchoalveolar lavage of patients with pulmonary tuberculosis. Cytokine 2023;171:156374. [Crossref] [PubMed]
- Yuswan MH, A, Jalil NH, Mohamad H, et al. Hydroxyproline determination for initial detection of halal-critical food ingredients (gelatin and collagen). Food Chem 2021;337:127762. [Crossref] [PubMed]
- Strong Rodrigues K, Oliveira-Ribeiro C, de Abreu Fiuza Gomes S, et al. Cutaneous Graft-Versus-Host Disease: Diagnosis and Treatment. Am J Clin Dermatol 2018;19:33-50. [Crossref] [PubMed]
- Xu Y, Pan X, Hu S, et al. Hepatocyte-specific expression of human carboxylesterase 2 attenuates nonalcoholic steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol 2021;320:G166-74. [Crossref] [PubMed]
- Rendon A, Schäkel K. Psoriasis Pathogenesis and Treatment. Int J Mol Sci 2019;20:1475. [Crossref] [PubMed]
- Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen 1994;2:165-70. [Crossref] [PubMed]
- Ning B, Zhang F, Song X, et al. Cardiac contractility modulation attenuates structural and electrical remodeling in a chronic heart failure rabbit model. J Int Med Res 2020;48:300060520962910. [Crossref] [PubMed]
- Shukla ST, Kaushik A, Auti SA, et al. Preclinical determination of wound-healing activity of halibut oil cream in rat model of burn wound. J Asian Nat Prod Res 2024; Epub ahead of print. [Crossref]
- Pezzella AT. History of Pulmonary Tuberculosis. Thorac Surg Clin 2019;29:1-17. [Crossref] [PubMed]
- Ravimohan S, Kornfeld H, Weissman D, et al. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev 2018;27:170077. [Crossref] [PubMed]
- Yablonskii PK, Kudriashov GG, Avetisyan AO. Surgical Resection in the Treatment of Pulmonary Tuberculosis. Thorac Surg Clin 2019;29:37-46. [Crossref] [PubMed]
- Tuberculosis Pearce L. Emerg Nurse 2017;24:13. [Crossref] [PubMed]
- Trajman A, Schwartzman K. Improving diagnosis of tuberculosis in children. Lancet Infect Dis 2021;21:302-3. [Crossref] [PubMed]
- Yu R, Hu S, Wang C, et al. Clinical diagnostic algorithm in defining tuberculous unilateral pleural effusion in high tuberculosis burden areas short of diagnostic tools. J Thorac Dis 2022;14:866-76. [Crossref] [PubMed]
- Xu F, Zhang H, Si X, et al. Assessment of CD27 expression on T-cells as a diagnostic and therapeutic tool for patients with smear-negative pulmonary tuberculosis. BMC Immunol 2021;22:41. [Crossref] [PubMed]
- Zhou W, Cheng G, Zhang Z, et al. Deep learning-based pulmonary tuberculosis automated detection on chest radiography: large-scale independent testing. Quant Imaging Med Surg 2022;12:2344-55. [Crossref] [PubMed]
- Grigorian VG, Polinkovskiĭ VI, Bradishtianu KIa. Serum hydroxyproline determination in pulmonary tuberculosis. Probl Tuberk 1980;42-5.
- Shkurupii VA, Kim LB, Potapova OV, et al. Study of fibrotic complications and hydroxyproline content in mouse liver at different stages of generalized BCG-induced granulomatosis. Bull Exp Biol Med 2014;157:466-9. [Crossref] [PubMed]
- Evtod'eva MIa, Alimova EK, Karapetian LP, et al. The clinical significance of urinary hydroxyproline and mucoprotein determination in childhood tuberculosis. Probl Tuberk 1970;48:27-30.
- Pawelec D. Urinary excretion of hydroxyproline in patients with chronic fibro-cavernous pulmonary tuberculosis. Gruzlica 1972;40:919-23.
- Amaral EP, Vinhaes CL, Oliveira-de-Souza D, et al. The Interplay Between Systemic Inflammation, Oxidative Stress, and Tissue Remodeling in Tuberculosis. Antioxid Redox Signal 2021;34:471-85. [Crossref] [PubMed]
- Sheedy FJ, Divangahi M. Targeting immunometabolism in host defence against Mycobacterium tuberculosis. Immunology 2021;162:145-59. [Crossref] [PubMed]
- Carriço F, Sousa MM, Duarte R. Nail Dystrophy due to Tuberculosis. Arch Bronconeumol (Engl Ed) 2020;56:392-3. [Crossref] [PubMed]
- Grobler L, Nagpal S, Sudarsanam TD, et al. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev 2016;2016:CD006086. [Crossref] [PubMed]
- Shen S, Xiao Y. Association Between C-Reactive Protein and Albumin Ratios and Risk of Mortality in Patients with Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis 2023;18:2289-303. [Crossref] [PubMed]
- Murakami R, Matsuo N, Ueda K, et al. Epidemiological and spatial factors for tuberculosis: a matched case-control study in Nagata, Japan. Int J Tuberc Lung Dis 2019;23:181-6. [Crossref] [PubMed]
- Badawi A, Gregg B, Vasileva D. Systematic analysis for the relationship between obesity and tuberculosis. Public Health 2020;186:246-56. [Crossref] [PubMed]
- Oxlade O, Huang CC, Murray M. Estimating the Impact of Reducing Under-Nutrition on the Tuberculosis Epidemic in the Central Eastern States of India: A Dynamic Modeling Study. PLoS One 2015;10:e0128187. [Crossref] [PubMed]
- Duan MM, Chen XR. Observation on the clinical effect of nutrition support for treatment of severe pulmonary tuberculosis. Modern Prevent Med 2017;44:4525-7.
(English Language Editor: J. Gray)


