The effectiveness of mediastinal lymph node evaluation in a patient with ground glass opacity tumor
Introduction
Lung cancer is the leading cause of cancer death globally (1). Recently, the use of chest computed tomography (CT) for lung cancer screening and ground glass opacity (GGO) detection has increased. The persistent presence of GGO nodules on CT usually suggests the presence of lung adenocarcinoma or a precancerous lesion (2). Since GGO tumor is considered to have a lepidic pattern, which is non-invasive in many cases, a tumor with more GGO content generally has a better prognosis (3-5). Since adenocarcinoma with GGO features may have a variety of prognoses due to amount of heterogeneity, options for surgical treatment (limited resection versus standard anatomical resection) is often selected depending on the degree of GGO.
Generally, standard anatomical resection (lobectomy) with mediastinal lymph node dissection (MLND) has been recommended for early stages of non-small cell lung cancer (NSCLC) (6-8). Performance of mediastinal lymph node evaluation (MLE) is recommended in most NSCLC, since occult lymph node metastasis may occur even in clinical N0 (9-12). MLE methods include MLND and mediastinal lymph node sampling (MLS). Even though MLND is generally considered to give more accurate nodal staging, whether or not MLND is associated with greater survival compared to MLS is controversial (9,10).
Many clinicians have recently opined that standard anatomical resection and MLND do not have to be performed for GGO tumors. Good results have been reported for limited resection of GGO-predominant nodules in particular, and randomized trials are underway (13-16). This suggests that the extent of surgery in GGO-predominant NSCLC may not need to be the same as in other lung tumors. It is important in decisions regarding surgical management to know the extent of resection and level of lymph node dissection. Although many studies have examined the results of limited resection of GGO nodules, there have not been many studies that have looked at the degree of surgical lymph node evaluation and association with GGO tumor outcomes. The aim of this study was to find out whether MLE is necessary for small (≤3 cm) and clinical N0 GGO-predominant NSCLC. We wanted to examine the utility of MLE by comparing the survival rates of cases in which MLE was and was not performed. We then compared the survival rates with solid-predominant tumor cases to elucidate any required conditions for MLE in GGO. We also wanted to determine whether GGO-predominant tumors were associated with occult lymph node metastases.
Methods
Patients
Between January 2004 and December 2013, a total of 958 consecutive patients at Seoul St. Mary’s Hospital in Korea were diagnosed with NSCLC and underwent surgical resection. Tumors of 484 patients were 3 cm or less and clinical N0 stage. Patients who underwent limited resections and incomplete resection were excluded (106 patients). Other types of carcinoma, such as large cell carcinoma, large cell neuroendocrine carcinoma, pleomorphic carcinoma, mucoepidermoid carcinoma, sarcomatoid carcinoma, and carcinoid were excluded (20 patients), and we performed analysis only for adenocarcinoma and squamous cell carcinoma (SqCC). Those patients’ (358 patients) charts were reviewed retrospectively. Surgical procedures included lobectomy, bilobectomy, and pneumonectomy. Mediastinal node evaluation was performed using two methods (MLND and MLS). MLND was en bloc resection of more than three mediastinal lymph node stations (9). MLS was partial node resection of more than one mediastinal node station selectively. MLND or MLS was performed according to surgeon preference.
According to radiologic findings, we classified all patients into two groups: GGO-predominant tumor and solid-predominant tumor. We then compared clinicopathological features and survival between the two groups, then classified each group into three subgroups: a group in which MLE was not performed [no mediastinal lymph node evaluation (NoMLE) group], a group in which MLS was performed (MLS group), and a group in which MLND was performed (MLND group). This study was approved by the institutional review board of Seoul St. Mary’s Hospital at the Catholic University of Korea (KC16RISI0163).
Radiologic evaluation and Preoperative staging
TNM staging was based on the 7th American Joint Committee on Cancer (AJCC) guidelines (17). Clinical staging was executed by contrast-enhanced chest CT and F-18-fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT scanning within 1 month before operation. Primary lesions were also evaluated using thin-section CT images. CT scans were obtained during full inspiration. GGO was defined on a CT scan by hazy increased opacities in lung parenchyma with preservation of bronchial structures and vascular margins (18). The diameter of GGO was defined as the largest axial diameter of the lesion on the lung window setting. Consolidation was defined as an area of increased opacification that completely obscured underlying bronchial structures and vascular markings, and the diameter of the consolidation area on the axial image on the lung window setting was also measured. We defined tumors with the diameter of consolidation to the diameter of tumor ratio (C/T ratio) less than 0.5 as GGO-predominant tumors, and tumors with C/T ratio greater than or equal to 0.5 as solid-predominant tumors. Each lung nodule on preoperative CT scans was reviewed blindly by two thoracic surgeons.
LNs were regarded as malignant when their short-axis diameters were greater than 10 mm on a CT scan and their FDG uptakes were greater than those of the surrounding mediastinal structures. However, high FDG in a LN was regarded as benign if the LN contained benign calcification or if unenhanced CT images showed high attenuation with a distinct margin (19). In cases in which there was general symmetric and equivocal FDG uptake in the mediastinal lymph nodes on a PET/CT scan, it was interpreted as reactive change of the lymph node by inflammation. In patients diagnosed with cN0 tumors by chest CT and PET/CT scanning, surgery was performed without preoperative invasive LN staging if complete resection was considered possible.
Histologic evaluation
All clinical specimens were examined by pathologists whose observations were recorded. Tumor studies included histologic characteristics like tumor size, location, differentiation, lymph node status, pleural invasion, lymphatic invasion, and vascular invasion. In the cases of adenocarcinoma, histologic subtypes were classified according to 2015 WHO classification system (20). Adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA) defined as small (≤3 cm) and solitary adenocarcinomas that consisted of lepidic growth pattern without invasion (AIS) or with ≤5 mm invasion (MIA) were identified (14).
Statistical analysis
Clinicopathological factors were compared. The Student’s t-test or the Wilcoxon rank-sum test was used for two groups of continuous variables, and ANOVA and Kruskal Wallis H test was used for three groups of continuous variables. In addition, the χ2 test or Fisher’s exact test was applied for categorical variables. Follow-up data for the interval between surgical resection and last follow-up visit were analyzed using confirmed recurrences/deaths to calculate RFS via Kaplan-Meier method. Survival of each group was compared by log-rank test, and the Cox proportional hazards model of multivariate analysis was engaged to determine risk of recurrence. Multivariate logistic regression was used to analyze factors influencing nodal upstaging after surgery. A value of P<0.05 was considered statistically significant.
Results
Of a total of 358 patients, the number with GGO-predominant tumor was 129 (36.0%) and that with solid-predominant tumor was 229 (64.0%). Clinicopathological characteristics were compared among the NoMLE, MLS, and MLND groups of GGO-predominant tumor and solid-predominant tumor (Table 1).
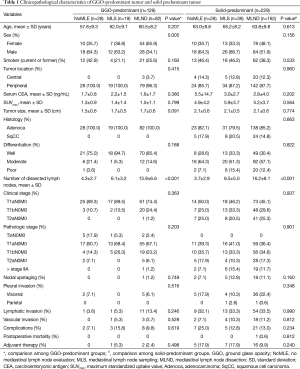
Full table
In the case of GGO-predominant tumor, there was no difference in age, smoking history, tumor location, preoperative mean serum carcinoembryonic antigen (CEA) level, or mean maximum standardized uptake value (SUVmax) of FDG on PET scanning among the NoMLE, MLS, and MLND groups; the only significant difference was that there were more females in the MLND group compared to the others. Mean tumor size was smaller in the NoMLE group (1.5 cm) than in the MLS (1.7 cm) or MLND (1.7 cm) groups, but it was not statistically significant (P=0.091). All GGO-predominant tumors were adenocarcinoma. There was no difference in tumor differentiation among the three groups. There was no difference in either clinical stage or pathologic stage (P=0.363, P=0.203, respectively). A patient with tumor beyond pathologic stage IIA was found in the MLND group (clinical T1aN0M0 to pathological T1aN1M0). In other words, the number of nodal upstaging in GGO-predominant tumor was 1 (0.8%). There was no difference among the three groups in visceral pleural invasion, lymphatic invasion, or vascular invasion of tumor. There was no difference in the incidence of postoperative complications, and there was no postoperative mortality. There was no significant difference among the three groups in terms of cases in which adjuvant chemotherapy was executed.
In the case of solid-predominant tumor, there was no difference among the three groups in age, sex, smoking history, tumor location, involved lobe, preoperative serum CEA, or SUVmax. There was also no difference in tumor size, histology, tumor differentiation, clinical stage, pathologic stage, incidence of nodal upstaging, visceral pleural invasion, lymphatic invasion, or vascular invasion. Nodal upstaging was observed in 25 patients (10.9%). There were 17 cases of N1 metastasis and 8 cases of N2 metastasis. There was no difference among the three groups in terms of postoperative complications, and there was one incidence of postoperative mortality in the MLND group.
GGO-predominant tumor was divided into AIS, MIA, and invasive adenocarcinoma made by postoperative histologic diagnosis (Table 2). There was no difference among the three groups even in cases when they were diagnosed with AIS or MIA after surgery (P=0.176).
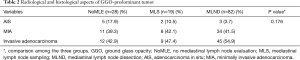
Full table
Median follow-up time for all patients was 1,153 days (range, 34–4,095 days), and recurrences were recorded in 63 patients. There was no difference in recurrence among the three groups in GGO-predominant tumor, however there was significantly more locoregional recurrence in the solid-predominant tumor (28.6% vs. 12.8% vs. 8.6%, P=0.002) in the NoMLE group vs. MLS vs. MLND, respectively (Table 3).
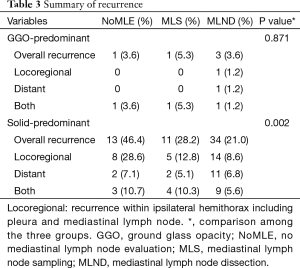
Full table
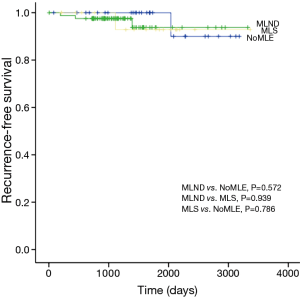
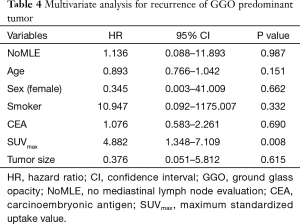
There was no statistical difference among the NoMLE, MLS, and MLND groups in terms 5-year RFS in the case of GGO-predominant tumor (100% vs. 92.9% vs. 93.8%, respectively, P=0.889; Figure 1). Survivals of NoMLE, MLS, and MLND groups of solid-predominant tumor were 48.6%, 72.8%, and 73.1%, respectively (Figure 2). There was statistically significant difference between NoMLE and MLND (P=0.007) in solid-predominant tumor. Multivariate Cox proportional hazards analysis of RFS in GGO-predominant tumor showed that NoMLE was not associated with recurrence [covariate factors = age, sex, smoking status, CEA, SUVmax, tumor size ; HR, 1.021; 95% confidence interval (CI), 0.088–11.893; P=0.987] (Table 4). SUVmax was a significant risk factor for recurrence in GGO-predominant tumor (HR, 3.096; 95% CI, 1.348–7.109; P=0.008).
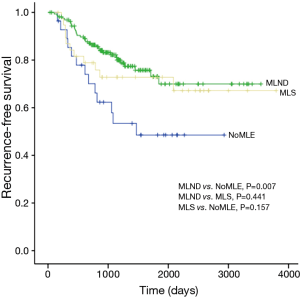
Full table
Multivariate analysis (via logistic regression model) was performed to identify risk factors for nodal upstaging (Table 5). Variables in the model included age, sex, smoking status, GGO-predominant tumor, adenocarcinoma, NoMLE, central location, CEA, and tumor size. Ultimately, GGO-predominant tumor [odds ratio (OR) =0.063; P=0.008] was identified as the sole parameter significantly impacting nodal upstaging in a negative fashion.
Full table
Discussion
Adenocarcinoma is heterogeneous and mostly exists in the form of mixed subtypes. According to the 2015 WHO classification of lung tumors, adenocarcinoma may be classified into various subtypes (20,21). AIS and MIA, in which a lepidic pattern is a major component, show a very good prognosis (22). Since a lepidic pattern is known to be non-invasive and often appears as GGO on CT, a patient with a GGO nodule on imaging is likely to be diagnosed with AIS or MIA (4,23). Although the standard surgical treatment of early lung cancer is major pulmonary resection with MLND, a lesser surgical procedure such as limited resection may be sufficient for GGO since there is a good possibility that it is AIS or MIA. In this study we focused on MLE rather than limited resection as the extent of the surgical procedure and we included patients who underwent standard anatomical resection over lobectomy. We chose our criteria of tumor size 3 cm or less because T1 comprises tumors sized 3 cm or less, and AIS and MIA, whose prognosis is favorable, also consist of tumors sized 3 cm or less (21). That is also why we chose to study tumors considered to be in early clinical stage.
Comparison of clinicopathological characteristics was made with the classification of GGO-predominant tumor into NoMLE, MLS, and MLND groups, and there was no statistically significant difference in all factors (except sex), particularly in pathologic stage and histological subtype (AIS or MIA). There was no difference among the three groups in 5-year RFS in GGO-predominant tumor. In other words, there was no effect on survival even if MLE was not conducted in GGO-predominant tumor. In addition, in multivariate analysis, which was conducted to identify factors related to recurrence of GGO-predominant tumor, NoMLE was not a significant factor related to recurrence. There was one incidence of nodal upstaging in the MLND group of GGO-predominant tumor. However, in that case, the metastasis was found in a lobar lymph node (N1 node, station 12), which was resected at lobectomy, and not in a mediastinal lymph node, so there was no case in which metastasis was found in a mediastinal lymph node in a GGO-predominant tumor. That fact supports the suggestion that MLE is not required in GGO-predominant tumor.
In previous studies, the incidence of mediastinal lymph node metastasis has been very low in GGO-predominant early stage NSCLC (11,24). This study also found that there was no mediastinal lymph node metastasis in GGO-predominant tumor. It may be reasonable to bypass MLE in cases with low risk of mediastinal lymph node metastasis. Although one study reported no difference in postoperative complications between selective LN sampling and MLND (25), it can be assumed that if MLE is not performed, the surgical risk and operative time can be lessened and postoperative status of patients improved.
In the case of solid-predominant tumor, there was no difference among the three groups in terms of clinicopathological characteristics, but the 5-year RFS was lowest in the NoMLE group. In particular, there was statistically significant difference in the survival between the NoMLE and MLND groups. For solid-predominant tumor, it is recommended that MLE be performed without fail as standard treatment, even in the case of clinical N0, since more accurate mediastinal lymph node staging is to be made through MLND or MLS in the end (9). In the case of solid-predominant tumor, there have been cases in which tumors previously diagnosed as clinical N0 were diagnosed with mediastinal lymph node metastasis after surgery (26). In this study, mediastinal lymph node metastasis was also found in eight patients; seven of those patients were found through MLND. If MLND had been performed in both NoMLE and MLS groups, more cases of N2 node metastasis could have been detected. This strongly suggests that MLE should always be performed in the case of solid-predominant tumor.
In this study, clinical stage and radiomorphologic features were the only preoperative factors used to choose the surgical procedure, because pathologic stage and histomorphologic characteristics of tumors could not be determined preoperatively. The C/T ratio was used as part of the radiomorphologic evaluation, because many studies have used the C/T ratio as an indicator of tumor invasiveness or indication for sublobar resection (14,15,27). Disease-specific prognosis better reflects cancer-related RFS as opposed to overall survival. Moreover, overall survival is a poor gauge of prognosis in stage I disease comparisons, because deaths are less likely a direct result of cancer (14). For this study, comparison of cancer-specific prognosis was our aim, and we examined RFS overall and separately at clinical stage I.
This study had a number of limitations: the first is that this was a retrospective review. Second, we obtained the data from a single institution, and there were an insufficient number of cases. A multicenter randomized trial may be required to validate our results. Third, a slightly more accurate cN0 could have been obtained if invasive LN staging had been performed for preoperative LN diagnosis rather than only chest CT and PET/CT scanning. However, because invasive LN staging very rarely yields positive results in cases of cN0 tumors found on chest CT and PET/CT scans and because of its high cost and invasiveness, it is generally performed only if N2 or N3 disease is suspected. Furthermore, invasive LN staging is considered unnecessary since the incidence of LN metastasis is extremely rare in GGO-predominant tumor.
In conclusion, NoMLE does not reduce the survival of clinical N0 non small cell lung cancer presenting as GGO-predominant nodule 3 cm or less in size. The incidence of mediastinal lymph node metastasis is extremely rare in GGO-predominant lung cancer. In particular, postoperative lymph nodal upstaging seldom occurs in GGO-predominant lung cancer. Future multicenter randomized controlled trials may provide more accurate results.
Acknowledgements
This article has been edited by native English-speaking experts of BioMed Proofreading, LLC.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board of Seoul St. Mary’s Hospital at the Catholic University of Korea (KC16RISI0163).
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Son JY, Lee HY, Kim JH, et al. Quantitative CT analysis of pulmonary ground-glass opacity nodules for distinguishing invasive adenocarcinoma from non-invasive or minimally invasive adenocarcinoma: the added value of using iodine mapping. Eur Radiol 2016;26:43-54. [Crossref] [PubMed]
- Nitadori J, Bograd AJ, Morales EA, et al. Preoperative consolidation-to-tumor ratio and SUVmax stratify the risk of recurrence in patients undergoing limited resection for lung adenocarcinoma ≤2 cm. Ann Surg Oncol 2013;20:4282-8. [Crossref] [PubMed]
- Wilshire CL, Louie BE, Manning KA, et al. Radiologic Evaluation of Small Lepidic Adenocarcinomas to Guide Decision Making in Surgical Resection. Ann Thorac Surg 2015;100:979-88. [Crossref] [PubMed]
- Takahashi M, Shigematsu Y, Ohta M, et al. Tumor invasiveness as defined by the newly proposed IASLC/ATS/ERS classification has prognostic significance for pathologic stage IA lung adenocarcinoma and can be predicted by radiologic parameters. J Thorac Cardiovasc Surg 2014;147:54-9. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Cho JS, Jheon S, Park SJ, et al. Outcome of limited resection for lung cancer. Korean J Thorac Cardiovasc Surg 2011;44:51-7. [Crossref] [PubMed]
- Darling GE. Current status of mediastinal lymph node dissection versus sampling in non-small cell lung cancer. Thorac Surg Clin 2013;23:349-56. [Crossref] [PubMed]
- Huang X, Wang J, Chen Q, et al. Mediastinal lymph node dissection versus mediastinal lymph node sampling for early stage non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2014;9:e109979. [Crossref] [PubMed]
- Ye B, Cheng M, Li W, et al. Predictive factors for lymph node metastasis in clinical stage IA lung adenocarcinoma. Ann Thorac Surg 2014;98:217-23. [Crossref] [PubMed]
- Moon Y, Kim KS, Lee KY, et al. Clinicopathologic Factors Associated With Occult Lymph Node Metastasis in Patients With Clinically Diagnosed N0 Lung Adenocarcinoma. Ann Thorac Surg 2016;101:1928-35. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Eguchi T, Kadota K, Park BJ, et al. The new IASLC-ATS-ERS lung adenocarcinoma classification: what the surgeon should know. Semin Thorac Cardiovasc Surg 2014;26:210-22. [Crossref] [PubMed]
- Cho JH, Choi YS, Kim J, et al. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg 2015;99:218-22. [Crossref] [PubMed]
- Sim HJ, Choi SH, Chae EJ, et al. Surgical management of pulmonary adenocarcinoma presenting as a pure ground-glass nodule. Eur J Cardiothorac Surg 2014;46:632-6; discussion 636. [Crossref] [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. editors. AJCC cancer staging manual. 7th ed. New York: Springer, 2010.
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Lu P, Sun Y, Sun Y, et al. The role of (18)F-FDG PET/CT for evaluation of metastatic mediastinal lymph nodes in patients with lung squamous-cell carcinoma or adenocarcinoma. Lung Cancer 2014;85:53-8. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. The correlation of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification with prognosis and EGFR mutation in lung adenocarcinoma. Ann Thorac Surg 2014;98:453-8. [Crossref] [PubMed]
- Cohen JG, Reymond E, Lederlin M, et al. Differentiating pre- and minimally invasive from invasive adenocarcinoma using CT-features in persistent pulmonary part-solid nodules in Caucasian patients. Eur J Radiol 2015;84:738-44. [Crossref] [PubMed]
- Haruki T, Aokage K, Miyoshi T, et al. Mediastinal nodal involvement in patients with clinical stage I non-small-cell lung cancer: possibility of rational lymph node dissection. J Thorac Oncol 2015;10:930-6. [Crossref] [PubMed]
- Okada M, Sakamoto T, Yuki T, et al. Selective mediastinal lymphadenectomy for clinico-surgical stage I non-small cell lung cancer. Ann Thorac Surg 2006;81:1028-32. [Crossref] [PubMed]
- Hattori A, Suzuki K, Matsunaga T, et al. Is limited resection appropriate for radiologically "solid" tumors in small lung cancers? Ann Thorac Surg 2012;94:212-5. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Oncological Characteristics of Radiological Invasive Adenocarcinoma with Additional Ground-Glass Nodules on Initial Thin-Section Computed Tomography: Comparison with Solitary Invasive Adenocarcinoma. J Thorac Oncol 2016;11:729-36. [Crossref] [PubMed]


